Abstract
A phosphazene-based flame retardant (PBFA) was synthesized by hexachlorocyclotriphosphazene and N-aminoethylpiperazine. To improve the flame retardancy of polypropylene (PP), PBFA and pentaerythritol derivatives (PEPA) were mechanically mixed to form a water resistance intumescent flame retardant (IFR). The flammability and thermal properties of the PP composites were analyzed by vertical burning test (UL-94), limit oxygen index, thermogravimetric analysis (TGA) and cone calorimeter test (CCT). The results indicate that there was a synergistic effect between PBFA and PEPA at a suitable mass ratio. When the mass ratio of PBFA/PEPA was 2:1, the PP composites can successfully achieve UL-94 V 0 rating. Compared with pure PP, the peak heat release rate and the total heat release were decreased by 75.40 and 15.38%, respectively; moreover, the total smoke production decreased by 22.11% during 0–465 s. The residual char after CCT was characterized by Fourier transform infrared spectrometer (FTIR). A strong pyrophosphate absorption peak was found in FTIR, indicating that the IFR mainly played a flame-retardant mechanism in the condensed phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the excellent mechanical property, electrical resistance, ease processing, low density and low cost, polypropylene (PP) has been widely used in various fields [1, 2]. However, the high flammability and melt dripping during combustion of PP limited its application. Therefore, improving the flame retardancy of PP is an important direction for studying the application of PP [3].
Traditionally, the combination of bromine-containing flame retardants and antimony trioxide is the most effective flame-retardant system. However, the corrosive smoke HBr and toxic gases will be produced during combustion which limited this synergistic system in application [4]. Hence, environmentally friendly halogen-free flame retardants such as metal hydroxides are the most widely used. But the mechanical properties of polymer matrix are greatly affected by the high loading of these metal hydroxides, for example, Mg(OH)2 was incorporated into PP with 60 mass% resulting in 80% decrease of mechanical property [5].
In the past decades, it has been found that intumescent flame-retardant system (IFRs) is very effective for thermoplastic resins such as PP and polyethylene (PE). IFRs is a kind of widely used flame retardant with low smoke, non-toxic, halogen free and low production of corrosive gases [6]. IFRs usually consist of three components: acid sources, carbon sources and gas sources. At present, the most common substances in acid sources are ammonium polyphosphate (APP), magnesium ammonium phosphate (MAP) and zinc borate. Carbon sources mainly include polyhydroxy compounds (such as pentaerythritol (PER), mannitol or sorbitol) and carbon-rich polymers. And the gas source is mainly melamine (MA), APP, dicyandiamide, etc. [7]. However, APP is soluble in water and PER contains strong hydrophilic hydroxyl group, which makes the commonly used intumescent systems (APP, PER and MA) easy to absorb moisture. This water absorption seriously affects the application of IFRs [8].
To break through the water absorption limitations of traditional acid sources and find a flame retardant both including acid and gas or carbon sources, scientists have made further research on phosphazene derivatives containing at least an acid source and a gas source or carbon source in IFRs. Wen et al. designed a cyclotriphosphazene-based char-forming agent (CPCFA) with a char yield of 86.5% by one-pot reaction of cyclotriphosphazene and piperazine and then applied it as an acid source and a carbon source in PP combining with the microcapsule ammonium polyphosphate (MAPP) to form an efficient IFRs with high water resistance [9]. The limit oxygen index (LOI) value of PP composites is as high as 37% (the LOI value of pure PP is 17%) at the mass ratio of MAPP: CPCFA = 2:1. Moreover, the composites can still reach V 0 rating after 72-h immersion in water. He et al. carried out SN2 nucleophilic substitution reaction to replace 3-chloropropyl (triethoxy) silane with hexachlorocyclotriphosphazene (HCCP) to form a phosphazene derivative APESP(N3P3[NH(CH2)3Si(OCH2CH3)3]6) [10]. Then, APESP and APP were mixed through an atomizing device to form APESP/APP, following it was added into PP [11]. The PHRR value of the PP composite was reduced by 53.1% and the LOI value was 26.5%. It can be concluded that cyclotriphosphazene can be used as matrix to form acid source, carbon source or gas source of IFRs by reacting with some excellent flame-retardant groups. In our previous research, we synthesized a phosphazene-based flame retardant (PBFA) using HCCP and N-aminoethylpiperazine (AEP) [14]. PBFA is a kind of cross-linked product which is insoluble in water and organic solvent. During combustion, it can release nonflammable gas and phosphorous substance. On the one hand, the released nonflammable gas can play the role of gas source—diluting combustible gas; on the other hand, phosphorous substance can be used as acid source to form char in condensed phase. Consequently, PBFA is an ideal gas source and acid source material in IFRs.
