Abstract
This paper deals with the study of excipient–excipient interactions, rather than more commonly studied drug–excipient interactions. It presents the compatibility study between two polymeric compounds used in pharmaceutical technology, namely polyvinyl alcohol and polyvinylpyrrolidone, with other commonly used pharmaceutical excipients, like starch, mannitol, sorbitol, magnesium stearate and hydroxyethyl cellulose. The compatibility investigations were carried out under ambient temperature by FTIR spectroscopy studies and later completed by the use of thermal analysis (TG/DTG/HF) data to study the influence of temperature over stability of binary mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the field of pharmaceutical technology, compatibility study is of great importance, since the stability of the active pharmaceutical ingredient can be dramatically changed by the selection of excipients. However, the solid-state stability of the final pharmaceutical formulation can be affected by the interactions occurring between excipients as well. Even if up to the date, these interactions weren’t investigated as much as drug–excipient ones, it is of great importance to analyze their occurrence in solid formulations.
Polyvinyl alcohol (PVA) is a synthetic polymer, with an increased solubility in highly polar and hydrophilic solvents such as water, dimethyl sulfoxide, ethylene glycol and N-methyl pyrrolidone. However, the solubility of PVA in water depends on factors such as the degree of polymerization and temperature. The PVA hydrogels (PVAH) have been used for a variety of biomedical and pharmaceutical applications [1–3], due to the fact that they do not have toxic or carcinogenic properties, as well as they are not bio-adhesive. PVA is mainly used as granulation binder and binding agent [4–7] or as a potential disintegrant, after functionalization [8] in solid or semisolid formulations. PVA is also used as a viscosity-increasing agent for ophthalmic formulations, but in oral formulations and sustained release transdermal patches as well [9, 10].
Copolymers, such PEG-PVA, are used mainly in the production of instant-release tablet coatings for pharmaceuticals and dietary supplements [11].
Polyvinylpyrrolidone (PVP) is a water-soluble polymer, available in various grades, based on the average molar mass. Nowadays, it is mainly used in pharmaceutical technology of solid formulations as a binding agent [12–14]. PVP is also employed in increasing the bioavailability of active pharmaceutical ingredients, by improving their dissolution, acting as a solubilizing agent [9]. PVP also modifies surface tension and it is used in pharmaceutical formulations to increase the water solubility of drugs, such as eprosartan, a low-solubility drug, which has been used to treat high blood pressure [15].
PVP is highly soluble in solvents of different polarities, and the physico-chemical properties made it one of the most important excipients in pharmaceutical technology [16].
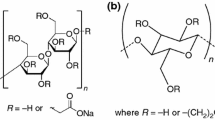
The chemical structures of PVA and PVP are presented in Fig. 1.
These two excipients were intensely studied in the field of pharmaceutical technology, mainly in preformulation study. In several papers, the compatibility of these excipients in binary [17–22] or ternary systems [23] was investigated, but always along with an active pharmaceutical ingredient. To our knowledge, the solid-state interactions between these polymeric compounds and other excipients used in pharmaceutical technology were not reported up to the date.
According to these considerations and the widespread presence of PVA and PVP in numerous pharmaceutical solid dosage forms, we set our goal in evaluating the compatibility/incompatibility of these polymeric excipients with five other currently used excipients, namely starch (St), mannitol (Man), sorbitol (Sorb), magnesium stearate (MgSt) and hydroxyethyl cellulose (HCel). The employed instrumental techniques are thermal analysis (TG/DTG/HF) and FTIR spectroscopy, in order to quantify the thermally induced interactions between the components. This study acts as a preliminary formulation study for analyzing the occurrence of interaction in commercial solid dosage forms between excipients, being known that a solid formulation generally contains more than two excipients.
