Abstract
Substitution of NdZn in Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19, (x = 0.00–0.10; y = 0.00–1.00) hexaferrites prepared by sol–gel method is investigated, and their effect on the microwave, structural, and magnetic properties is reported. The XRD patterns reveal single-phase M-type hexaferrite structure. The lattice parameters were found to increase by the substitution of NdZn. The increase in lattice parameters is due to the difference in ionic sizes of the cations involved. The average grain size was found between 16 and 29 nm by Scherer formula and was also confirmed by SEM and TEM. Magnetic behavior of selected sample was observed up to a magnetic field of 8T taken at temperature ranges from 4.2 to 373 K. The coercivity of the sample decreased from 2300 to 1210 Oe with increasing temperatures in a linear fashion up to 373 K. The grain boundaries, and the associated pinning fields, seem to have a resolute role in the magnetic behavior of these hexaferrites. Microwave measurements of the ferrite sample have been measured in the frequency range 0.5–12 GHz. The frequency dispersion of ferrites is credited to the phenomenon of natural magnetic resonance and domain wall pinning.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hexaferrites due to their unique electrical and magnetic properties belong to very important form of attractive magnetic materials for technological applications. They have many applications, such as in magnetic recording media, as magneto-optical devices, and permanent magnets. The magneto-dielectric property of hexaferrites is of particular interest in microwave and radio frequency applications. Many studies on the magnetic properties of hexagonal ferrites have been reported, which shows enhanced magnetic, structural, and microwave properties due to incorporation of rare earth elements [1–4]. Moreover, low cost, easy manufacturing, and high power at low loss of hexaferrite materials have greatly increased their production and effectiveness at commercial level. The studies on BaFe12O19 show that nanosized hexagonal BaFe12O19 has a single magnetic domain and high anisotropy, due to which it offers excellent magnetic properties, which strongly depend on the mean grain size and morphology of the synthesized materials. [5–9]. As communication and electronic systems are moving up to higher working frequencies, so the demand on spinel ferrites seems to be less, due to intrinsic restrictions imposed by the occurrence of their natural ferromagnetic resonance up to 1 GHz. This technological progress has set off the potential of hexaferrites to replace spinel ferrites in applications comprising ferrite devices which operate in microwave frequencies for microwave absorbers. Research growth in the field of microwave-absorbing materials working at higher frequency range is driven by the ever-increasing requirement of exploiting electromagnetic waves for wireless telecommunication systems and reduction of radar signatures, as in stealth technology. The intrinsic magnetic properties resulted from the specific site occupancy of the Fe ions and rare earth (RE) elements along with divalent cations enhance the electromagnetic properties of ferrites [10, 11]. These reasons make the manipulation of Fe and RE in the lattices, as a promising way to improve magnetic properties of hexagonal ferrites. In this work, the sol–gel method was used to fabricate calcium–barium hexaferrites. Ca-based M-type hexaferrites have not been extensively studied so far; however, very few studies have been performed, indicating positive results of hexaferrites [12, 13]. Calcium is chosen as base due to its intrinsic properties such as the reduction in the sintering temperature and improvement in the structural properties; meanwhile, the combination of rare earth and divalent improves magnetic and electrical properties [14, 15]. The aim of the present study was to investigate the effect of NdZn substitution on the magnetic properties of Ca-based M-type hexaferrites. The synthesis of single-phase RE-doped calcium–barium hexaferrite is thought to be difficult, and properties of such materials have not been studied systematically so far. The present work reports the synthesis of single-phase calcium–barium hexaferrites and the effect of simultaneous substitution of NdZn ions on the electrical, magnetic, and microwave properties of calcium–barium hexaferrite.