The modification of polyhydroxy compounds has received more and more attention for increasing the water resistance of traditional carbon source. In the middle and late twentieth century, Verkade et al. synthesized a PER derivative with cage structure for the first time, namely 1-oxo-4-hydroxymethyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane (PEPA) [12]. It still has a hydroxyl group in its structure which has lower hydrophilicity than multiple hydroxyl groups in PER. Then, a macromolecular carbonization agent PEPADC with a water resistance was synthesized by PEPA, diethylenetriamine and cyanuric chloride, and was added with APP to flame-retardant PP [13]. When the mass ratio of APP:PEPADC is 4:1, the LOI value of PP composites reached 35.5% and the char residue rate of the flame-retardant PP composites can reach 29.1%,
To build a water resistance and high-efficiency IFRs, PBFA [14] and PEPA were designed and evenly mixed by mechanical method. Then, the flammability and thermal properties of PP composites were investigated by vertical burning test (UL-94), LOI, thermogravimetric analysis (TGA) and cone calorimeter test (CCT). The flame-retardant mechanism was studied by Fourier transform infrared spectrometer (FTIR) of the residual char after the CCT.
Experimental section
Materials
AEP was purchased from Aladdin Industrial Co. Ltd. (Shanghai, China). 1,4-dioxane was obtained from Tianjin Kemiou Chemical Reagent Co. Ltd. (Tianjin, China). Triethylamine (TEA) was provided by Tianjin Fuchen Chemical Reagent Factory (Tianjin, China). PP particles were supplied by Shanghai Secco Petrochemical Co., Ltd. (Shanghai, China). Pentaerythritol phosphate was obtained from Jiangsu Vicotrey Chemical Co., Ltd. (Jiangsu, China). HCCP was purchased from Zibo Lanyin Chemical Co. Ltd. (Zibo, China).
Preparation of PBFA
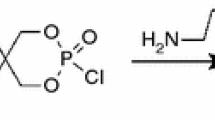
Phosphazene-based flame retardant was prepared according to the steps of our previous research. PBFA was synthesized by substituting AEP for the active chlorine atom on the cyclophosphazene The results of X-ray photoelectron spectroscopy, FTIR, solid-state 31P nuclear magnetic resonance and TGA showed that PBFA was a water-insoluble and cross-linking flame retardant with thermal decomposition temperature of 247.2 °C [14].
Preparation of different mass proportions of PBFA/PEPA
First, 24-g PBFA and 6-g PEPA were mixed together in a crusher to prepare evenly flame-retardant PBFA/PEPA with a mass proportion of 4:1 noting PBFA/PEPA (4:1). Then, the flame retardants PBFA/PEPA with different mass ratios of 1:2, 1:1, 2:1 and 3:1 were obtained in the same preparation method of PBFA/PEPA (4:1), which were noted as PBFA/PEPA (1:2), PBFA/PEPA (1:1), PBFA/PEPA (2:1) and PBFA/PEPA (3:1), respectively.
Preparation of PP-PBFA/PEPA
A typical preparation process of PP composite with 30 mass% PBFA/PEPA (2:1) was listed below: first, 18-g PBFA/PEPA (2:1) and 42-g PP were dried in a vacuum oven at 60 °C for 6 h. Second, the prepared samples were blended at 160 °C for 8 min in a two-roll mill and hot pressed at 170 °C for 8 min in a flat vulcanizer, followed by cold pressing for 8 min. Finally, the prepared sheet was cut into the appropriate specimens for the following test. The preparation of pure PP, PP-PBFA/PEPA (1:2), PP-PBFA/PEPA (1:1), PP-PBFA/PEPA (3:1) and PP-PBFA/PEPA (4:1) composites followed the same process.
