Abstract
Via a simple sol-gel approach, the high photocatalytic efficiency of Bi1-xSmxFeO3 nanoparticles were realized when x was 0, 0.05, 0.10, 0.15 and 0.20. Microstructural characterizations including X-ray diffraction (XRD) spectroscopy, Raman spectroscopy, field emission scanning electron microscope (FESEM), x-ray photoelectron spectroscopy (XPS) and ultraviolet-visible (UV-Vis) spectrophotometer, demonstrated a transformation from the rhombohedral structure of BiFeO3 to the orthorhombic structure of Bi0.80Sm0.20FeO3. Moreover, the experimental findings discovered that the photocatalytic efficiency of Bi0.80Sm0.20FeO3 was the highest, reaching 98.5% within 2 h based on visible light degradation. The underlying mechanisms, involved to phase transition, size effect, band energy and Fenton reaction, were discussed. The research presented in the paper is crucial in offering a fundamental strategy for utilizing BiFeO3 to address the issue of water pollutants.
Graphical Abstract

Highlights
-
Bi1-xSmxFeO3 nanoparticles with varying Sm doping levels (x = 0, 0.05, 0.10, 0.15, and 0.20) were successfully synthesized in this study.
-
X-ray diffraction and Raman spectroscopy demonstrated that the transformation of the rhombohedral crystal structure (R3c space group) to the orthorhombic structure (Pnma space group) in Bi0.80Sm0.20FeO3.
-
Experimental results indicated that the photocatalytic efficiency of Bi0.80Sm0.20FeO3 reach to 98.5%, with superior chemical stability.
-
These findings offer a fundamental strategy for utilizing BiFeO3 in addressing water pollutant issues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to its moderate band gap (2.1–2.8 eV), superior chemical stability and low cost [1,2,3,4,5,6], BiFeO3 (BFO) is generally considered one of the most assuring multifunctional materials with numerous applications, including photocatalytic, ferroelectricity, magnetoelectric and piezoelectric devices [7,8,9,10]. However, practical limitations, such as cycloidal (spiral) spin structure and poor photogenerated electron-hole pair production efficiency, largely offset the photocatalytic properties of BFO [11,12,13,14,15,16]. Great efforts have been devoted to enhance photocatalytic and other physical activities via doping metal ions at the A(B) sites in the ABO3 lattice structure of BFO [7, 8, 17,18,19,20]. For instance, exotic metallic elements can effectually increase the photocatalytic performance of BFO by promoting the efficacy of splitting photo-induced carriers during the catalytic process [12, 21]. In particular, rare-earth element-doped BFO has been verified to expedite the splitting of photo-generated electron-hole pairs due to its unusual 4 f electron configuration [22, 23]. For example, lanthanum substitution, which lowers bandgap energy and increases charge carrier concentrations in BFO nanoparticles obtained by sol-gel methods, can result in enhanced photocatalytic efficiency [23, 24]. Furthermore, it is worth noting that replacing Bi3+ cations with tinier foreign elements can induce an adequate lattice distortion in BFO, e.g., numerous studies have investigated on Samarium doped into BFO to improve its ferroelectric and magnetic characteristics [25,26,27,28,29] due to lattice twist generated by substituting Bi3+ by Sm3+ (0.958 Å) with reduced ionic radius. However, there have been few studies on the degradation of dye Rhodamine B (Rh B) by Sm-doped BFO nanoparticles [30, 31]. Thus, it is of great significance to investigate the effect of Sm doping elements on the photocatalytic performance of BFO.
On the other aspect, the preparation of single-phase BFO and its doped series of BFO nanoparticles remains a challenging task because of the limited temperature range of phase stability. Based on the above facts, Bi1-xSmxFeO3 nanoparticles with x ranging from 0 to 0.20 were prepared by the sol-gel approach, and this work significantly enhanced the photocatalytic characteristics of the prepared samples. The underlying physical mechanisms were also thoroughly discussed, according to the lattice structure transition, size effect, band gap, Fenton reaction, and the efficiency of photogenerated electrons and holes, etc.
2 Experimental procedures
The chemicals were of analytical grade and were dissolved in a nitric acid solution, with tartaric acid serving as a chelating agent. Specifically, Sm(NO3)3·6H2O, Bi(NO3)3·5H2O, and Fe(NO3)3·9H2O were weighed consistent with the stoichiometric ratio x: (1-x) : 1 (x = 0.05, 0.10, 0.15, 0.20) into the prepared nitric acid solution and stirred for 30 min to ensure complete dissolution. Next, the same stoichiometric amount of tartaric acid with Fe was added and the mixture was kept at 50 °C for 60 min to attain a light-yellow sol. Subsequently, it was transferred to a silicone oil container and maintained at 150 °C until the solution evaporated completely, resulting in the formation of a dry colloid. Finally, the xerogel was sintered in a high-temperature furnace at 800 °C for 2 h and naturally cooled to 25 °C. The prepared samples were then taken out and ground to obtain the Sm-doped BiFeO3 powders, which were denoted as nBSFO (n = 5, 10, 15, 20, n value equal to 100x). Pure BFO powders were synthesized without adding Samarium nitrate, and the more detailed procedures can be referred to refs. [32, 33].
