Abstract
A rapid method was developed to analyze 237Np and Pu isotopes in steel and concrete samples using HCl–HNO3–NH4HF2 digestion, CaF2/LaF3 co-precipitation, AG MP-1 M resin column separation and SF-ICP-MS measurement. Compared to acid digestion using HCl–HNO3–HF, the whole procedure not only avoids the use of toxic HF, but can be completed within 1 day. The decontamination factor of U was estimated to be 1.1 × 106. The limits of detection of 237Np, 239Pu, and 240Pu were 0.25, 0.16, and 0.14 fg g−1, respectively, allowing method users to sufficiently detect low level Pu and Np in steel and concrete samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nuclear energy as a clean and economical electrical energy is favored by many countries. At present, there are more than 400 nuclear power reactors in operation, which provide about 11% of the electrical power generated worldwide [1, 2]. However, emergencies and accidents that come with the operation of nuclear power plants need to be faced, such as the Fukushima Daiichi Nuclear Power Plant accident that occurred in March 2011. In addition, the number of first-generation nuclear power plants that need to be decommissioned has also been increasing gradually, since the typical design life of a nuclear power reactor was 30 − 50 years [2, 3]. During the decommission and decontamination, the resulting various types of radioactive wastes need to be safely managed and disposed of; these include steel and concrete materials used in buildings, shielding and the pressure vessel of the nuclear reactor, etc. [4, 5]. In order to establish appropriate disposal strategies, the inventory and distribution of the target radionuclides (including fission products and actinides) should be measured. Actinide radionuclides, especially Np and Pu are the key radionuclides of concern for the characterization of nuclear decommissioning samples due to their long half-lives (e.g., 237Np (t1/2 = 2.14 × 106 y), 239Pu (t1/2 = 2.41 × 104 y), and 240Pu (t1/2 = 6.56 × 103 y)), radioactivity, and toxicity. Weinreich et al. [5] have reported that the concentration of U is at the μg g−1 level in bio-shielding concrete materials; and 237Np and 239Pu will be generated via the following reactions [5, 6]:
Therefore, rapid and simple methods to analyze Np and Pu in steel and concrete samples are highly desired for preparing responses to a nuclear accident during reactor operation and decommission based on reliable risk assessment.
Steel and concrete can be divided into materials that have induced internal radioactivity and those that have only surface radioactivity depending on the source of the radionuclides [4]. If the buildings of concern are far away from the nuclear reactor, the radioactive substances will be only deposited loosely onto the bare surface of the building materials after a nuclear accident. By contrast, for the steel and concrete materials in the pressure vessel and shielding of the nuclear reactor, they are close to the reactor core and have had long-term exposure to the neutron flux of the reactor fuels, resulting in the radionuclides being present inside and on the surfaces of the steel and concrete. Therefore, total digestion of analysis samples is critical to release radionuclides completely from the steel and concrete materials. Aqua regia and dilute H2SO4 (3–6 M) have been reported to be effective in complete digestion of steel samples [4, 7, 8]. However, the presence of sulfate ions will lead to CaSO4 precipitate formation during the process of CaF2/LaF3 co-precipitation, which cannot be dissolved rapidly in the following HNO3 solution prior to resin column chromatography. Digestion of concrete only using aqua regia cannot release Pu completely due to potential hot particles or refractory matrixes [9, 10]. Although many methods for the analysis of Np and Pu isotopes in soil or sediment have been reported [6, 11,12,13,14,15,16], they are not feasibly applicable to analyze Np and Pu in concrete, due to not only the potential hot particles or refractory matrixes in the concrete samples, but also the time-consuming procedures of these methods, which cannot meet the requirement for the rapid response in an emergency [17, 18]. To date, a few studies have reported methods to digest concrete samples for the analysis of Pu [5, 10, 17, 18], such as HNO3–H3BO3–HF digestion [5], HNO3–HClO4–HF digestion [17], and HCl–HNO3–HF digestion or NaOH fusion [10, 18]. However, it should be noted that the previous methods for the total digestion of concrete samples are tedious, time-consuming and hazardous as they have: 1) repeated acid digestions, 2) fusion with NaOH (high temperature (600 °C)), and 3) use of toxic HF. Therefore, a proper decomposition approach avoiding these disadvantages is highly required for the measurement of Np and Pu isotopes in steel and concrete samples. Ammonium hydrogen fluoride (NH4HF2) has been used to digest soil or rock samples at low temperature [19–21], since it partially decomposes into HF at temperatures between ~ 120 °C and 220 °C [19], and it also can directly decompose the sample by breaking the Si–O bond and Pu–O bond [20]. In addition, Yang et al. [13] found that NH4HF2 could release F− in H+ solutions. Therefore, NH4HF2 is a potential replacement reagent for toxic HF to get complete digestion of concrete samples and then to form CaF2/LaF3 co-precipitate to remove matrix and interfering elements, especially U, for the final 237Np and Pu isotopes analysis by sector field-inductively coupled plasma-mass spectrometry (SF-ICP-MS).