Characterization
TGA was performed on a SAT 449C thermal analyzer (NETZSCH, Germany) instrument under nitrogen atmosphere. The FTIR (TENSOR 27, Bruker, Germany) was collected at room temperature. The combustion behaviors of PP composites were investigated by CCT (Fire Testing Technology, UK).The specimen was 100 mm × 100 mm × 3 mm. The LOI was tested on a JF-3 oxygen index meter (China Jiangning Analytical Instrument Co., Ltd.) at room temperature with sample size of 130 mm × 6.5 mm × 3 mm. The PX-03-001 instrument (PHINIX, China) was used to obtain the results of UL-94 with specimen of 130 mm × 6 mm × 3 mm.
Results and discussion
Flammability of PP-PBFA/PEPA composites via UL-94 and LOI
To examine the flame-retardant effect of PBFA and PEPA on PP, LOI and UL-94 test were carried out. The results are shown in Table 1.
The results suggest that the LOI of PP-PBFA/PEPA composites were significantly improved than pure PP. And PP-PBFA/PEPA (2:1) composite had the best effect with LOI value of 31.2%. In all PP-PBFA/PEPA samples, PP-PBFA/PEPA (2:1) and PP-PBFA/PEPA (4:1) composites reached the V 0 rating, indicating that they have good flame-retardant effect. Both LOI and UL-94 results show that the flame retardancy of PP can be improved by PBFA/PEPA.
Combustion behaviors of PP-PBFA/PEPA composites via CCT
Cone calorimeter test is a very effective flame-retardant test method for simulating and estimating material combustion behavior in fire under laboratory conditions. Many valuable data can be acquired from the CCT displaying the combustion status of the prepared flame-retardant composites [15]. The combustion data, such as time to ignition (TTI), peak heat release rate (PHRR), total heat release (THR), peak heat release time (tPHRR), fire performance index (FPI), fire growth index (FGI), peak smoke production rate (PSPR), total smoke production (TSP) and residual mass are shown in Table 2. According to the LOI and UL-94 results, PP-PBFA/PEPA ratio 1:1, 2:1, 4:1 composites were selected for analysis.
Heat release analysis
The HRR curves of PP-PBFA/PEPA composites are shown in Fig. 1. The HRR value of pure PP increased rapidly after ignition and reached the PHRR value of 870 kW m−2 at 145 s. However, the PHRR values of PP composites incorporating with PBFA/PEPA decreased drastically. The PHRR values of PP-PBFA, PP-PEPA, PP-PBFA/PEPA (1:1), PP-PBFA/PEPA (2:1) and PP-PBFA/PEPA (4:1) decreased by 53.58%, 52.69%, 71.95%, 75.38% and 75.76%, respectively. From Fig. 1, all the curves of flame-retardant PP composites except that of pure PP have two peaks with a typical “M” characteristic peak which appears in high-efficiency intumescent flame-retardant system [16]. After ignition, the PP composites burn rapidly with the release of heat. At the same time, PBFA and PEPA catalyze PP to form char layer, which acts as a barrier of heat and oxygen, resulting in the reduction of HRR. As the combustion proceeds, the char layer is not unstable; so it can further burn and release heat under the radiation.
The decrease of PHRR value in PP-PEPA can be described as the catalytic carbonization of PEPA. PEPA can be used not only as a carbon source, but also as an acid source promote PP to form a stable and heat-resistant char layer in condensed phase. The char layer coated on the surface of the matrix can prevent heat transfer and flammable gas release during combustion. Moreover, PEPA produces PO· in thermal decomposition, which can capture the high-energy free radical H· produced by PP decomposition to further reduce the degree of combustion [17].
The decrease of PHRR value in PP-PBFA can be described as the large amount of cyclotriphosphate and piperazine in PBFA. One the one hand, the cyclotriphosphate and piperazine can produce a large amount of N2 and NH3 during combustion to dilute flammable gas and oxygen and foam the char layer. On the other hand, phosphazene structure can promote the formation of char residue and produce PO· working as the free radical capturing mechanism in the combustion process [14]. PBFA acted as gas source and acid source to slow down the fierce combustion of PP composites.
The PHRR values of PP corresponding with PBFA/PEPA were significantly lower than that of PP composites only adding PBFA or PEPA. The PHRR values of PP-PBFA/PEPA (2:1) and PP-PBFA/PEPA (4:1) composites were almost the same, and they were better than that of PP-PBFA/PEPA (1:1). This is because PBFA/PEPA not only has the characteristics of PBFA as acid source and gas source, but also PEPA as carbon source and acid source. Within a certain mass mixing ratio, PBFA/PEPA can form a IFRs to play a good synergistic role.