3 Results and discussion
3.1 Microstructure analysis
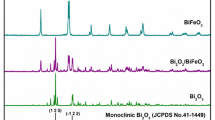
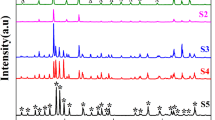
X-ray diffraction (XRD) spectra of Bi1-xSmxFeO3 were obtained in Fig. 1. The XRD peaks for BFO match the JCPDS standard card #71-2494, indicating a rhombohedral crystal structure (R3c space group). For nBSFO nanoparticles, the diffraction patterns are similar to those of pure BFO, but the peaks of (104) and (110) corresponding to a diffraction angle of 30–33° are changed. Briefly, the (104) peak gradually weakens and merges into the (110) peak, while the merged peak shifts to a higher angle. When the doping concentration is increased to 20%, as indicated by the XRD spectrum of 20BSFO, the diffraction pattern indicates that the space group R3c of rhombohedral crystal structure has transformed into the space group Pnma orthorhombic structure with a new characteristic peak (111) [34, 35], which can be further supported by the Rietveld fittings in Supplemental material [36,37,38,39,40,41]. In addition, the lattice parameters extracted from Rieveld analysis indicate that the 20BSFO nanoparticles are smaller in size compared to pure BFO. Moreover, Rieveld refinement proves that Sm doping can lead to changes in the phase structure of the prepared nanoparticles. Also, it is noteworthy that when the Sm doping amount is in the range of 0–15%, there are small amounts of Bi25FeO40 and Bi2FeO9 impurities observed in Fig. 1, while 20BSFO shows no impurity peaks. For more details, refer to Fig. S1 in the Supplemental material.
The Goldschmidt tolerance factor, denoted as G, can characterize ABO3 structure, as indicated in Equation (1) [35].
where RA, RB, and RO are corresponding to the ionic radii of Bi, Fe and O, respectively. Since the Sm3+ ion has a radius of 0.958 Å, which is smaller than the radius of the Bi3+ ion (1.03 Å) [42], its substitution will decrease the value of G. This results in stronger Fe-O bonds and weaker of Bi-O bonds, thereby causing lattice alterations in nBSFO. Besides, the XRD peaks (110), (012), and (204) of nBSFO become wider relative to the undoped particles, indicating a reduction in the crystal dimension of nBSFO.
Next, Raman spectra were collected to verify the structure variations in the synthesized nanoparticles. Briefly, BFO and nBSFO samples were electrophoretically deposited on platinum substrates to diminish background signals, as shown in Fig. 2a. The Raman curves demonstrate the observation of 13 vibrational modes, comprising 4 Al and 9 E. Specifically, four symmetrical longitudinal optical modes were located at 135.60, 167.50, 216.00 and 464.6 cm−1, while 71.50, 91.50, 262.00, 273.00, 340.50, 363.87, 433.71, 542.00 and 599.50 cm−1 were corresponding to nine transverse optical (TO) modes (E-1 to E-9), correlated to phonons [35]. Usually, Bi-O and Sm-O vibrations belong to Al modes and mainly participate at low frequencies, while Fe-O vibrations contribute to E modes, corresponding to higher frequencies. When the Sm doping level varied from x = 0 to 0.20, the modes of A1-1, A1-2, A1-3, E-1 and E-3 affected by Bi-O covalent bonds [43, 44] gradually shifted towards the higher frequency region, with broadening peaks. It is well known that the frequencies in Raman spectra are correlated to force constant and atomic mass [45]. For instance, Sm’s atomic mass is 150.36 amu, which is lighter compared to 208.98 amu of Bi, so the substitution of Sm results in a blue shift in frequency. The accumulative doping-level of Sm in BFO is accompanied by an increase in lattice distortion, as evidenced by Raman shifts and broadening [27, 42, 46], which further reinforces the XRD results. Consequently, through Raman and XRD tests, with increasing x, nBSFO nanoparticles gradually change from the rhombohedral structure of space group R3c to the orthorhombic crystal of Pnma structure [46, 47], as depicted in the schematic drawing in Fig. 2b.
After magnification 30 k times using a field emission scanning electron microscope (FESEM), the FESEM micrographs of BFO and nBSFO were obtained in Fig. 3, and the inset was the size distribution of the nanoparticles. Both BFO and nBSFO exhibited asymmetrical shapes and narrow size distributions. For BFO, the particle size distribution ranged from 20 to 110 nm, with an average particle size of 47.92 nm. Compared with BFO, nBSFO indicated comparable morphological characteristics, with particle sizes ranging from 10 to 100 nm. The average particle sizes of differently doped nBSFO were 44.00, 34.18, 26.13, and 22.12 nm, respectively. nBSFO with smaller particle sizes are more likely to agglomerate, but the sizes further confirm that the doping of Sm reduces the size of BFO system, which is consistent with the XRD outcomes.