SF-ICP-MS was employed for the analysis of 237Np and Pu isotopes in this study, based on its characteristics of high sensitivity and short measurement time [22]. It should be noted that high removal of U is required for accurate measurement 237Np and Pu isotopes in steel and concrete samples by SF-ICP-MS, due to the tailing effect of the 238U signal overlapping the 237Np signal and the dominant polyatomic interferences of the 238UH+ and 238UH2+ on 239Pu and 240Pu [12, 23, 24]. It was reported that about 70% of Fe and 60% of U was removed by CaF2/LaF3 co-precipitation [23], and a high decontamination factor (1.6 × 107) of U was achieved using CaF2/LaF3 co-precipitation combined with AG MP-1 M resin column chromatography in analysis of 237Np and Pu isotopes in soil and sediment samples [12]. Therefore, CaF2/LaF3 co-precipitation combining with the AG MP-1 M resin column chromatography was selected here to separate and purify Np and Pu isotopes in steel and concrete samples. Furthermore, there is no proper yield tracer for 237Np analysis by ICP-MS, since most Np isotopes have short half-lives [6]: for example, 239Np (t1/2 = 2.4 d) and 235Np (t1/2 = 396 d). Although 236Np (t1/2 = 1.54 × 105 y) is an ideal tracer, it is not commercially available currently. 242Pu with a long half-life (3.76 × 105) has often been used as a yield tracer for the determination of Pu isotopes by ICP-MS. Tetravalent Np and Pu have been reported to have similar chemical behavior [17] and the first ionization potentials of Np and Pu are similar (6.26 eV and 6.03 eV, respectively) [25]. Meanwhile, 242Pu as a yield tracer for the analysis of 237Np has been proposed in previous studies [11,12,13,14,15,16]. Therefore, the non-isotopic 242Pu was also chosen to see if it could act as a yield tracer for the simultaneous measurement of 237Np and Pu isotopes by SF-ICP-MS after HCl–HNO3–NH4HF2 total digestion, CaF2/LaF3 co-precipitation, and AG MP-1 M resin column separation.
In this work, a novel HCl−HNO3−NH4HF2 total digestion method for steel and concrete samples was developed to analyze 237Np and Pu isotopes simultaneously by SF-ICP-MS. Since no steel and concrete standard reference material is commercially available, one marine sediment was used (collected from the Sea of Japan in Ishikawa Prefecture), with known amounts of 239Pu, 240Pu, and 237Np measured by the method of Huang et al. [12] and treated at high temperature (1000 °C) as standard reference material to assess the accuracy of the developed method.
Experimental
Reagents and materials
Analytical grade reagents including HNO3 (60–61%), HCl (35–37%), HF (46–48%), HBr (47–49%), NH4HF2, H3BO3, Ca(NO3)2, La(NO3)3, TiCl3 (20%), NaNO2, and ascorbic acid were purchased from Kanto Chemicals (Tokyo, Japan). Ultrapure HNO3 (68%) was purchased from TAMA Chemicals (Tokyo, Japan). Milli-Q water (18.2 MΩ⋅ cm) was used to prepare required solutions. The AG MP-1 M resin (100–200 mesh, chloride form) was purchased from Bio-Rad Laboratories (Hercules, CA, USA). For SF-ICP-MS measurement, 242Pu (CRM 130, plutonium spike assay and isotopic standard, New Brunswick Laboratory, USA) and 233U (AEA Technology, 92/233/23, Institute for Reference Materials and Measurements, Geel, Belgium) were used as yield tracer and internal standard, respectively.