2 Experiment details
2.1 Sample preparation
M-type hexaferrites with a nominal composition of Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19 (x = 0.00–0.10; y = 0.00–1.00) were synthesized by sol–gel autocombustion method [19]. Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19 samples were synthesized using analytical-grade citric acid, iron chloride (FeCl3·6H2O), calcium chloride (CaCl2), barium nitrate Ba (No3)2, nickel nitrate Ni (NO3)2, and neodymium oxide (Nd2O3) by dissolving each of them one by one in deionized water except Nd2O3. As Nd2O3 is not soluble in water so it was first dissolved in nitric acid at 80 °C and converted into nitrate and poured into the prepared solution. To maintain the pH value at 7, an aqueous ammonia solution at a ratio of 40:60 was added drop-wise, while stirring at 60 °C. Then the sols were allowed to gel at 60 °C. Powdered gel was formed after drying at 200 °C. The dried gel was ground homogenously in an agate mortar and pestle for 20 min. Finally, the homogenized powder was then sintered at 1100 °C for 8 h.
2.2 Characterization
2.2.1 FTIR spectroscopy and X-ray diffraction
Fourier transform for infrared has been done by (FTIR) spectrophotometer (BioRad Merlin FTS 3000MX). X-ray diffraction analysis was performed using shimadzu X-ray diffractometer equipped with CuKα radiation (λ = 1.5406 Å). The crystallite size was determined by Scherrer formula [14].
where K is shape constant, λ is the wavelength, β is FWHM intensity, and θ is the Bragg angle. The values of X-ray density (ρ X-ray), bulk density (ρ m ), porosity (P), and the unit cell volumes (V cell) were calculated by the following equations [15]:
where M is the molar mass, a and c are lattice constants, m is the mass of pellets, r is radius of the pellets, N A is Avogadro’s number, and V cell is the unit cell volume. The vibrating sample magnetometer (VSM) Lake Shore (7400, USA) was used to measure M-H loops at room temperature.
2.2.2 Electron microscopy
TEM was used for the estimation of the crystallite size and aggregation. The analysis of the samples was investigated using a TEM:JEOL Japan 2011 (with LaB6 filament). Samples were prepared by sonicating the particles in ethanol for 1 h and placing drops of the suspension on carbon-coated copper TEM grids before allowing them to dry in air. Femto electronic diener plasma surface technology plasma cleaner model 2011 has been used to remove any contamination from TEM grids before analyzing. SEM analysis of the samples was performed using an FEI SIRION SEM operating at 20 kV and was used to observe the microstructure of the sintered specimens.
2.2.3 Radio frequency and microwave attenuation
The radio frequency and microwave attenuation characteristics of Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19 were measured from 0.5 to 12 GHz using an ASTM4935 coaxial transverse electromagnetic wave cell and an HP 8753D vector network analyzer [16]. The system was calibrated using a mechanical short-open-load-through (SOLT) calibration kit. The conventional ASTM D4935 reference sample technique for samples with poor surface contact impedance is not suitable for powdered materials. An in-line sample holder was therefore constructed in a 3.2-mm length of the coaxial cell using two very thin 0.1-mm, low-permittivity, lossless sheets of acetate to accommodate the Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19 powder. It was found necessary to render the powder into a paste in order to stabilize its distribution within the sample holder and thereby obtaining reproducible results. The paste was prepared by adding 4.04 g of the powder into 20 ml of super spray adhesive liquid and placed in the sample holder, giving an average sample density of 0.338 g cm−3. The insertion loss of the cell was determined from the decrease in transmission when the paste was introduced into the sample holder.
3 Results and discussions
3.1 FTIR spectroscopy
Figure 1 shows FTIR profile of three sintered samples of Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19 (x = 0.02, 0.06, 0.10; y = 0.20, 0.60, 1.00) hexaferrite materials. They have dominant absorption bands on 574, 586, and 595 cm−1, respectively. The shoulder on the peaks above 1000 cm−1 results due to some bending vibrations of Fe–O–H [17]. However, the indication of these peaks of FTIR confirmed the formation of hexagonal structure.