To further verify the binding energy and chemical composition of nBSFO, X-ray photoelectron spectroscopy (XPS) was performed. For example, the comprehensive XPS spectra of 20BSFO can be found in Fig. 4. Specifically, Fig. 4a contains the XPS spectrum of the core energy levels Bi 4f, Sm 3d, Fe 2p and O 1s of the sample 20BSFO. Figure 4b–e demonstrate the high-resolution spectra of Bi, Sm, Fe, O, and Sm elements. The two characteristic peaks emerging at 159.93 and 165.26 eV correspond to Bi 4f7/2 and Bi 4f5/2, respectively, verifying that the Bi ions in the 20BSFO sample are in a trivalent oxidation state [48,49,50]. In Fig. 4c, the XPS spectrum of Sm 3d5 is obtained. The double peaks of Sm 3d3/2 and Sm 3d5/2 are correspondingly positioned at 1110.49 and 1084.00 eV, respectively, suggesting that Sm in 20BSFO exists in the same trivalent oxidation state as Bi [46]. As shown in Fig. 4d, the Fe 2p core energy spectrum presents peaks at 725.0 and 711.25 eV, which are corresponding to Fe 2p1/2 and Fe 2p3/2, while the satellite summit emerges at 720.8 eV (9 eV higher than Fe 2p3/2 peak) is characteristic of the Fe valence state, implying that the iron element exists in the form of Fe3+ of 20BSFO. In Fig. 4e, the O 1s spectrum can be decomposed to the central peaks near 530.48 and 532.11 eV, corresponding to chemically and physically adsorbed oxygen [51]. Accordingly, the XPS findings indicate that in the obtained 20BSFO samples, Bi, Sm, and Fe exist in the desired forms of Bi3+, Sm3+, and Fe3+, meaning that the Sm elements have been successfully doped into the BFO powders.
3.2 Light absorption and photocatalytic performance of nBSFO
In order to clarify the energy band gaps of nBSFO, the nanoparticle absorption spectra were collected using an ultraviolet-visible spectrophotometer (UV-Vis, scanning range from 375 to 800 nm), as shown in Fig. 5. According to the Kubelka-Munk theory [8],
where α, h, ν, A and Eg are corresponding to the absorption coefficient, Planck’s constant, optical frequency, proportionality constant and the energy gap. The parameter n is determined by the photocatalytic transition type and is normally equal to one for BFO with an indirect transition. The calculated energy gaps of the prepared nanoparticles are derived from 2.04 to 1.92 eV, as shown in Fig. 5b. Specifically, the inset gives that the energy gaps of nBSFO decrease with increasing Sm level, as indicated in Fig. 5c. These findings demonstrate that 20BSFO has the smallest energy gap, which impacts its photocatalytic properties. In conclusion, relative to BFO, the light absorption spectra of nBSFO have a clear ascendent shift overall, implying that the Sm doping level can tune the energy gap of nBSFO.
The photocatalytic performance of nBSFO nanoparticles was studied by degrading Rh B in a 30 mol/L aqueous solution under visible light from a 500 W halogen lamp. Meanwhile, to confirm that Rh B arrived adsorption stability before visible light treatment, a dark reaction was conducted for 20 min, during which 250 μl of 30% hydrogen peroxide solution was added. Figure 6a-e indicates the absorption spectra of photocatalytic concentration of Rh B as a function of time. Notably, the specific adsorption strength of Rh B at 553 nm unceasingly drops with increasing exposure time. For instance, when the doping concentration of Sm was 5%, this absorption peak basically disappeared after 80 min under the synergistic photocatalytic action of hydrogen peroxide, achieving a degradation rate of approximately 98%. Moreover, at the concentration of Sm of 10%~20%, the characteristic absorption peak of Rh B vanished at 60 min, with the degradation of Rh B reaching an equilibrium state after 80 min.