Digestion and co-precipitation
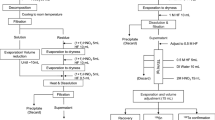
The chemical procedures are presented in Fig. 1. Two different kinds of samples, a sample of about 1 g stainless steel (304L) or a mixed sample of about 1 g stainless steel (304L) and 0.5 g concrete (hereafter called the 1.5 g steel + concrete mixed sample), were investigated in this study. The steel sample or steel + concrete mixed sample was transferred to a 250 mL Teflon beaker and spiked with 0.57 pg 242Pu. For total digestion, 20 mL conc. HCl, 5 mL conc. HNO3 and 5 g NH4HF2 were used. After covering the Teflon beaker with a Teflon lid, the sample and acids were heated at 200 °C for 30 min on a hot plate. Subsequently, the sample solution was transferred to a 250 mL centrifuge tube. The Teflon beaker was rinsed three times using 5 mL Milli-Q water and this rinse water was added to the tube. The sample solution and rinse water were then diluted to 100 mL using Milli-Q water. After addition of 3 mL 20% TiCl3 to adjust Pu to Pu (III) and Np to Np (IV), 100 mg Ca2+, 100 mg La3+, and 3 g NH4HF2 were added into the centrifuge tube to co-precipitate Pu and Np by CaF2/LaF3 co-precipitation after shaking. The supernatant was discarded after centrifugation at 3000 rpm for 15 min. Finally, ca. 0.5 g H3BO3 and 20 mL of 7.2 M HNO3 were added to dissolve the fluoride precipitate, and the sample solution was transferred to a 50 mL centrifuge tube after filtration.
AG MP-1 M resin column chromatographic separation
The valences of Pu and Np were again adjusted to Pu(III) and Np(IV) by adding 0.3 g ascorbic acid along with a small amount of residual Fe, waiting for about 3 min to ensure complete valence adjustment. Subsequently, 0.3 g NaNO2 was added to the sample solution followed by heating at 40 °C for 30 min to oxidize Pu(III) to Pu(IV). After that, a 2 mL AG MP-1 M resin column was preconditioned with 20 mL of 7.2 M HNO3—0.4 M NaNO2, and the sample was loaded onto this column followed by washing the centrifuge tube twice with 5 mL of 7.2 M HNO3 and loading the washes onto the column. To remove the interfering elements, 20 mL conc. HCl—0.3 mL conc. HNO3 and 60 mL of 7.2 M HNO3 were used to rinse the resin. Afterwards, 10 mL of 10 M HCl was used to wash out the residual HNO3 and remove the Th and U completely. Finally, 20 mL of conc. HBr was used to elute Pu and Np from the resin and the eluate was collected in a 50 mL Teflon beaker.
SF-ICP-MS measurement
The eluate was evaporated to dryness at 250 °C on a hot plate to remove HBr, then 1 mL TAMA pure HNO3 was added to dissolve the residue and the solution was heated to near dryness. After that 0.7 mL of 4% HNO3 with 0.0022 ng mL−1 233U as internal standard was used to dissolve the sample. Finally, the Np and Pu isotopes were determined with a SF-ICP-MS instrument (Element XR, Thermo Fisher Scientific Inc., Germany). To improve the sensitivity of SF-ICP-MS, a high efficiency sample introduction system of APEX-Q/ACM (Elemental Scientific Inc, USA) was used, and operation was in a low-resolution mode (m/∆m = 300) [12]. The detailed parameter settings of the SF-ICP-MS instrument and the sample introduction system have been described elsewhere [12, 13, 22].
