Abstract
This paper describes the nuclear-forensic characterization of materials provided by the Nuclear Forensic International Technical Working Group within the 7th Collaborative Material Exercise. The characterized materials were two powdered uranium material samples labelled ES-1 and ES-3 and two uranium metal samples labelled ES-2 and ES-4. A combination of several analytical techniques was used by a group of Czech laboratories to the identification of chemical compounds and the determination of the uranium isotopic and elemental composition of all four samples. Those results allowed for an unambiguous conclusion that ES-1 was depleted uranium trioxide, ES-2 was depleted uranium-vanadium alloy, ES-3 was depleted uranium nitrate, and ES-4 was depleted uranium metal. A further conclusion was that ES-2 cannot originate from the same source as the other three samples and that ES-1, ES-3, and ES-4 had a common source material. The highlights of this work were in obtaining the isotopic composition of uranium, including minor isotopes, in the early (24-hour) phase of the exercise using the Secondary Ion Mass Spectrometry in a single-particle measurement mode and in the application of the Neutron Activation Analysis with uranium separation prior to irradiation for the elemental screening of the uranium samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A country that is advanced in nuclear technology needs a nuclear forensic capacity to support an investigation of criminal acts that involve nuclear or radioactive substances, to deter or combat illicit trafficking of nuclear material, and to verify the correctness and completeness of material holdings reported by the licensees. In the Czech Republic (CR), most seized materials out of regulatory control (MORC) were abandoned legacy samples of uranium compounds or radioactive sources found at various institutions or public areas during cleanup or decommissioning processes or by chance. Those MORC were identified and categorized by first responders, then stored or disposed of under the supervision of the regulatory body. In most cases, the criminal investigation and thereby specialized nuclear forensic laboratories were not involved.
In the absence of frequent criminal cases nuclear forensic laboratories need regular exercises to sustain or improve their skills in nuclear forensics and to demonstrate credible nuclear forensic performance that can serve as a deterrence against nuclear smuggling.
To that end, a group of Czech laboratories with nuclear forensic capabilities has been assembled to cover a broad range of skills, know how, and analytical infrastructure and to take part in the 7th Collaborative Material Exercise (CMX-7) organized by the Nuclear Forensic International Technical Working Group (ITWG).
The nuclear forensic investigation involves a broad range of techniques, including chemical and physical analytical methods and radio analytical methods [1] and relies on knowledge in areas such as radiochemistry, reactor physics, material science, and nuclear fuel cycle. The aim of the nuclear forensic investigation is to answer questions on the origin of the investigated nuclear material, its production process, its owner, the smuggling route, and the intended use. The key characteristics to answer those questions are isotopic, elemental, and molecular composition, microscopic structure, as well as macroscopic features.
In this work, the nuclear forensic characterization of uranium materials, the analytical workflow, and the decision-making leading to answering the investigator’s questions from the exercise scenario are described. The CMX-7 exercise had also a conventional forensic part, but its description is beyond the scope of this paper.
Experimental
Samples and exercise investigative questions
Four samples were received by a shipping Point of Contact (POC), who was not participating in the exercise and did not share the information about the samples from the packing list with the members of the participating laboratories. The POC transported samples to Research Centre Rez, where the boxes with the samples were opened and the exercise on the Czech side began.
After initial inspection and setting up the radiation protection measures the materials in transparent glass bottles were taken out of the transport containers and the first physical characteristics were recorded. The sample labelled ES-1 was a yellow powder in a glass vial. Sample ES-2 was composed of four pieces of black foil in a glass bottle. Sample ES-3 was a green-yellow powder in an Erlenmeyer flask. Sample ES-4 was composed of two rectangular ~ 1 mm thick dark pieces in a glass vial.
In the fictional scenario of the exercise, ES-1 and ES-2 were found on the crime scene as MORC, ES-3 was found also as MORC during the follow-up investigation in the workplace of the main suspect, and ES-4 was retrieved for comparison from a laboratory licensed to handle nuclear material [2]. The investigator requested the support of the investigation by answering the following questions:
-
(1)
Can the yellow powder of ES-1 be linked with the black foils of ES-2?
-
(2)
Can either ES-1 or ES-2 be associated with the green-yellow powder of ES-3?
-
(3)
Are any MORC (ES-1, ES-2, ES-3) consistent with the dark pieces of comparative sample ES-4?