THR curves of PP-PBFA/PEPA composites are shown in Fig. 2. THR values within 800 s of pure PP, PP-PBFA, PP-PEPA, PP-PBFA/PEPA(1:1), PP-PBFA/PEPA (2:1) and PP-PBFA/PEPA(4:1) were 116.75, 111.37, 102.80, 111.95, 99.27 and 107.87 MJ m−2, respectively, decreasing by 4.61%, 11.95%, 4.10%, 14.97% and 7.61%, respectively, than pure PP. Among the PP-PBFA/PEPA composites, the THR value of PP-PBFA/PEPA (2:1) reduced the most, while that of PP-PBFA/PEPA (1:1) was basically unchanged. This indicated that 2:1 of PBFA/PEPA is suitable mass proportion, in which the rate of gas produced of PBFA just keeps space with the charring rate of PEPA to form a closed foaming char layer to prevent heat release. Combined with the analysis of UL-94, LOI and heat release of PP-PBFA/PEPA composites, it was concluded that the PP-PBFA/PEPA (2:1) has the best fire resistance.
Smoke release analysis
In general, smoke is the main cause of fire deaths. SPR and TPS curves of PP-PBFA/PEPA composites are depicted in Figs. 3 and 4, respectively. The peak of pure PP in SPR was 0.10 m2 s−1 occurring at 155 s. In Fig. 3, the SPR values of PP-PBFA and PP-PBFA are higher than that of pure PP in the initial stage of combustion. It can be interpreted as that PBFA and PEPA can promote the formation of more char residue, but some of the char residues could not stay in the condensed phase but enter into the gas phase to form smoke. While, the peak SPR value of PP-PBFA/PEPA (1:1), PP-PBFA/PEPA (2:1) and PP-PBFA/PEPA (4:1) decreased to 0.05 m2 s−1 at 205 s, 0.06 m2 s−1 at 235 s and 0.05 m2 s−1 at 330 s, respectively. This phenomenon indicated that different mass proportion of PBFA/PEPA can reduce the SPR value and extend the time to reach the peak. This is because the synergistic effect of PBFA and PEPA. PBFA/PEPA can form a phosphate ester in thermal decomposition, which are closely attached to the char layer and reduced the gaps and voids in the char layer [18].
In general, the TSP is discussed for a certain period. Figure 4 shows that pure PP released 13.93 m2 kg−1 of smoke during 0 ~ 465 s. The TSP value of PP-PBFA/PEPA (1:1) during 0–465 s was 14.75 m2 kg−1 increased by 5.89% than that of pure PP, which is corresponded to the result of LOI and UL-94. While, the TSP values of PP-PBFA/PEPA (2:1) and PP-PBFA/PEPA (4:1) were 10.85 m2 kg−1 and 13.02 m2 kg−1, respectively, which decreased by 22.11% and 6.53% comparing with pure PP.
The TSP value of PP-PBFA/PEPA (2:1) is the lowest in that of PP-PBFA/PEPA. That is because the synergistic effect of PBFA and PEPA in mass proportion of 2:1 can effectively catalyst the formation of more stable char layer, which could suppress the release of smoke. However, with complete combustion of the PP composites, the TSP value of pure PP is lower than PP composites. On the one hand, pure PP is mainly a linear chain composed of propylene, which is easy to fully burn with releasing small amount of smoke during combustion. On the other hand, the mass of residual chars for PP-PBFA/PEPA composites were increased. The residual chars were unstable and part of them in high-temperature irradiation of CCT entered the gas phase resulting in the increase of TSP.