3.2 X-ray diffraction analysis
Figure 2 shows X-ray diffraction analysis of the Ca0.5Ba0.5−x Nd x Zn y Fe12−y O19 (x = 0.00–0.10; y = 0.00–1.00) hexaferrite materials. The given XRD pattern reveals that all the peaks match well with the standard pattern ICDD 00-051-1879 [18]. The parameters such as cell constants (a and c) and crystallite size are also calculated from XRD data. The substitution of NdZn leads to increase in the lattice constants as shown in Fig. 3, and these results are in agreement with already reported [18]. This increase is due to the higher ionic radius of Zn2+ (0.74 Å) as compared to that of Fe3+ (0.64 Å).
The bulk density has been calculated using the following equation:
where m is the mass of the pellet, r is the radius of pellet, and h is the thickness of the pellet. It can be observed that the bulk density of the substituted samples is greater than that of the undoped samples as listed in Table 1. The larger value of bulk density may be attributed to larger density of Nd3+ (7.24 g cm−3) as compared to that of Ca2+ (1.55 g cm−3). The bulk density is less than theoretical density due to the presence of pores created during sintering process [19].
The crystallite size has also been calculated by Debye–Scherer formula:
where K is the shape constant having a value of 0.89 for hexagonal system, λ is the wavelength of the X-rays used, β is the broadening of diffraction line measured at half width of its maximum intensity, and θ B is the Bragg’s angle of diffraction [16]. The crystallite size is found in the range of 16–29 nm as given in Table 1. It has been reported that the crystallite size <50 nm is required to obtain the suitable signal-to-noise ratio in the high-density recording media [21]. The crystallite size is <50 nm, so these materials can be used for applications in high-density recording media in obtaining suitable signal-to-noise ratio.
3.3 Temperature-dependent M-H loop
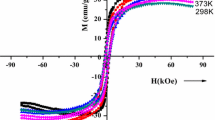
The temperature dependence of M-H loops between 4.2 and 373 K of a selected Ca0.5Ba0.5NdZnFe12O19 hexaferrite sample is shown in Fig. 3, and corresponding values are listed in Table 2. The M-H loops were taken at temperatures of 4.2, 77, 273, 298, and 373 K. Between these temperatures, the increased thermal disorder decreases the coercivity in the samples; as can be seen in Fig. 4, the coercivity of the sample decreased from 2300 to 1210 Oe with increasing temperatures in a linear fashion up to 373 K. The decrease in coercivity is consistent with reduction in grain size during heat treatment. The grain boundaries, and the associated pinning fields, seem to have a resolute role in the behavior of oxide magnets. However, it has been inferred that large pinning fields exist at grain boundaries, and their origin must be responsible for perturbations in the magnetic properties on a very fine scale, and these changes in coercivity resulted due to domain wall pinning fields. Generally, the results show that substitutions of NdZn lead to the decrease in Hc through the reduction of the magneto-crystalline anisotropy of calcium–barium ferrite. Figure 5 shows the corresponding observed magnetic parameters of the NdZn-doped ferrites and reveals that the saturation magnetization M s and M r of the as-prepared ferrites decreased in response to the heat treatments. However, it is earlier reported that for hexagonal ferrites, M s continuously decreases with increase in temperature, and almost a linear behavior of saturation magnetization has been observed over a wide range of temperature. It may be concluded that the diffusion of the dopant in the anoxic conditions is responsible for the changes in the coercivity. The effect of rare earth element Nd3+ and Zn substitution on the magnetic properties of the material is in general useful [20–23].