The degradation rate, denoted as D, can be written as Ci/C0, where C0 is Rh B concentration before light irradiation, and Ci is the concentration difference before and after illumination. Impressively, as depicted in Fig. 7a, the addition of 250 μl of hydrogen peroxide, barely 0.5% of the Rh B volume, significantly impacts on the photocatalytic characteristics of nBSFO. From Fig. 7c, it is evident that with the increase of Sm doping level, adding H2O2 synergistically enhanced the degradation ability. For instance, after 2 h of irradiation, the degradation rates of pure and Sm-doped BFO nanoparticles were corresponding to 72.2%, 98.4%, 98.4%, 98.5% and 98.5%, with negligible errors. In contrast, the degradation efficiencies of BFO, 5BSFO, 10BSFO, 15BSFO, and 20BSFO were 30%, 74.4%, 76.4%, 79.4%, and 81.4%, respectively, without adding H2O2. In addition, the quality of photocatalysts can be characterized by their photochemical stability. Hence, 20BSFO, exhibiting the maximum photocatalytic productivity, was subjected to cyclic testing at 25 °C in Fig. 7b to reveal its degradation efficiency. Given that BiFeO3 doped with 20% Sm reached degradation equilibrium within 80 min under the synergistic photocatalysis of H2O2, the photocatalytic degradation time for the cyclic experiment was adjusted accordingly to 80 min. Figure 7c illustrates that the degradation rates for 20BSFO on Rh B over six cycles were 98.5%, 98.2%, 98.0%, 97.3% and 96.2%, respectively. Despite some losses during the recycling process, the degradation rates were basically stable. After six cycles, the 20BSFO samples were recovered and tested by XRD analysis. Through XRD comparison, it was observed that the phase structure did not change before and after photocatalytic degradation, indicating its structural stability (see Fig. 7d). In summary, after 6 cycles, the photocatalytic properties of Bi0.80Sm0.20FeO3 did not meaningfully decline, suggesting that it simultaneously possessed the highest efficiency and excellent stability.
a Degradation rate variation for H2O2, BFO, BFO + H2O2, and nBSFO+H2O2, (b) is a cyclic photocatalytic degradation diagram of 20BSFO, (c) gives error analysis diagram for photocatalytic degradation of BFO and nBSFO with and without H2O2 and (d) shows XRD spectra for 20BSFO with and without illumination
The nBSFO nanoparticles investigated in this paper for Rh B degradation have received limited attention from previous researchers, who mainly focused on light absorption [8] or degradation of different pollutants. For instance, Orudzhev et al. reported that 10BSFO exhibited the highest degradation efficiency for the degradation of methyl orange (MO) solution, reaching 90% efficiency in 30 min [52]. However, Hu et al. came to a different conclusion, stating that the largest degradation rate of MO via 3BSFO can reach 86.9% in 2 h [53]. In addition, their research found that the photocatalytic efficiency of 16BSFO on typical organic contaminant Methylene blue (MB) dye attained 81% in 2.2 h [54]. Besides, 5BSFO can degrade Methylene Orange (MEO) by up to 80% within 3 h [55]. Moreover, the Sm and Mn co-doped BFO nanoparticles were explored on the degradation of the organic dyes Congo red (CR), MB and methyl violet (MV), and after 2 h of visible light irradiation, the catalytic efficiency increased to 97%, 65%, and 64%, respectively [56]. Similarly, doping Sm and Co into BFO achieved 92.1% degradation of MB [57]. Compared with the photocatalytic efficiencies reported in these works, our work significantly enhances the Rh B degradation efficiency from 30% of BFO to 98.5% of 20BSFO with a steady performance. This positions 20BSFO as one of the most promising catalysts for treating water pollutants [58,59,60,61,62].
3.3 Photocatalytic mechanism
On the basis of the experimental findings of the photocatalytic performance of 20BSFO, we propose the working principle as follows. First, nanoparticle dimension can appreciably affect photocatalytic characteristics of nBSFO. As the Sm concentration increases, the particle size decreases, implying that the surface area-to-volume values correspondingly increased. It would produce extra opportunities for nBSFO photocatalysts to contribute photochemical reactions with Rh B, i.e., especially by growing the surface reaction spots. This assertion of size effect can also be further supported by the magnetic behavior of nBSFO nanoparticles, and the more details can be referred to Supplemental material [36].
The photocatalytic mechanism of degrading Rh B by BFO and 20BSFO is drawn schematically in Fig. 8. Briefly, the Sm 3d5 energy level in 20BSFO is smaller than that in BFO, acting as a collector for photogenerated electrons. Consequently, relative to BFO, the lowered band gap of 20BSFO can facilitate separation and transport of photoinduced carriers, thereby improving its photocatalytic performance. The mechanism by which 20BSFO enhances photocatalysis could be further elucidated by the creation of semiconductor-separated electron-hole pairs, as well as the growth of surface reaction locations described previously for the degradation of Rh B. In short, the electron carriers in nBSFO can be optically stimulated from the valence band to the conduction band. This process leaves holes in the valance band, thereby forming e−-h+ pairs transferred to the surface to enhance the reaction probability, as summarized in Fig. 4 [63, 64]. The electrons effectively transported from 20BSFO could bond to oxygen molecules to generate *O2− in solution, resulting in strong oxidation, which can decompose Rh B into H2O and CO2. In addition, H2O molecules will be decomposed into positive proton and hydroxyl groups (*OH) due to the catalytic action from valence band holes. Moreover, Rh B* can be produced from Rh B under the photo-Fenton effect of hydrogen peroxide in the degradation solution, and Fe2+ can be attained from Fe3+ and Rh B*. Next, Fenton reaction occurred between Fe2+ and H2O2, creating Fe3+, *OH and OH−[65], eventually resulting in the reduction of Rh B into H2O and CO2.