Thermal degradation properties of PP-PBFA/PEPA composites
The thermal degradation behavior of PP-PBFA/PEPA composites was investigated by TGA. Thermogravimetric (TG) and differential thermogravimetric (DTG) curves of PP-PBFA/PEPA composites under N2 atmosphere are shown in Fig. 5 and the related data are shown in Table 3. The onset of decomposition temperature (Td) (Table 3) was the temperature corresponding to 5% mass loss. The temperature of maximum rate (Tmax) of mass loss could be acquired from DTG curves. Pure PP began to decompose at 417.7 °C and rapidly degraded in the temperature range of 417.7–486.0 °C with a sharp DTG peak at 462.3 °C (Tmax). And the residual char of pure PP was only 2.53% at 800 °C. These indicated that the pure PP was thermally decomposed quickly and completely. The Td of PP composites with PBFA/PEPA, pure PBFA and pure PEPA were all advanced to the range of 310–330 °C. This is because the stability of PBFA and PEPA was lower than PP, which make PBFA and PEPA decompose in advance. However, the char residual mass was improved by adding PBFA/PEPA, pure PBFA and pure PEPA (the lowest was 10.23% compared to 2.53% in pure PP). The reason why the amount of char was increased is that PBFA and PEPA can generate phosphoric acid during the combustion, which can catalyze the formation of stable expanded char layer, thereby reducing the combustion intensity. Meanwhile, as the mass proportion of PBFA/PEPA increases, the Tmax value also increases. This is because of that as the PBFA increases, the more phosphoric acid produced in the decomposition can make the char layer tighter and enhance the heat insulating ability.
From the DTG curve, the Tmax of pure PP was the largest. And the Tmax of PP-PBFA/PEPA composites were all lower than that of pure PP. This is mainly because more non-combustible gas was generated with the increasing of the nitrogen content in the IFRs and more phosphoric acid was produced by the decomposition of PBFA and PEPA, which both can make the char layer tighter and enhance the heat insulating ability, thereby decreasing the mass decomposition rate of PP-PBFA/PEPA composites.
Practical security application analysis
It is also meaningful to consider whether the flame retardant can give a better escape condition in the combustion. Two important parameters were analyzed for the assessment of fire hazard, one for the FPI and the other for the FGI [19]; FPI (m2 s−1 kW−1) = TTI/PHRR; FGI (kW m−2 s−1) = PHRR/tPHRR [20]. The greater the FPI, the higher the safety factor of the flame retardant. Conversely, the greater the FGI, the lower the safety factor of the flame retardant.
To understand the fire hazard clearly, the FPI and FGI were obtained in Fig. 6. It showed that pure PP had the smallest FPI value and the largest FGI value, indicating that PP needs to add flame retardant to improve its fire safety. The FPI and FGI of PP-PBFA/PEPA composites were superior to the PP-PBFA and PP-PEPA. Among the different mass proportion of PP-PBFA/PEPA composites, the PP-PBFA/PEPA (4:1) is the safest proportion with the lowest FGI value and the largest FPI value when fire occurring.
Residual char analysis
Residual char curves of PP-PBFA/PEPA composites were shown in Fig. 7. The mass loss rate of pure PP was very fast and the char residue was 4.32%, indicating that the char-forming property was very poor. This is also indirectly seen from the HRR curve. The slope of the PP-PBFA/PEPA composites residual char curve of different mass proportions was smaller than that of pure PP, probably because a heat-resistant char layer was formed on the surface of the substrate, which prevents the rapid burning of the PP composites. Moreover, the char residue of PP-PBFA/PEPA composites with a mass ratio of 1:1, 2:1 and 4:1 were increased by 20.10%, 37.86% and 21.46%, respectively. The char residue of PP-PBFA and PP-PEPA composites was 8.68% and 11.29%. The results show that PBFA/PEPA can inhibit the combustion of PP.
Analysis of char residue
Figure 8 shows digital photos of char residues after CCT. The pure PP in Fig. 8a1 was almost completely burned and there was no residual char. The TGA and CCT also confirm this Fig. 8b–f showed that PP-PBFA/PEPA had more char residue than PP-PBFA and PP-PEPA. Figure 8b1–f1 showed that the residual char of PP-PBFA and PP-PEPA was relatively compact with relatively regular structure; and the residual char of PP-PBFA/PEPA weasloose, expansive and foaming, indicating that PBFA and PEPA work synergistically to form IFRs.