Materials and methods
Polyvinyl alcohol (PVA) was purchased from Merck (MW approx. 145000, fully hydrolyzed, lot: S6585194331). Polyvinylpyrrolidone (PVP) was purchased from BASF Aktiengesellschaft, Germany (lot: 95658675LO). Both compounds were used as received, without further purification. The excipients (pharmaceutical grade) were used as received, as follows: starch (Grain Processing Corporation, USA), mannitol (Merck, Germany), sorbitol (Sigma-Aldrich, Germany), magnesium stearate (Sigma, Germany) and hydroxyethyl cellulose (Sigma, Germany).
The binary mixtures of PVP + excipient and PVA + excipient were prepared by manual grinding of equal masses of polymeric compounds and each excipient in an agate mortar for approximately 5 min. The 1:1 (m/m) ratio was chosen in order to maximize the probability of observing the interaction(s).
The thermoanalytical TG/DTG/HF curves were determined using a PerkinElmer DIAMOND equipment, in air atmosphere (100 mL min−1), under non-isothermal conditions at a heating rate β = 10 °C min−1. Samples were put into aluminum crucibles and heated by increasing temperature from ambient up to 400 °C. For determining the thermal effects, the DTA data (µV) were converted in normalized HF (Heat Flow) data (Wg−1).
Universal Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (UATR-FTIR) spectra of the samples were registered on a PerkinElmer Spectrum 100 device. Spectra were collected after 32 co-added scans, with a resolution of 4 cm−1, on the spectral domain 4000–600 cm−1.
Results and discussion
Thermoanalytical investigations
All the samples were investigated by thermal analysis (Figs. 2, 3). In order to determine if any interactions occur between the polymeric samples PVA and PVP and selected excipients, the thermoanalytical profile of each pure component was investigated versus the 1:1 (m/m) binary mixture (Table 1).
Binary mixtures of PVA
The thermoanalytical profile of PVA + St shows small endothermic peaks corresponding to the superimposing of thermal events observed in the case of pure components. According to thermal analysis, no interaction was evidenced between the samples during heating.
The analysis of PVA + HCel revealed two separate degradative processes on the TG curve, with a total mass loss of 92.0 %. The HF curves revealed three maximums, and they were also found in the HF curves of the pure samples. Following this, no interaction occurs between these components during heating.
For the binary mixture of PVA + Man, the total mass loss is 95 %. No interactions took place at temperatures under 200 °C. However, the melting peak of PVA at 226 °C disappeared from the curve of the binary mixture. Also, the degradation event of Man from 344 °C is shifted to 325 °C. In this case, it can be said that these two excipients are compatible only up to 200 °C.
The behavior of mixture between PVA + Sorb is similar to the behavior of PVA + Man sample. This fact can be explained by the similarity of structures of the two sugar alcohols, Man and Sorb. PVA is compatible with Sorb only up to 170 °C.
For PVA + MgSt, the melting peak of PVA appears at the same temperature as in the case of pure sample, but the thermoanalytical profile of the salt is totally modified in the binary mixture. It can be said that the two components are incompatible, namely that PVA determines the disappearance of thermal events corresponding to MgSt, i.e., its chemical degradation.
Binary mixtures of PVP
The physical mixture PVP + St shows no interactions at temperatures below 325 °C. At higher temperatures, the degradation of starch occurs, the binary mixture having a similar HF profile to the one of pure PVP.
The thermoanalytical curves of PVP + HCel show thermal stability up to 251 °C. The HF curve also sustains this compatibility, since no exothermal/endothermal events took place.
For the PVP + Man, no interactions are observed up to 300 °C. Above this temperature, the thermal event observed on the HF curve of pure Man is shifted from 344.8 to 332 °C, while the characteristic peak of PVP is unshifted.
In the case of PVP + Sorb mixture, the binary mixture is stable up to 250 °C. Also, with the increasing of temperature, modification of the PVP structure took place. The characteristic HF peaks of Sorb are at the same temperature in the binary mixture as in the case of pure Sorb, while the characteristic HF peak of PVP from 362 °C disappeared.