-
(4)
What is the chemical composition/phase of the samples?
The answers were sent to the investigator played by the ITWG organizers after the completion of each of the three phases of the exercise (24-hour, 1-week, 2-month).
Analytical plan and the exercise workflow
A generic analytical plan prepared for the exercise following the guidelines from the ITWG [3] has been continually adjusted as the exercise was evolved. The final version of the analytical plan is in Fig. 1. Within the initial inspection in the 24-hour phase, basic dosimetry measurements (dose rate, surface contamination) were conducted. Within the conventional forensic inspection step, the photographic evidence, fingerprints, and basic physical description were recorded and relevant information was inserted into the chain of custody forms. Then the samples in unopened bottles were transported to the Gamma Spectroscopy (GS) laboratory and after finishing the GS measurement to the Micro-Raman Spectroscopy (RAMAN) laboratory. Then each bottle was opened in a flow box or fume hood in a different radiochemical laboratory to avoid cross-contamination and the swipes of microscopic particles released from the samples were taken from the inner surface of the neck of the glass bottles without touching the samples inside. The swipes went to the Secondary Ion Mass Spectrometry (SIMS) laboratory. Next, sets of subsamples from the powdered samples (ES-1, ES-3) were prepared for the X-Ray Fluorescence (XRF), the X-Ray Diffraction (XRD), and the Scanning Electron Microscopy–Energy Dispersive X-ray Spectroscopy (SEM–EDS). The latter subsamples were shared with the visible-light Optical Microscopy (OM). The XRF and the XRD subsamples were placed using a micro-spatula inside the cups made of a Mylar-foil forming a circular base fixed on a Teflon ring. The OM/SEM–EDS subsamples were placed also with a spatula onto a double-sided carbon tape attached to an aluminum alloy SEM stub. In the case of the metallic pieces (ES-2, ES-4) no subsampling was needed in the 24-hour phase. The metallic pieces were simply placed inside the cups or onto a double-sided carbon tape on a SEM stub.
The analyses in the 1-week and 2-month phases were designed to refine the answers or to increase the level of confidence in the answers either by repeating the analyses from the 24-hour phase at different measurement conditions or by employing other methods. In the 1-week phase, the other methods were the Alpha Spectrometry (AS) and the Inductively Coupled Plasma Mass Spectrometry (ICP-MS), in the 2-month phase it was the Pre-Irradiation Separation–Neutron Activation Analysis (PS-NAA).
Methods and instruments
The instruments and methods used in the exercise are listed in Table 1, including a basic description of instrumental specifications and the main parameters or settings used in the exercise. The procedures using SIMS and PS-NAA will be described in more detail in the next sections.
The sample characteristics obtained using the methods in Table 1 were processed using the Graded nuclear forensic decision framework (GDF) [3] – a statistical tool that enabled to assign a level of confidence to answers to investigative questions. A clustering analysis was used to quantify linkage among samples in terms of Euclidean distance \(\left( {x,y} \right) = \left[ {\sum {\left( {x_{i} {-}y_{i} } \right)^{2} } } \right]^{1/2}\), where xi and yi were concentrations of element “i” in samples x and y.
SIMS in a 24-hour phase of nuclear forensic investigation
The SIMS as a destructive technique and a demanding method in many respects (throughput, cost of ownership, availability) is usually employed in the 2-month phase or the 1-week phase at best of a nuclear forensic investigation. Here, the use of SIMS in the 24-hour phase is described. The procedure is based on the experience obtained in the analyses of individual dust particles containing uranium for international nuclear safeguards [4].
Shortly after removing the cap, swipe samples were taken from the inner surface of the neck of each bottle (Fig. 2). This was done without touching or disturbing the samples inside the bottles. The extraction of particles from the swipes onto silicon planchets (1″ wafers) was done with a vacuum impactor [5]. This method consists of impacting particles on a silicon planchet beforehand covered with an organic compound, polyisobutylene in nonane, acting as a sticky agent [6]. This operation was carried out in a disposable plastic glove bag. Each sample had its own glove box to prevent cross-contamination.