In the end, it is noteworthy that the phase structure transition is the dominant factor in improving the photocatalytic performance of 20BSFO. Generally, if smaller sized Sm cations replace Bi ions, the dopants do not perfectly match the original rhombohedral perovskite lattice of BFO, leading to the phase structure transition. Subsequently, this transition causes a reduction in the size of the synthesized nBSFO nanoparticles, bringing about size effects. Meanwhile, the decrease in the size of the synthesized BSFO particles will also increase the strain inside the nanoparticles, which influences the lattice constant and thus the periodic field of the crystal. This ultimately results in a decline in band gaps, as observed in Fig. 5 [66]. Consequently, we conclude that the phase transition is the main reason for the enhanced photocatalytic efficiency of 20BSFO. In addition, previous studies have shown that the formed BiFeO3/Bi25FeO40, Bi25FeO40/Bi2Fe4O9 or BiFeO3/Bi2Fe4O9 heterojunction can have larger photocatalytic performance compared to their mixture [67,68,69,70]. This is because the heterojunction surfaces can effectively separate electron-hole pairs, thereby increasing the generation of superoxide and hydroxyl groups. Besides, the defect concentration observed in Fig. 1 is small and is simply mixed with the BFSO particles. Consequently, defects in the XRD patterns are not expected to significantly affect the overall catalysis. The improved efficiency in this paper is primarily attributed to the successful doping of Sm.
4 Conclusions
In this study, the photocatalytic performance of Bi1-xSmxFeO3 samples was investigated via the sol-gel approach, and the Sm doping level x was selected as 0, 0.05, 010, 0.15 and 0.20 accordingly. Based on microstructural examination, including XRD, Raman spectroscopy, FESEM, XPS and UV-Vis spectra, it was observed that the rhombohedral structure gradually changed into an orthorhombic structure. For BFO and nBSFO, the experimental findings also demonstrate that the photocatalytic efficiency of Bi0.80Sm0.20FeO3 can reach to maximum photocatalytic efficiency of 98.5%. Compared to the original, non-doped BFO nanoparticles, the photocatalytic efficiency has increased by approximately threefold. Moreover, the increased photocatalytic properties can be attributed to the phase transition, the reduced dimension of nanoparticle, which can appreciably reduce band gap energy from 2.04 to 1.92 eV. Consequently, it can provide extra chances to depart e−-h+ pairs and eventually improves the photocatalytic efficacy of 20BSFO.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhang J, Gondal MA, Wei W, Zhang TN, Xu QY, Shen K (2012) Preparation of room temperature ferromagnetic BiFeO3 and its application as an highly efficient magnetic separable adsorbent for removal of Rhodamine B from aqueous solution. J Alloy Compd 530:107–110
Ćirković J, Radojković A, Luković Golić D, Tasić N, Čizmić M, Branković G et al. (2021) Visible-light photocatalytic degradation of Mordant Blue 9 by single-phase BiFeO3 nanoparticles. J Environ Chem Eng 9(1):104587
Long JJ, Ren TT, Han J, Li NJ, Chen DY, Xu QF et al. (2022) Heterostructured BiFeO3@CdS nanofibers with enhanced piezoelectric response for efficient piezocatalytic degradation of organic pollutants. Sep Purif Technol 290:120861
Zhang LT, Li XJ, Chen JG, Jin DR, Cheng JR (2022) Enhanced photocatalytic activity in ferroelectric BiFeO3 nanoparticles treated by a corona poling method. Ceram Int 48(11):15908–15912
Wang YX, Chen J, Wu J, Armutlulu A, Xie RZ (2023) Constructing durable BiFeO3@SrBi2B2O7 p-n heterojunction for persulfate enhanced piezo-photocatalytic water purification. Sep Purif Technol 324:124479
Gupta G, Kansal SK, Umar A, Akbar AS (2023) Visible-light driven excellent photocatalytic degradation of ofloxacin antibiotic using BiFeO3 nanoparticles. Chemosphere 314:137611
Higuchi T, Liu YS, Yao P, Glans PA, Guo JH, Chang CL et al. (2008) Electronic structure of multiferroic BiFeO3 by resonant soft x-ray emission spectroscopy. Phys Rev B 78(8):085106
Gao T, Chen Z, Niu F, Zhou DT, Huang QL, Zhu TX et al. (2015) Shape-controlled preparation of bismuth ferrite by hydrothermal method and their visible-light degradation properties. J Alloy Compd 648:564–570
Parida S, Nanda J, Sarangi B (2023) Investigation of structural, optical, magnetic and photocatalytic properties of Eu–Mg co-doped BiFeO3 nanoparticles. J Sol Gel Sci Technol https://doi.org/10.1007/s10971-023-06257-w
Bossini D, Juraschek DM, Geilhufe RM, Nagaosa N, Balatsky AV, Milanović M et al. (2023) Magnetoelectrics and multiferroics: theory, synthesis, characterisation, preliminary results and perspectives for all-optical manipulations. J Phys D Appl Phys 56:273001