PVP + MgSt thermoanalytical profile is the superimposing of the curves determined for pure components. MgSt is compatible with PVP up to 300 °C, and with the increasing of temperature over this limit, the behavior of mixture is totally dissimilar to the one of pure excipients.
Spectroscopic investigations
Initially, the spectra of polymeric samples PVA and PVP were investigated by UATR-FTIR technique at 25 °C versus thermally treated samples up to 350 °C (Fig. 4). The spectra of pure PVA is relatively simplistic, since the structure consists of few types of bonds. The broadband between 3600 and 3000 cm−1 is due to O–H stretching vibrations of intermolecular polymeric association by H-bond from the structure. The C–H stretching vibrations from polymers appear around 2910–2930 cm−1, with peaks at 2919 and 2911 cm−1. The C–H deformation vibrations appear at 1421 and 1321 cm−1. The C–O stretching deformation from the secondary alcohol appears at 1141 and 1083 cm−1, while the hydrocarbonated chain skeletal vibrations appear at 834 cm−1. After thermal treatment at 350 °C, some modifications appear in the FTIR spectrum, as follows: modification of the broad signal corresponding to stretching vibration of O–H groups, and appearance of new bands at 1700 and 1598 cm−1. These bands can be attributed to the formation of oxidation products of PVA at the secondary alcohol, probably to a keto group (intense sharp band at 1700 cm−1), while the other band can be associated with intramolecular dehydration and formation of C=C bonds. Also, the characteristic vibrations of C–O bonds are no longer present in the spectrum, suggesting their chemical modification, as previously stated. The skeletal vibrations of the hydrocarbonated chain are still visible in the spectrum, suggesting that at this temperature, the advanced thermooxidations do not occur.
In the case of PVP spectra, a more complex pattern is observed and is correlated with the chemical structure of the polymeric chain. The broadband between 3700 and 3100 cm−1 is due to the presence of water in the structure, while the C–H vibrations appear at 2954, 2929 and 2911 cm−1. The C=O characteristic keto band appear as a sharp intense band at 1644 cm−1. These bands appear also in the case of thermally treated sample at 350 °C, with some minor modifications: the broadband around 3700–3100 cm−1 decreased in intensity due to water loss, while the keto band is shifted to 1649 cm−1. This comparative study revealed that the thermal treatment at 350 °C determines increased modifications in the case of PVA sample, while in the case of PVP sample, these are not that relevant. These results are also correlated with the mass loss in these temperature ranges, being 39 % for PVA and 23 % for PVP.
The FTIR spectra of pure excipients (Fig. 5) are presented for a better comparison and interpretation of the spectral data registered for binary mixtures (Figs. 6, 7). In the case of binary mixtures PVA + excipients and PVP + excipients, the simple grinding under ambient temperature do not induce any interactions. Practically, all the FTIR spectra of the samples consist of the superimposition of the FTIR spectra of pure components. These results can be correlated with the ones from thermal analysis, leading to the main conclusion that PVA and PVP are compatible under ambient conditions with all selected excipients.
In order to evaluate the thermolysis of the binary samples after thermal treatment up to 500 °C, UATR-FTIR spectra were collected. In the case of PVA, the thermal treatment at 500 °C determined an advanced degradation of the polymeric structure of the excipient. However, several IR bands were evidenced in the analysis of the chars: 2879 and 2848 cm−1 corresponding to the stretching vibrations of C–H bonds from hydrocarbonated chain, 1428 cm−1 for C–H deformation vibrations and 879 for the skeletal vibrations of the –(CH2) n – moieties. FTIR spectra also showed that under advanced thermal treatment, the C–O and O–H bonds were broken, since no bands appear in the characteristic spectral region for these moieties. The same bands are present in the spectra of binary mixtures after thermal treatment, suggesting that the degradation of the added excipients to PVA occurs more rapidly, and the remaining char is solely due to the presence of the PVA. Solely in the case of PVA + St, the FTIR spectra were determined after thermal treatment at 400 °C, due to the fact that the remaining char after thermolysis at 500 °C was insufficient for the analysis (Fig. 8).