Char residues were characterized by FTIR to determine functional groups. As shown in Fig. 9a, the curves of PP-PBFA and PP-PEPA were almost the same, and both had infrared peaks around 3421 cm−1 (stretching vibration of –NH– or –OH) [21]. –NH– was mainly derived from the piperazine structure in the PBFA, while –OH was due to the P–OH bond generating from PBFA with O2; The char of PP-PEPA showed characteristic absorptions at 2955 cm−1 (stretching vibration of –CH2–) and 983 cm−1 (P–O–P characteristic peak) [22], indicating that PBFA and PEPA form P–O–P structure during combustion. Since the peaks appearing in the infrared characterization of char residue of PBFA/PEPA composites were basically the same, the char residue of PP-PBFA/PEPA (2:1) composite was selected for analysis. It can be seen in Fig. 9b that the peak at approximately 986 cm−1 was attributed to the P–O–P groups, the peak appearing at 3417 cm−1 was assigned to –NH– and –OH stretching vibration and the peak appearing at 2955 cm−1 was due to the absorption of –CH2–. In general, combining with the increase in the mass of PP-PBFA/PEPA residual char with different proportions, the char residue photos and the residual char infrared indicate that there was a synergistic effect between PBFA and PEPA during the combustion process. The pyrophosphoric acid produced by PBFA and PEPA can catalyze the formation of multiple foaming char layer to block the heat release and prevent the combustion in the condensed phase.
Flame-retardant mechanisms
The av-EHC was used to analyze the working mechanism of a gas-phase flame retardant. The av-EHC is the ratio of HRR to mass loss referring to the heat released by the volatilization of each unit mass of gaseous substance into the gas phase. A low av-EHC value indicates that these flame retardants have gas-phase flame-retardant effect with nonflammable gases existing during combustion. In Table 4, the av-EHC for different mass proportions of PP-PBFA/PEPA, PP-PBFA and PP-PEPA were decreased. This is because PBFA and PEPA can release nonflammable gases, such as N2 and NH3 during the combustion process [23]. These gases reduce the risk of fire by diluting flammable gases to hinder the spread of the fire. Moreover, since PBFA and PEPA both contain P element, it can be ascribed to the fact that PBFA and PEPA were thermally decomposed to form PO· during combustion [17].
According to the image of char residue in Fig. 8 and the FTIR curve of char residue in Fig. 9, it can be concluded that the phosphoric acid and pyrophosphoric acid produced by the PBFA and PEPA during the combustion process can catalyze the formation of char. These results also show that there is indeed a gas-phase flame-retardant mechanism, but the condensed-phase flame-retardant mechanism plays a major role.
Conclusions
In this research, water resistance IFRs were formed by mechanical mixing of a synthesized cross-linked PBFA and PEPA, which greatly enhances the flame retardancy of PP. The PP-PBFA/PEPA (2:1) composite has the best flame-retardant efficiency with the LOI value of 31.2% and the UL-94 V 0 rating. The CCT results showed that the PHRR, THR and TSP (during 0–465 s) of PP-PBFA/PEPA (2:1) decreased by 14.97%, 75.38% and 22.11%, respectively. The TGA results demonstrated that PBFA/PEPA could improve the amount of char residue at 800 °C. The digital photos and FTIR spectrum of residual char after CCT were carried out to explain the flame-retardant mechanism. On the one hand, PBFA and PEPA can produce the phosphoric acid and pyrophosphoric acid during the combustion, which can increase the amount of char and catalyze the formation of multi-layered and foaming char layer to block the heat release. On the other hand, PBFA and PEPA can thermally decompose to form PO·, which can capture the high-energy free radical H· produced by PP decomposition to further reduce the degree of combustion.
References
Song P, Fang Z, Tong L, Xu Z. Synthesis of a novel oligomeric intumescent flame retardant and its application in polypropylene. Polym Eng Sci. 2009;49(7):1326–31.
Matzen M, Kandola B, Huth C, Schartel B. Influence of flame retardants on the melt dripping behaviour of thermoplastic polymers. Materials. 2015;8(9):5621–46.
Hu S, Song L, Pan H, Hu Y. Effect of a novel chitosan-based flame retardant on thermal and flammability properties of polyvinyl alcohol. J Therm Anal Calorim. 2013;112(2):859–64.
Peng HQ, Zhou Q, Wang DY, Chen L, Wang YZ. A novel charring agent containing caged bicyclic phosphate and its application in intumescent flame retardant polypropylene systems. J Ind Eng Chem. 2008;14(5):589–95.
Meng WH, Dong YL, Li JH, Cheng LY, Xu JZ, Hao JW, Qu HQ. Bio-based phytic acid and tannic acid chelate-mediated interfacial assembly of Mg(OH)2: concurrently synergism improved flame retardancy, smoke suppression and mechanical properties of PVC. Compos Part B-Eng. 2020;8368(19):34360–4.