Yan YJ, Wang RH, Maimaitituersun M, Sun HJ, Liu XF, Mater J (2022) Built-in electric field enhanced BiFeO3 photo-Fenton degradation Rhodamine B solution. J Mater Sci 57(13):6900–6913
Rusevova K, Köferstein R, Rosell M, Richnow HH, Kopinke FD, Georgi A (2014) LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions. Chem Eng J 239:322–331
Moreau JM, Michel C, Gerson R, James WJ (1971) Ferroelectric BiFeO3 X-ray and neutron diffraction study. Phys Chem Solids 32(6):1315–1320
Chen JR, Wang WL, Li JB, Rao GH (2008) X-ray diffraction analysis and specific heat capacity of (Bi1−xLax)FeO3 perovskites. J Alloy Compd 459(1):66–70
Ahmad T, Jindal K, Tomar M, Jha PK (2023) Theoretical insight of origin of Rashba–Dresselhaus effect in tetragonal and rhombohedral phases of BiFeO3. Phys Chem Chem Phys 25:5857–5868
Gervits NE, Tkachev AV, Zhurenko SV, Gunbin AV, Lomanova NA, Danilovich DP et al. (2023) The size effect of BiFeO3 nanocrystals on the spatial spin modulated structure. Phys Chem Chem Phys 25(37):25526–25536
Yu JD, Koshikawa N, Arai Y, Yoda S, Saitou H (2001) Containerless solidification of oxide material using an electrostatic levitation furnace in microgravity. J Cryst Growth 231(4):568–576
Wang YP, Zhou L, Zhang MF, Chen XY, Liu JM, Liu ZG (2004) Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl Phys Lett 84:1731–1733
Quan ZC, Hu H, Xu S, Liu W, Fang GJ, Li MY (2008) Surface chemical bonding states and ferroelectricity of Ce-doped BiFeO3 thin films prepared by sol–gel process. J Sol-Gel Sci Technol 48:261–266
Xu JH, Ke H, Jia DC, Wang W, Zhou Y (2009) Low-temperature synthesis of BiFeO3 nanopowders via a sol–gel method. J Alloy Compd 472:473–477
Mao WW, Wang XF, Han YM, Li X, Li YT, Wang YF et al. (2014) Effect of Ln (Ln = La, Pr) and Co co-doped on the magnetic and ferroelectric properties of BiFeO3 nanoparticles. J Alloy Compd 584:520–523
Liu Y, Zuo RZ, Qi SS (2013) Controllable preparation of BiFeO3@carbon core/shell nanofibers with enhanced visible photocatalytic activity. J Mol Catal A Chem 376:1–6
Nie Y, Ma HE, Wang Z, Lan CY, Zhang Wei, Chen LM et al. (2023) New insights into the photocatalytic and magnetic activity of Co-doped BiFeO3 nanoparticles via competing structures. J Phys D: Appl Phys 56:234002–234015
Vanga PR, Mangalaraja RV, Ashok M (2015) Structural, magnetic and photocatalytic properties of La and alkaline co-doped BiFeO3 nanoparticles. Mater Sci Semicond Process 40:796–802
Arya GS, Sharma RK, Negi NS (2013) Enhanced magnetic properties of Sm and Mn co-doped BiFeO3 nanoparticles at room temperature. Mater Lett 93:341–344
Li Q, Bao SX, Liu YL, Li YX, Jing YL, Li J (2016) Influence of lightly Sm-substitution on crystal structure, magnetic and dielectric properties of BiFeO3 ceramics. J Alloy Compd 682:672–678
Yotburut B, Thongbai P, Yamwong T, Maensiri S (2017) Synthesis and characterization of multiferroic Sm-doped BiFeO3 nanopowders and their bulk dielectric properties. J Magn Magn Mater 437:51–61
Karpinsky DV, Pakalnis A, Niaura G, Zhaludkevich DV, Zhaludkevich AL, Latushka SI (2021) Evolution of the crystal structure and magnetic properties of Sm-doped BiFeO3 ceramics across the phase boundary region. Ceram Int 47:5399–5406
Zhao HH, Lai YJ, Feng LS, Shen JD, Jia XY, Mi W (2023) Ferroelectric, ferromagnetic and magneto-capacitance properties of Sm-doped BiFeO3-BaTiO3 solid solution. Appl Phys 129:77
Kebede MT, Devi S, Dillu V, Chauhan S (2022) Effects of Sm and Cr co-doping on structural, magnetic, optical and photocatalytic properties of BiFeO3 nanoparticles. Mater Sci Eng B-Adv 283:115859
Vishwakarma AK, Hussain M, Verma SK, Shukla V, Shaz MA, Srivastava ON (2021) Synthesis and characterizations of graphene/Sm doped BiFeO3 composites photoanode for efficient photo-electrochemical water splitting. Int J Hydrog Energy 46(29):15550–15560
Huang CF (2019) Structural and magnetic characterization of Bi1–xLaxFeO3 and BiFe1–yMnyO3 nanoparticles synthesized via a sol–gel method. Phase Transit 92(2):164–171
Huang CF, Zhang XL, Zhang H, Zhang W, Lan CY, Li MX (2020) Enhanced photoelectrocatalytic performance from size effects in pure and La-doped BiFeO3 nanoparticles. Appl Phys A 126(4):273
Lin TK, Chang HW, Sung YH, Wang CR, Wei DH, Tu CS et al. (2020) Multiferroic properties of Bi0.95R0.05FeO3 polycrystalline films on the glass substrates (R = La, Pr, Nd, Sm, and Ho). Mater Lett 276:128216
Singh HH, Sharma HB (2019) Enhanced electrical and magnetic properties of samarium (Sm) doped multiferroic bismuth ferrite (BFO) ceramics. Integr Ferroelectr 203(1):120–132
Supplemental material for Rietveld analysis and magnetic characteristics of the prepared nanoparticles.