In the case of binary samples with PVP content, UATR-FTIR revealed a more advanced thermal degradation of the polymeric structure. Only for the binary mixture PVP + St, a band was observed around 1625 cm−1, while in the case of PVP + MgSt, three bands were observed around 1610, 1440 and 1150 cm−1 and can be associated with magnesium-containing residues (Fig. 9).
Conclusions
In this paper, the thermal behavior of binary mixtures of two polymeric compounds currently used in pharmaceutical technology was investigated. The compounds (polyvinyl alcohol and polyvinylpyrrolidone) were studied by thermoanalytical techniques as pure components, and as binary mixtures with other currently used pharmaceutical excipients. It was shown that under ambient conditions, both polyvinyl alcohol and polyvinylpyrrolidone are compatible with starch, mannitol, sorbitol, magnesium stearate and hydroxyethyl cellulose, while under heating, no interactions occur below 170 °C in all ten studied binary mixtures. These observations can be useful in preformulation studies in pharmaceutical technology, suggesting that in solid dosage forms, both PVA and PVP can be used along with previously mentioned excipients.
References
Hansen K, Kim G, Desai KGH, Patel H, Olsen KF, Curtis-Fisk J, Tocce E, Jordan S, Schwendeman SP. Feasibility investigation of cellulose polymers for mucoadhesive nasal drug delivery applications. Mol Pharmaceut. 2015;12(8):2732–41.
Fernandez-Ronco MP, Salvalaglio M, Kluge J, Mazzotti M. Study of the preparation of amorphous itraconazole formulations. Cryst Growth Des. 2015;15(6):2686–94.
Gift AD, Southard LA, Riesberg AL. Influence of polymeric excipient properties on crystal hydrate formation kinetics of caffeine in aqueous slurries. J Pharm Sci. 2012;101(5):1755–62.
Paduszyński P, Krusiński T, Han T, Musiał W. Early stage of drug release non-classified in BCS influenced by coating excipients: hydroxypropyl methylcellulose and polyvinyl alcohol. Lat Am J Pharm. 2016;35(1):84–90.
Puri V, Dantuluri AK, Bansal AK. Barrier coated drug layered particles for enhanced performance of amorphous solid dispersion dosage form. J Pharm Sci. 2012;101(1):342–53.
Diluccio RC, Hussain MA, Coffinbeach D, Torosian G, Shefter E, Hurwitz AR. Sustained-release oral delivery of theophylline by use of polyvinyl-alcohol and polyvinyl alcohol-methyl acrylate polymers. J Pharm Sci. 1994;83(1):104–6.
Gutch PK, Jitendra S, Alankar S, Anurekha J, Ganesan K. Thermal analysis of interaction between 2-PAM chloride and various excipients in some binary mixtures by TGA and DSC. J Therm Anal Calorim. 2011;111(3):1953–8.
Patel AR, Vavia PR. Evaluation of synthesized cross linked polyvinyl alcohol as potential disintegrant. J Pharm Pharm Sci. 2010;13(2):114–27.
Sinko PJ, Singh Y. Physical chemical and biopharmaceutical principles in the pharmaceutical sciences, 6th Edn. Pharmaceutical polymers. 2009. p. 492.
Fundueanu G, Constantin M, Bortolotti F, Cortesi R, Ascenzi P, Menegatti E. Cellulose acetate butyrate-pH/thermosensitive polymer microcapsules containing aminated poly(vinyl alcohol) microspheres for oral administration of DNA. Eur J Pharm Biopharm. 2007;66(1):11–20.