Then the SIMS performed two-dimensional image scans 500 μm × 500 μm for 235U and 238U isotopes (Fig. 3) and an automated sample-stage movement in discrete steps of 500 μm to cover a sufficiently large part of the planchet—2 mm × 2 mm composing of 16 fields in the center of the silicon planchet (Fig. 4). The coordinates and preliminary enrichment of uranium particles was the result of that step. Additionally, various enrichment populations of particles can be observed if the sample is not isotopically homogenous. Next, several representative particles were chosen one by one, and an accurate and precise measurement of isotopic composition, including minor isotopes 234U and 236U, of each individual particle was obtained.
The calibration of the isotopic measurement was carried out using the certified reference material of uranium oxide particles CRM U010 (New Brunswick Laboratory, Argonne, IL, USA) analyzed under identical conditions as the samples.
The main difference in the SIMS procedure used in this work compared to [4] was in speeding up the particle search and identification step by screening substantially smaller areas of the planchets. Thus, the SIMS measurements for all four samples could have been completed within a “record-breaking” time of ~ 14 hours.
NAA in the 2-month phase of nuclear forensic investigation
NAA can be used for the screening and determination of trace elements in various materials. However, in the case of uranium matrix, number of fission products is created on neutron irradiation, so that their activity prevents determination of other major, minor, and trace elements. Therefore, uranium must be selectively separated from the samples prior to irradiation, which is a challenging task [7].
Within this work, uranium powdered samples ES-1 and ES-3 with masses of 30–50 mg were dissolved in 3M HNO3. The chunks with masses of 50–70 mg cut out of the metal samples ES-2 and ES-4 were first etched to lose about 5–10% of their mass to remove a possible external contamination and then dissolved. Three 2-ml-columns of UTEVA resin in succession in Fig. 5 were used for pre-irradiation removal of uranium from the samples. A quality control sample Chanterelle (powdered U3O8) [9] with mass of ≈ 50 mg was analyzed in the as received state. The same analytical procedure was used for a processing blank. Aliquots of the effluent were transferred into cleaned polyethylene and quartz (Suprasil®, Heraeus) tubes for short- and long-time irradiation, respectively, and sealed. A separation factor of uranium between 103 and 104 was achieved.
All laboratory ware (Teflon, Polypropylene, Polyethylene) was soaked for several days in sub-boiled 3M HNO3 and washed with demi-water. For sample dissolution and uranium separation using UTEVA resin, 3M HNO3 prepared from sub-boiled HNO3 was used. All operations were performed in a Clean Lab ISO 5—6.
The irradiation has been performed in the LVR-15 reactor in Rez, Czech Republic [8]. The thermal neutron flux density and the timing for the short-time irradiation were Φth = 3·1013 cm−2 s−1, ti = 90 s (irradiation time), td = 600 s (decay time), tc = 600 s (counting time), respectively. The thermal neutron flux density and the timing for long-time irradiation were Φth = 4·1013 cm−2 s−1, ti = 3 hours, td = 2–3 days or 25–30 days, tc = 1 hour, or 3 hours, respectively.
The quantification was carried out by the comparator method with in house calibrators prepared from solutions Astasol with certified element contents (Analytika, s.r.o., Czech Republic) irradiated and counted as the samples.
The counting of short-lived radionuclides of the elements Al, V, Mn, and Cu was done with a coaxial BEGE detector (PGT, FWHM 1.75 keV, relative efficiency 20.3%, both for 1332.5 keV line of 60Co) using dual LFC module to perform dynamic correction of dead time and pile-up losses. The counting geometry was 3 cm from the detector cap. For the counting of medium- and long-lived radionuclides of the elements Na, As, Sb, W, Cr, Fe, Co, Ni, Zn, Sb and Ba another coaxial BEGE detector (Canberra, FWHM 1.87 keV, relative efficiency 77.8%, both for 1332.5 keV line of 60Co) was used, again with the LFC module in the associated electronic chain. The counting geometry was 2 cm from the detector cap.
The cluster analysis was employed to quantify linkage among the samples analyzed using Euclidean distance \(\left( {x,y} \right) = \left[ {\sum {\left( {x_{i} {-}y_{i} } \right)^{2} } } \right]^{1/2}\), where xi and yi were concentrations of element “i” in samples x and y.
Results and discussion
24-hour phase
The measurements, data interpretation, and creation of the report for each analysis from Fig. 1 were running in parallel with continually streaming the data to the coordinator of the engaged laboratories.
The very first results came from the GS laboratory indicating that all samples contained depleted uranium (DU).