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B 192:55–69
Roisnel T, Rodríguez-Carvajal J (2001) WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater Sci Forum 378:118–123
Kumar P, Kar M (2015) Mater Sci Semiconductor Process Mater Sci Semicond Process 31:262–271
Kumar P, Shankhwar N, Srinivasan A, Kar M (2015) Oxygen octahedra distortion induced structural and magnetic phase transitions in Bi1-xCaxFe1-xMnxO3 ceramics. J Appl Phys 117:194103
Kumar P, Panda C, Kar M (2015) Effect of rhombohedral to orthorhombic transition on magnetic and dielectric properties of La and Ti co-substituted BiFeO3. Smart Mater Struct 24:045028–045040
Rhaman MM, Matin MA, Hossain MN, Mozahid FA, Hakim AM, Rizvi MH et al. (2018) Bandgap tuning of Sm and Co co-doped BFO nanoparticles for photovoltaic application. J Electron Mater 47(12):6954–6958
Dutta DP, Mandal BP, Naik R, Lawes RG, Tyagi AK (2013) Magnetic, Ferroelectric, and magnetocapacitive properties of sonochemically synthesized Sc-doped BiFeO3 nanoparticles. J Phys Chem C 117(5):2382–2389
Zhang JY, Xue W, Chen XY, Hou ZL (2020) Sm doped BiFeO3 nanofibers for improved photovoltaic devices. Chin J Phys 66:301–306
Kothari D, Reddy VR, Sathe VG, Gupta A, Banerjee A, Awasthi AM (2008) Raman scattering study of polycrystalline magnetoelectric BiFeO3. J Magn Magn Mater 320(3-4):548–552
Suresh P, Babu PD, Srinath S (2014) Effect of Ho substitution on structure and magnetic properties of BiFeO3. J Appl Phys 115:17D905
Dieguez O, Gonzalez-Vazquez OE, Wojdel JC, Iniguez J (2011) First-principles predictions of low-energy phases of multiferroic BiFeO3. Phys Rev B 83:094105
Liu HM, Wei GL, Xu Z, Liu P, Li Y (2016) Quantitative analysis of Fe and Co in Co-substituted magnetite using XPS: the application of non-linear least squares fitting (NLLSF). Appl Surf Sci 389:438–446
Gao T, Chen Z, Zhu TX, Niu F, Huang QL, Qin LS (2014) Synthesis of BiFeo3 nanoparticles for the visible-light induced photocatalytic property. Mater Res Bull 59:6–12
Jamil H, Dildar IM, Ilyas U, Hashmi JZ, Shaukat S, Sarwar MN et al. (2021) Microstructural and optical study of polycrystalline manganese oxide films using Kubelka-Munk function. Thin Solid Films 732:138796
Xun BW, Song AZ, Yu JR, Yin Y, Li JF, Zhang BP (2021) Lead-free BiFeO3-BaTiO3 ceramics with high curie temperature: fine compositional tuning across the phase boundary for high piezoelectric charge and strain coefficients. ACS Appl Mater Interfaces 13:4192–4202
Orudzhev FF, Alikhanov NMR, Ramazanov SM, Sobola DA, Murtazali RK, Ismailov EH et al. (2022) Morphotropic Phase Boundary Enhanced Photocatalysis in Sm Doped BiFeO3. Mol 27(20):7029–7048
Hu ZJ, Chen D, Wang S, Zhang N, Qin LS, Huang YX et al. (2017) Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater Sci Eng: B 220:1–12
Kumar P, Chand P (2022) Sm3+-BiFeO3 nano catalyst: A synergetic effect of Sm3+ on enhanced multiferroic properties and photocatalysis. J Alloy Compd 891:161896
Gu YH, Zhou Y, Zhang WY, Guo GY, Zhang XH, Zhao JG et al. (2021) Optical and magnetic properties of Sm-doped BiFeO3 nanoparticles around the morphotropic phase boundary region. AIP Adv 11:045223
Irfan S, Shen Y, Rizwan S, Wang HC, Khan HB, Nan CW (2016) Band-Gap Engineering and Enhanced Photocatalytic Activity of Sm and Mn. J Am Ceram Soc 100(1):31–40
Arya G, Yogiraj J, Negi NS, Shah J, Kotnala RK (2017) Observation of enhanced multiferroic, magnetoelectric and photocatalytic properties in Sm-Co codoped BiFeO3 nanoparticles. J Alloy Compd 723:983–994