3.4 Scanning electron microscopy, EDS, and transmission electron microscopy
SEM and TEM analyses on three selected samples, i.e., Ca0.5Ba0.48Nd0.02Zn0.20Fe11.8O19, Ca0.5Ba0.44Nd0.06Zn0.60Fe11.4O19, and Ca0.5Ba0.4Nd0.10Zn1.00Fe11O19 were performed. Figure 6a, b, and c reveals the effect of the substitution of NdZn on the microstructure and grain size of M-type hexaferrites. It is clear from the SEM profiles that most of the particles have well-defined shape and boundaries. The grains are regular hexagonal platelets with homogeneous grain size between 0.5 and 20 µm in average. The surface morphology shows that as substitution increases, the grains also become clearly hexagonal and regular in shape. The diameter of the hexagonal grains seems to be decreased as the substitution level increased. This result suggests that NdZn substitutions encouraged the grain growth in the M-type hexaferrites. Figure 6d, e, and f shows the TEM profiles of Ca0.5Ba0.48Nd0.02Zn0.20Fe11.8O19, Ca0.5Ba0.44Nd0.06Zn0.60Fe11.4O19, and Ca0.5Ba0.4Nd0.10Zn1.00Fe11O19 samples. The values of particle size calculated by TEM were found to be 5–20, 5–10, and 1–10 nm, respectively. It is obvious from the images that as the substitution level of NdZn increases, the average grain size decreases, which shows that substitution of NdZn enhances the grain growth and helps in the formation of smaller grain sizes. The grain size obtained by SEM lies in the range of 5–20, 1–5, and 0.5–5 µm, respectively, for the Ca0.5Ba0.48Nd0.02Zn0.20Fe11.8O19, Ca0.5Ba0.44Nd0.06Zn0.60Fe11.4O19, and Ca0.5Ba0.4Nd0.10Zn1.00Fe11.4O19 hexaferrites. The particle size for the samples synthesized in the present study is much smaller compared with those of the reported earlier for the M-type hexaferrites. These particles are small in size and are highly suitable for improving signal-to-noise ratio in high-density recoding media [24–30].
3.5 Complex permittivity and permeability
Figures 7 and 8 illustrate the representative complex permittivity (εr = ε′ − jε″) and complex permeability (µr = µ′ − jµ″) spectra versus frequency having both real and imaginary parts in the range from 0.5 to 12 GHz. It is obvious from Fig. 7 that the value of ε′ decreases with the frequency, but the value of ε″ remains almost constant in the entire range of frequency. The value of permittivity for the ferrites at high frequencies mainly depends upon the atomic and electronic polarization within the ceramic grains. The mechanism of electric polarization is considered as due to hopping electrons between Fe2+ and Fe3+ on the octahedral sites [31]. The smaller the number of hopping electrons, the lower would be the permittivity [32–35], which is favorable for impedance matching. In addition, the complex permeability of composite versus frequency is shown in Fig. 8. The value of µ′ shows a peak at about 2 GHz. This can be due to the demagnetizing field caused by magnetic poles in the surface of ferrite particles, which causes to increase the resonance frequency compared with sintered ferrite [37]. The value of µ′ slightly decreases from 2 to 12 GHz. This is favorable for microwave surface impedance match because the wavelength in microwave absorber decreases as the frequency increases. The magnetic spectra of µ″ show a weak frequency dispersion phenomenon in Fig. 8, which can be interpreted as [37, 38]. The frequency dispersion of ferrites is credited to the phenomenon of natural magnetic resonance and domain wall pinning. In the weak alternative magnetic field, domain wall pinning is the dominant magnetization process. Moreover, the crystal grains of ferrite are enclosed by non-magnetic coating of epoxy, which is known to increase the effective reluctance of the ferrite sample. Consequently, a weak dispersion phenomenon in the spectra of µ″ has been observed for the investigated ferrite sample.
3.6 Microwave absorption
The normalized input impedance, Zin, of a metal-backed microwave absorption layer is derived from the following expression [39].
Where f is the frequency of the EM wave, d is the thickness of the absorber, ε r and µ r are relative permittivity and permeability, respectively, t is the thickness of the sample, and c is the velocity of light in a vacuum.