The RAMAN laboratory provided the first clue about the composition of the powdered samples while keeping the materials inside the glass bottles (Fig. 6). After the receipt of the XRD data the main composition of ES-1 and ES-3 could be determined with high confidence due to the match between RAMAN and XRD data in Table 2. ES-1 was identified as a mixture of uranium trioxide UO3 and uranium oxo hydroxide UO2(OH)2 with the possible presence of minor elements or compounds containing N or Na (not shown in Table 2 due to low score). It should be noted that the stoichiometry of UO2(OH)2 from the XRD/RAMAN databases is identical to that of uranium trioxide monohydrate UO3·H2O. ES-3 was identified as a mixture of trihydrate and hexahydrate of uranyl nitrate UO2(NO3)2·nH2O (n = 3, 6). In the case of ES-2 and ES-4, XRD identified UO2 and Fe but if the ductility of the samples and possible contamination from the manipulating metallic tools was considered, it was conjectured that both samples were uranium metals with oxidized surfaces and trace Fe surface contamination. XRF added Cu, Zn, and V to the list of possible surface contaminants. SEM–EDS did not add any new information besides confirming uranium, oxygen, and in the case of ES-2 nitrogen as well.
The SEM together with the OM allowed for morphological characterization of the material. However, these generally important characteristics did not contribute to answering the investigator’s questions.
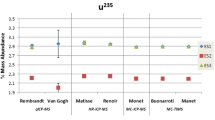
The most important information came from the SIMS laboratory in the third quarter of the 24-hour phase. In the automated particle screening mode, the SIMS identified 1540 particles containing 235U and 238U from the ES-1 sample, 330 particles from ES-2, 218 particles from ES-3, and 1012 particles from ES-4. The outputs of that step were coordinates of uranium particles on the planchets and preliminary estimates of the enrichments. It was found that all four samples contained particle population with only one enrichment, i.e., the samples were isotopically homogenous. Several uranium particles from each sample were chosen for isotopic measurement at high precision mode. The choice was based on the 235U signal intensities. The results in the graphical form are in Fig. 7. Each point corresponds to one analyzed particle. The difference between the uranium isotopic composition of ES-2 and the other samples has been clearly established.
To summarize the 24-hour phase, it was found (at various levels of confidence) that
-
All samples are made of depleted uranium and are isotopically homogeneous
-
ES-1, ES-3, and ES-4 have the same isotopic composition, but different from ES-2
-
ES-1 is a mixture of uranium trioxide UO3 and uranium oxo hydroxide UO2(OH)2
-
ES-2 is an oxidized uranium metal
-
ES-3 is a mixture of trihydrate and hexahydrate of uranyl nitrate UO2(NO3)2·3H2O and UO2(NO3)2·6H2O
-
ES-4 is an oxidized uranium metal
-
The same isotopic composition of ES-1, ES-3, and ES-4 gave rise to the conjecture that ES-3 could have been made from ES-4 and ES-1 from ES-3.
1-week phase
The most important contribution in the 1-week phase of the exercise came from the ICP-MS measurements. Besides confirming the isotopic composition of the samples at a high level of confidence, it provided uranium assay for all four samples in Table 3. Though almost an exact match between the measured uranium assay for ES-1 and the theoretical value for UO3 must have been incidental, it narrowed down the compound identification to UO3. The uranium assay for ES-3 is in between the theoretical values for the trihydrate and hexahydrate of uranyl nitrate, suggesting a refinement of ES-3 identification to a mixture of the trihydrate/ hexahydrate = 2/3.
For comparison, the samples were also measured using AS, but the uncertainties of isotopic concentrations were much larger than those for SIMS or ICP-MS, so the AS results were not considered in solving the exercise questions.
2-months phase
After the completion of 24-hour and 1-week phases of the nuclear forensic part of the exercise the only missing critical information was the trace element content in ES-1, ES-3, ES-4, which could provide further confirmation about the established linkage among the samples or disprove it.