Results and discussion
Digestion of steel and concrete to release Np and Pu
Stainless steel 304L with high temperature resistance and low corrosion rate has been commonly used in light water reactor nuclear power plants and site buildings [26,27,28]. The steel and concrete samples contaminated by a nuclear accident and from nuclear facilities undergoing decontamination or decommission have the following characteristics: 1) lots of metal ions will be released after complete digestion of steel samples and they will affect the separation efficiency of resin column chromatography; 2) steel samples from building materials will inevitably have small amounts of concrete attached; and 3) the activity concentrations of Pu and Np in the samples after decontamination or decommission are much higher than their environmental level. Therefore, two different kinds of samples, 1 g of steel and 0.5 g of concrete mixed with 1 g of steel (1.5 g steel + concrete mixed sample), were prepared to explore the digestion effects of different kinds of steel and concrete samples.
The HCl − HNO3 − NH4HF2 digestion system was used to completely digest steel and concrete samples, and the HCl−HNO3−HF digestion system was also introduced for comparison. For HCl−HNO3−HF acid digestion, 20 mL of conc. HCl, 5 mL of conc. HNO3, and 5 mL conc. HF were chosen according to the report by Maxwell et al. [10]. For HCl−HNO3−NH4HF2 acid digestion, 20 mL of conc. HCl, 5 mL of conc. HNO3, and 5 g of NH4HF2 were chosen, where about 5 g of NH4HF2 was chosen according to the molar concentration of F− in HF (28 M, 5 mL) and the mass ratio (4:1) of NH4HF2 and sample [19, 21]. The results of 242Pu yield after using the two different acid digestion conditions are shown in Fig. 2. For the digestion of steel samples, the use of NH4HF2 resulted in similar chemical recoveries of Pu (84 ± 6%, n = 3) to that of using HF (86 ± 2%, n = 3). For the digestion of steel + concrete mixed samples, the use of NH4HF2 in place of HF presented better chemical recoveries of Pu (71 ± 4%, n = 3) than when using HF (59 ± 1%, n = 3). These findings indicate that NH4HF2 can not only replace HF, but it also provides better digestion than HF for steel + concrete mixed samples. Therefore, 5 g of NH4HF2 combined with 20 mL of conc. HCl and 5 mL of conc. HNO3 at 200 °C were applied to digest steel + concrete mixed samples.
In digestion, the steel began to be dissolved when the acids were added to the Teflon beaker, and dissolution speed was significantly improved via heating. A 1 g steel sample was dissolved completely by the time the temperature reached 200 °C and this process took only 10 min. For 0.5 g of concrete, with the continuous heating at 200 °C, the NH4HF2 fusion and/or HF that was produced by NH4HF2 decomposition would break the Pu–O and Si–O bonds during this process to achieve the total digestion [19, 20], within 30 min. Finally, the sample solution was transferred to a 250 mL centrifuge tube before it had cooled to room temperature completely. Unless that was done a small amount of black substance would sometimes appear on the beaker inner surface. The occurrence of a black substance during steel digestion has also been observed in previous studies [4, 29], and presented insignificant influence on the Pu recovery, but its composition needs further study.
Elimination of matrix effect and interference
Stainless steel (type 304L) chosen in this work, consists of 17.5–20% chromium, 8–11% nickel, < 0.08% carbon, < 2% manganese, < 1% silicon, < 0.045% phosphorus, < 0.03% sulfur, balance iron [10, 30]; and these elements, especially the iron, will be released after complete digestion and will affect the separation efficiency of resin column chromatography. In addition, elements including U, Pb, Bi, Tl, Hg and Pt from the matrix can cause polyatomic interferences at the same m/z for the SF-ICP-MS measurement of Np and Pu [12, 13, 17]. Therefore, the CaF2/LaF3 co-precipitation and AG MP-1 M resin column separation were introduced to eliminate matrix and interfering elements as much as possible. HF and Ca2+/ La3+ have often been used to get CaF2/LaF3 coprecipitation [10, 12, 23], in which about 70% of the Fe and 60% of the U can be removed [23]. However, it has been found in previous studies that NH4HF2 could release F− in H+ solution [12, 19]. Therefore, NH4HF2 can also replace toxic HF to get CaF2/LaF3 co-precipitation. In this work, although F− was already present in the digestion solution, a