According to the transmission line theory, the reflection coefficient RL (dB) of electromagnetic wave under perpendicular wave incidence at the surface of a single-layer material backed by a perfect conductor can be expressed as [40, 41].
where Z 0 is the characteristic impedance of vacuum Z 0 = 120π.
The thickness dependence of the RL of the composite was studied. The theoretical value reflection loss of the Ca0.5Ba0.4Nd0.10Zn1.00Fe11O19 with different thickness was calculated based on formulas (9) and (10). Figure 9 shows the variation of the reflection loss versus frequency of Ca0.5Ba0.4Nd0.10Zn1.00Fe11O19 with layer thicknesses ranging from 2 to 3 mm. It is obvious that the peak for the RL shifted to lower frequencies with increasing thickness; under this condition, the peak value and absorption band width first increased and then decreased. The reflection loss is measured at the values of the thickness (t m ) [42] and is expressed by equation;
where λ a is the wavelength inside the absorber, f is the incident frequency of the free space.
The above equation shows that the thickness is inversely proportional to peak frequency; due to this reason, the peak frequency has been shifted toward lower frequency band as the thickness increases [43]. The reflection loss has been found strongly dependent on the thickness of the sample. The similar type of phenomenon was observed previously in case of U-type hexaferrites [44–46], which demonstrates that attenuation peak frequency of the ferrite samples can be controlled easily by changing the thickness of materials to use these materials at a desired frequency.
4 Conclusions
-
NdZn-substituted Ca0.5Ba0.5Fe12O19 samples exhibit a single magneto-plumbite phase with slightly increasing values of lattice parameters a and c.
-
The crystallites size was found in the range 13–24 and 16–29 nm calculated by TEM and Scherer equation, respectively. The particle size is smaller enough to obtain a suitable signal-to-noise ratio in the magnetic recording media.
-
Coercivity can be tuned at lower substitution level. The magnetization behavior has been characterized by the preferential site occupancy of sublattice sites by substituted cations, magnetic dilution, and spin canting.
-
The attenuation peak frequency of the ferrites can be controlled by changing the thickness of materials to use these materials at a desired frequency. Moreover, in NdZn set of samples, 3-mm sample indicates low loss and more absorption.
References
Albnese G, Dariu A, Licci F, Rinaldi S (1978) IEEE Trans Magn MAG-14:710
Kown HJ, Shin JY, Oh JH (1994) J Appl Phys 75:6109
Naiden E, Maltsen V, Ryabtsen G (1990) Phys Status Solidi (a) 120:209
Yoon K, Lee D, Jung H, Yoon S (1992) J Mater Sci 27:2941
Ding J, Maurice D, Miao WF, McCormick PG, Street R (1995) J Magn Magn Mater 150:417
Mendoza-Suarez G, Matutes-Aquino JA, Escalante-Garcıa JI, Mancha-Molinar H, Rıos-Jara D, Johal KK (2001) J Magn Magn Mater 223:55
Abbas SM, Dixit AK, Chatterjee R, Goel TC (2007) J Magn Magn Mater 309:20
Kulikowski J (1984) J Magn Magn Mater 41:56
Ruan S, Xu B, Suo H, Wu F, Xiang S, Zhao M (2000) J Magn Magn Mater 212:175