The chosen method for the trace-element screening—PS-NAA provided concentrations of more than 10 elements at ppm levels for all four samples. Due to an unfavourable operation schedule of the LVR-15 reactor (usually 10–11 operation campaigns of 4 weeks with breaks for 2–3 weeks for the reactor maintenance and the active core adjustment for upcoming experiments) during the CMX-7 exercise and a long decay time needed for the determination of long-lived radionuclides (4–5 weeks), only the results for the short-lived radionuclides were available before the end of the exercise. However, since the measurement of long-lived radionuclides was completed only a couple of days after the end of the 2-months phase (and in the case of a more favourable LVR-15 operation schedule could be managed within 2-months phase), the results obtained from the measurement of both short- and long-lived radionuclides have also been included in this work for the sake of completeness. These results are presented in Table 4, which shows a completely different elemental composition of sample ES-2 compared to samples ES-1, ES-3 and ES-4, especially much higher V content. The results for the quality control sample–reference material Chanterelle agree with the CETAMA certified values within uncertainty margins, proving the accuracy of our PS-NAA results, which also means that none of elements determined was significantly retained in uranium separation by UTEVA resin. No significant element levels in the processing blank were found (indicating good contamination control), so that no correction was needed.
The cluster analysis of the PS-NAA data (cf. dendrogram in Fig. 8) revealed short linkage distance (a high similarity of elemental composition) between the ES-4 metal and the ES-3 powder, which supported the conjecture that the uranium nitrate powder ES-3 was created from the uranium metal ES-4. The other powdered sample ES-1 exhibits somewhat longer linkage distance to both ES-3 and ES-4, therefore the proposed reaction path between the uranium nitrate powder and the uranium trioxide powder remained neither confirmed nor excluded. The ES-2 metal forms a unique, completely separated cluster, indicating completely different elemental composition. Noteworthy, the PS-NAA results obtained on bulk samples, and results of the subsequent cluster analysis, are fully consistent with the results of uranium isotopic composition in individual particles of the samples achieved by SIMS already in the 24-hour phase of the CMX-7 exercise (cf. Fig. 1).
Conclusions
Coordinated application of a range of analytical methods allowed for answering all nuclear forensic investigator’s questions within 24 hours at varying level of confidence.
A notable point of this work was the application of the SIMS technique in the earliest phases of the exercise in a non-destructive manner using the sub-sampling via swiping the surfaces of the samples or their transport containers. Besides precise isotopic composition, the SIMS results showed that the particles released from each sample contained only a single enrichment. In case of the presence of several enrichments in the sample, early use of SIMS could give valuable clue for the next investigative steps.
Another innovative part was the application of PS-NAA to the trace-element screening of the uranium samples, which supported the SIMS results. Though the potential of PS-NAA for survey analysis has not been fully explored in this work, it can be already concluded that PS-NAA could be a useful tool for trace level elemental screening of samples with high content of uranium and its application potential in nuclear forensics deserves further exploration.
Data availability
The data that support the findings of this study are available within the article or from the corresponding author, Jan Lorincik, upon reasonable request.
References
Mayer K, Wallenius M, Ray I (2005) Nuclear forensics-a methodology providing clues on the origin of illicitly trafficked nuclear materials. Analyst 130:433–441
Project Hebenon - Inject 1, Collaborative Materials Exercise 7, ITWG, December 2020
Taylor F, Higginson M, Marsden O, Schwantes J (2020) Participant information package collaborative materials exercise. J Radioanal Nuclear Chem 323:415–430
Peres P, Hedberg PML, Walton S, Montgomery N, Cliff JB, Rabemananjaraa F, Schuhmacher M (2012) Surf Interface Anal 45:561–565
Donohue DL (2002) Peer reviewed: strengthened nuclear safeguards. Anal Chem 74(1):28A-35A
Esaka F, Watanabe K, Fukuyama H, Onodera T, Esaka KT, Magara M, Sakurai S, Usuda S (2004) J Nucl Sci Technol 41:1027–1032
Rosenberg RJ (1993) J Radioanal Nucl Chem 169:113–124
Koleška M, Lahodová Z, Šoltés J, Viererbl L, Ernest J, Vinš M, Stehno J (2015) J Radioanal Nucl Chem 305:51–59
(2018) Reference materials catalogue, chanterelle, CEA, CETAMA, 30207 bagnois-sur-cèze cedex, France
Acknowledgements
The larger part of the presented results was obtained using the CICRR infrastructure, which is financially supported by the Ministry of Education, Youth and Sports—project LM2023041.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lorincik, J., Sihelska, K., Vesela, D. et al. Participation of Czech laboratories in isotopic, structural, and elemental characterization of uranium nuclear forensic samples within the 7th collaborative material exercise. J Radioanal Nucl Chem 333, 3675–3684 (2024). https://doi.org/10.1007/s10967-023-09336-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09336-y