Wang X, Wang Z, Li J. Effects of a semi-bio-based triazine derivative on intumescent flame-retardant polypropylene. Polym Adv Technol. 2019;30(5):1259–68.
He W, Qi F, Wang N, Chen X, Zhang K, Guo J. The influence of thermal oxidative ageing on flame retardancy, thermal and mechanical properties of LGFPP/IFR composites. J Therm Anal Calorim. 2018;131(2):1017–24.
Yu S, Xiao S, Zhao Z, Huo X, Wei J. Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene. Chin J Chem Eng. 2019;27(7):1735–43.
Wen P, Tai Q, Hu Y, Yuen RKK. Cyclotriphosphazene-based intumescent flame retardant against the combustible polypropylene. Ind Eng Chem Res. 2016;55(29):8018–24.
He LL, Zhang Y, Qin ZL, Lan YH, Li DH, Yang RJJAMR. Study on synthesis of cyclotriphosphazene containing aminopropylsilicone functional group as flame retardant. Adv Mater Res. 2013;683(4):25–9.
Qin Z, Li D, Lan Y, Li Q, Yang R. Ammonium polyphosphate and silicon-containing cyclotriphosphazene: synergistic effect in flame-retarded polypropylene. Adv Mater Res. 2015;54(43):10707–13.
Xu Y, Claiden P, Zhu Y, Morita H, Hanagata N. Effect of amino groups of mesoporous silica nanoparticles on CpG oligodexynucleotide delivery. Sci Technol Adv Mater. 2015;16(4):045006(1–11).
Yang R, Ma B, Zhao H, Li J. Preparation, thermal degradation, and fire behaviors of intumescent flame retardant polypropylene with a charring agent containing pentaerythritol and triazine. Ind Eng Chem Res. 2016;55(18):5298–305.
Yang G, Wu WH, Wang YH. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of epoxy resin. J Hazard Mater. 2019;366(9):78–87.
Wang W, Wen P, Zhan J. Synthesis of a novel charring agent containing pentaerythritol and triazine structure and its intumescent flame retardant performance for polypropylene. Polym Degrad Stabil. 2017;144(13):454–63.
Zhang NE, Zhang M, Zhang J. Flexible water-resistant intumescent coatings: fabrication, characterization, and fire protective performance. Prog Org Coat. 2019;137(9):105322–33.
Zhang W, Wu H, Meng W. Investigation of nickel ammonia phosphate with different morphologies as a new high-efficiency flame retardant for epoxy resin. High Perform Polym. 2019; https://doi.org/10.1177/0954008319867369.
Song Q, Wu H, Liu H, Han X, Qu H, Xu J. Synergistic flame-retardant effects of ammonium polyphosphate and AC-Fe2O3 in epoxy resin. J Therm Anal Calorim. 2019;138(2):1259–67.
Schartel B, Hull TR. Development of fire-retarded materials—interpretation of cone calorimeter data. Fire Mater. 2007;31(5):327–54.
Wen P, Wang X, Wang B, et al. One-pot synthesis of a novel s-triazine-based hyperbranched charring foaming agent and its enhancement on flame retardancy and water resistance of polypropylene. Polym Degrad Stabil. 2014;110(2):165–74.
Liu L, Xu Y, Xu M, Li Z, Hu Y, Li B. Economical and facile synthesis of a highly efficient flame retardant for simultaneous improvement of fire retardancy, smoke suppression and moisture resistance of epoxy resins. Compos Part B-Eng. 2019;167(2):422–33.
Jin Y, Huang G, Han D. Functionalizing graphene decorated with phosphorus-nitrogen containing dendrimer for high-performance polymer nanocomposites. Compos Part A-Appl S. 2016;86(5):9–18.
Xu MJ, Xu GR, Leng Y, Li B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym Degrad Stabil. 2016;123(7):105–14.
Acknowledgements
This work was supported by the Higher Education Science and Technology Research Project of Hebei Province [Grant numbers ZD2018011]; Key Research and Development Projects of Hebei Province [Grant numbers 19211205D].
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, K., Wu, H., Wang, T. et al. Flame-retardant effect of cross-linked phosphazene derivatives and pentaerythritol derivatives on polypropylene. J Therm Anal Calorim 145, 3067–3075 (2021). https://doi.org/10.1007/s10973-020-09898-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09898-z