Stergiou CA, Manolakis I, Yioultsis TV, Litsardakis G (2010) J Magn Magn Mater 322:1532
Ramasamy DSR (1997) International conference electromagnetic interference and compatibility INCEMIC-97. New Jersey 7B-7, 459
Iqbal MJ, Ashiq MN, Gul IH (2010) J Magn Magn Mater 322:1720
Hussain Shahid, Maqsood A (2007) J Magn Magn Mater 316:73–80
Valanzuela R (2009) Magnetic ceramics. In: Chemistry of solid state materials, vol 4. Cambridge University Press, p 44
Iqbal MJ, Ashiq MN (2008) Chem Eng J 136:383
ASTM International, Standard test method for measuring the electromagnetic shielding effectiveness of planar materials, standard number D4935-10, 1 May 2010. doi:10.1520/D4935-10
Rezlescu N, Doroftei C, Rezlescu E, Popa PD (2006) Phys Status Solidi 15:3844
Iqbal MJ, Farooq S (2011) Mater Res Bull 46:662
Popa PD, Rezlescu E, Doroftei C, Rezlescu N (2005) J Optoelectron Adv Mater 7:1553
Lechevallier L, Le Breton JM, Wang JF, Harris IR (2004) J Magn Magn Mater 269:192
Che S, Wang J, Chen Q (2003) J Phys Condens Matter 15:L335
Wagner TR (1998) J Solid State Chem 136:120
Chang Sun, Kangning Sun, Pengfei Chui (2012) J Magn Magn Mater 324:802
Costa ACFM, Tortella E, Morelli MR, Kiminami RHGA (2003) J Magn Magn Mater 256:174
Khan HM, Islam MU, Ali I, Rana MA (2011) Mater Sci Appl 2:1083–1089
Smit J, Wijn HPJ (1959) Ferrites. Wiley, New York
Vasambekar PN, Kolekar CB, Vaigankar AS (1999) Mater Res Bull 34:863
Lee SW, An SY, Shim I, Kim CS (2005) J Magn Magn Mater 290–291:231
Rashad MM, Radwan M, Hessien MM (2008) J Alloys Compd 453:304
Mu C, Chen N, Pan X, Shen X, Gu X (2008) Mater Lett 62:840
Khan HM, Islam MU, Xu Y, Ashiq MN, Ali I, Iqbal MA, Ishaque M (2014) Ceram Int 40:6487–6493
Khan HM, Islam MU, Xu Y, Iqbal MA, Ali I (2014) J Alloys Compd 589:258–262
Iqbal MJ, Ashiq MN, Gul IH (2010) J Magn Magn Mater, 1720–1726
Zhang HJ, Liu ZC, Yao X (2003) Mater Sci Eng B 97:160
Haijun Z, Jia XL, Yao X et al (2004) J Rare Earth 22(3):338
Kim YJ, Kim SS (2002) IEEE Trans Magn 38(5):3108
Gao B, Qiao L, Wang JB, Liu QF, Li FS, Feng J, Xue DS (2008) J Phys D Appl Phys 41:35005
Tsutaoka T, Ueshima M, Tokunaga T (1995) J Appl Phys 78:3983
Zhang XF, Guan PF, Dong XL (2010) Appl Phys Lett 96:102505
Singh P, Babbar VK, Razdan A, Srivastava SL, Goel TC (2000) Mater Sci Eng B 78:70
Wu MZ, Zhang YD, Hui S, Xiao TD, Ge SH, Hines WA, Budnick JI, Taylor GW (2002) Appl Phys Lett 23:4404
Naito Y, Suetake K (1971) IEEE Trans Microw Theory Tech 19:65–72
Michielssen E, Sajer J, Ranjithan S, Mittra R (1993) IEEE Trans Microw Theory Tech 41:1024
Wessling B (1991) Polym Eng Sci 16:1200–1206
Oikonomou A, Giannakopoulou T, Litsardakis G (2007) J Magn Magn Mater 316:e827–e830
Feng Y, Qiu T (2012) J Alloys Compd 513:455–459
Meena RS, Bhattachrya S, Chatterjee R (2010) Mater Des 31:3220–3226
Qing Y, Zhou W, Luo F, Zhu D (2011) J Magn Magn Mater 323:600
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, H.M., Islam, M.U., Xu, Y. et al. Structural, magnetic, and microwave properties of NdZn-substituted Ca0.5Ba0.5Fe12O19 hexaferrites. J Sol-Gel Sci Technol 75, 305–312 (2015). https://doi.org/10.1007/s10971-015-3700-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3700-x