Heuschmid FF, Schneider S, Schuster P, Lauer B, van Ravenzwaay B. Polyethylene glycol-g-polyvinyl alcohol grafted copolymer: reproductive toxicity study in Wistar rats. Food Chem Toxicol. 2013;51:S24–35.
Prudic A, Ji YH, Luebbert C, Sadowski G. Influence of humidity on the phase behavior of API/polymer formulations. Eur J Pharm Biopharm. 2015;94:352–62.
Paus R, Prudic A, Ji YH. Influence of excipients on solubility and dissolution of pharmaceuticals. Int J Pharm. 2015;485(1–2):277–87.
Veronez IP, Daniel JSP, Junior CEC, Garcia JS, Trevisan MG. Development, characterization, and stability studies of ethinyl estradiol solid dispersion. J Therm Anal Calorim. 2015;120(1):573–81.
Halake K, Birajdar M, Kim BS, Bae H, Lee CC, Kim YJ, Kim S, Kim HJ, Ahn S, An Y. Recent application developments of water-soluble synthetic polymers. J Ind Eng Chem. 2014;20:3913–8.
Rasekh M, Karavasili C, Soong YL, Bouropoulos N, Morris M, Armitage D, Li X, Fatouros DG, Ahmad Z. Electrospun PVP-indomethacin constituents for transdermal dressings and drug delivery devices. Int J Pharm. 2014;473(1–2):95–104.
Ledeti I, Vlase G, Vlase T, Ciucanu I, Olariu T, Todea A, Fulias A, Suta LM. Instrumental analysis of potential lovastatin–excipient interactions in preformulation studies. Rev Chim (Bucharest). 2015;66(6):879–82.
Ledeti I, Vlase G, Ciucanu I, Olariu T, Fulias A, Suta LM, Belu I. Analysis of solid binary systems containing simvastatin. Rev Chim (Bucharest). 2015;66(2):240–3.
Ledeti I, Vlase G, Vlase T, Suta LM, Todea A, Fulias A. Selection of solid-state excipients for simvastatin dosage forms through thermal and nonthermal techniques. J Therm Anal Calorim. 2015;121(3):1093–102.
de Melo CM, Vieira ACQD, do Nascimento ALD, Figueiredo CBM, Rolim LA, Soares-Sobrinho JL, Veras LMC, Leite JRDD, Neto PJR, Soares MFD. A compatibility study of the prototype epiisopiloturine and pharmaceutical excipients aiming at the attainment of solid pharmaceutical forms. J Therm Anal Calorim. 2015;120(1):689–97.
Vieira ACQD, Marques GS, de Melo CM, da Silva KER, Rolim LA, de Lima MDA, Galdino SL, Pitta ID, Neto PJR. Physical-chemical characterization of new anti-inflammatory agent (LPSF/GQ-130) and evaluation of its thermal compatibility with pharmaceutical excipients. J Therm Anal Calorim. 2014;115(3):2339–49.
Chaves LL, Rolim LA, Goncalves MLCM, Vieira ACC, Alves LDS, Soares MFR, Soares-Sobrinho JL, Lima MCA, Rolim-Neto PJ. Study of stability and drug–excipient compatibility of diethylcarbamazine citrate. J Therm Anal Calorim. 2013;111(3):2179–86.
Bruni G, Berbenni V, Milanese C, Girella A, Marini A. Drug–excipient compatibility studies in binary and ternary mixtures by physico-chemical techniques. J Therm Anal Calorim. 2010;102(1):193–201.
Acknowledgements
Roxana Blajovan (Ph.D. student) was cofinanced in this work from the European Social Fund through Sectoral Operational Programme Human Resources Development 2007–2013, Project Number POSDRU/187/1.5/S/155559, Competitive Multidisciplinary Doctoral Research in Europe.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blajovan, R., Ledeţi, I., Vlase, G. et al. Study of thermal induced excipient–excipient interactions. J Therm Anal Calorim 126, 171–179 (2016). https://doi.org/10.1007/s10973-016-5348-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5348-3