Mohamed WAA, Mousa HA, Abd El-Gawad HH, Handal HT, Galal HR, Ibrahim IA et al. (2023) Photophysical effects of TiO2 quantum dots on phytotoxicity, recycling for solar and photocatalytic processes of industrial effluent. Results Phys 46:106316
Kleiman A, Meichtry JM, Xaubet M, Grondona D, Litter MI, Márquez A (2023) Efficiency of cathodic arc-grown N-doped TiO2 films for the photocatalytic reduction of Cr(VI) under UV-Vis irradiation. J Phys D Appl Phys 56:495303
Ullah S, Shabir M, Rasheed MA, Ahmad I, Ahmed E, Ahmad M et al. (2023) Silver and yttrium co-doped ZnO nanoparticles as a potential water splitting photocatalyst for the H2 evolution reaction. J Sol-Gel Sci Technol 108:756
Xu YH, Han JN, Li ZH, Zhang ZH (2023) Two-dimensional g-GeC/PtSe2 van der Waals heterostructure: a visible light-driven direct Z-scheme photocatalyst for overall water splitting. J Phys D: Appl Phys 56:365504
Yang H, Yang M, Hu TP, Guo LJ, Meng RQ, Shi Y et al. (2023) Heterostructured Mo and Co-based phosphides as high-performance bifunctional electrocatalysts for overall water splitting. Phys Chem Chem Phys 25:17186–17196
Meng WW, Hu RS, Yang J, Du YF, Li JJ, Wang HY (2016) Influence of lanthanum-doping on photocatalytic properties of BiFeO3 for phenol degradation. Chin J Catal 37:1283–1292
Chen Y, Liu KR (2017) Fabrication of Ce/N co-doped TiO2/diatomite granule catalyst and its improved visible-light-driven photoactivity. J Hazard Mater 324:139–150
Yuan GL, Or SW, Chan HLW, Liu ZG (2007) Reduced ferroelectric coercivity in multiferroic Bi0.825Nd0.175FeO3 thin film. J Appl Phys 101(2):024106
Wang BJ, Li XH, Zhao RQ, Cai XL, Yu WY, Li WB et al. (2018) Electronic structures and enhanced photocatalytic properties of blue phosphorene/BSe van der Waals heterostructures. J Mater Chem A 6:8923–8929
Wang GM, Cheng D, He TC, Hu YY, Deng QR, Mao YW et al. (2019) Enhanced visible-light responsive photocatalytic activity of Bi25FeO40/Bi2Fe4O9 composites and mechanism investigation. J Mater Sci: Mater Electron 30:10923–10933
Gadhi TA, Hernandez S, Cástellino M, Chiodono A, Husak T, Barrera G et al. (2018) Single BiFeO3 and mixed BiFeO3/Fe2O3/Bi2Fe4O9 ferromagnetic photocatalysts for solar light driven water oxidation and dye pollutants degradation. J Ind Eng Chen 6:437–448
Zhang T, Shen Y, Qiu YH, Liu Y, Xiong R, Shi J et al. (2017) Facial Synthesis and Photoreaction Mechanism of BiFeO3/Bi2Fe4O9 Heterojunction Nanofibers. J Am Chem Soc 6:4630–4636
Alkan S, DemirbasÖ, Celikcapa S, Doğan M (2004) Sorption of acid red 57 from aqueous solution onto sepiolite. J Hazard Mater 116(1-2):135–145
Acknowledgements
Our research was supported by the National Natural Science Foundation of China under Grant No. 12004424, and the Key Academic Discipline Project of China University of Mining and Technology under Grant No. 2022WLXK07 and National Laboratory of Solid State Microstructures, Nanjing University, Nanjing 210093, China under Grant No. M35012.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: CFH, YN, JGW; data collection: YN, RZH, XFY; analysis and interpretation of results: CFH, YN, RZH, XFY, LZ, JW, XWX; draft manuscript preparation: CFH, YN, JGW. All authors reviewed the results and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, C., Nie, Y., Han, R. et al. Preparation, characterization, and mechanism for enhanced photocatalytic performance in Bi1-xSmxFeO3 nanoparticles. J Sol-Gel Sci Technol 110, 736–746 (2024). https://doi.org/10.1007/s10971-024-06392-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-024-06392-y