small amount of F− will still be lost as SiF4 gas because the Teflon beaker was only covered by a Teflon lid [19]. In addition, it also was found here that the concentration of F− should be at least 2 M, otherwise CaF2/LaF3 co-precipitate could not form sufficiently. Therefore, an additional 3 g of NH4HF2 was added to ensure the concentration of F− was above 2 M in a 100 mL solution. Before the co-precipitation, Pu and Np were reduced to Pu(III) and Np(IV) using 3 mL of 20% TiCl3 to ensure complete co-precipitation of Pu and Np [6]. In this step, some fraction of Fe was also reduced to Fe2+, and the redox-couple Fe3+/Fe2+ as a redox buffer in solution will protect Np(IV) [11, 31]. Furthermore, the 2 mL AG MP-1 M resin column was chosen to separate and purify Np and Pu from the sample solution [12−14]. The Fe and other matrix elements were further removed during the loading and separation processes [32, 33], where these matrix elements have a tiny influence on the separation efficiency of Pu in the resin column, as shown in Fig. 2; after HCl−HNO3−NH4HF2 digestion, high chemical recovery of 242Pu (84 ± 6%, n = 3) in the steel samples was obtained, and the chemical recovery of 242Pu (71 ± 4%, n = 3) in steel + concrete mixed samples was only decreased by ca.13%, which was less than the loss using the HCl−HNO3−HF digestion (ca. 27%). For 238U, which is ubiquitous, the concentrations were < 10 pg mL−1 in the final sample solution (0.7 mL) after CaF2/LaF3 co-precipitation and AG MP-1 M resin column separation, and the decontamination factor (DF) of 238U was estimated to be about 1.1 × 106 for 0.5 g of the in-house standard reference material (from the Sea of Japan in Ishikawa Prefecture). The details on eliminating matrix and interfering elements have been reported elsewhere [12, 13]. In this work, the low-resolution mode (m/∆m = 300) and the APEX-Q/ACM high efficiency sample introduction system were used to maximize the instrument sensitivity (about 3 × 107 cps ppb−1). For the SF-ICP-MS measurement, the formation rate of 238UH+/U+ and the ratio of 237Np/U+ due to the U tailing effect were all in the level of 10–5; therefore, there were only several cps contributions for 239Pu and 237Np from U, which can be easily corrected.
Simultaneous determination of 237Np and Pu isotopes in steel and concrete
To verify whether 242Pu is suitable as the yield tracer for the simultaneous determination of Np and Pu isotopes in steel and concrete samples by SF-ICP-MS after HCl−HNO3−NH4HF2 total digestion, CaF2/LaF3 co-precipitation, and AG MP-1 M resin column separation, the same samples (1 g steel sample or 1.5 g steel + concrete mixed sample) with spiking by an in-house 237Np + 242Pu mixed standard solution (0.405 pg 237Np and 0.285 pg 242Pu) were studied following the chemical procedure presented in Fig. 1. The results are listed in Table 1. The average yield ratios of Np/Pu were 0.922 ± 0.025 and 0.948 ± 0.006 using NH4HF2 for steel samples and steel + concrete samples, respectively, and similar yield ratios of 237Np/242Pu were obtained using HF (0.929 ± 0.017 for steel samples; 0.928 ± 0.002 for steel + concrete samples), suggesting that approximately 5−8% more Np get lost in the chemical separation procedure compared with 242Pu. Compared to use of the AG MP-1 M resin column for the purification of Np and Pu isotopes in soil, sediment, and urine samples [12 − 14], the loss of Np in the present study was mainly attributed to the metal ions (e.g., Fe) that affect the separation efficiency of Np during AG MP-1 M resin column chromatography, since large amounts of metal ions were produced after steel digestion; for example, ca. 600 mg of Fe was released from 1 g of steel. Although most of the metal ions are removed by the CaF2/LaF3 co-precipitation and they go through the resin column during the sample loading process [23, 33], the high amount of Fe can compete with trace Np and Pu isotopes in the CaF2/LaF3 co-precipitation and column chromatography processes, resulting in tiny fractionation between Np and Pu. Therefore, the results of Np should be corrected as described by Eq. (1):
where (Np)measured is the measured counting rate (cps). (Np/Pu) yield ratio is the average yield value of 237Np/242Pu that is obtained under the corresponding conditions of sample and digestion.
Evaluation of the developed method
To assess the accuracy of the developed method, 1 g steel and 0.5 g in-house sediment standard reference material (from the Sea of Japan in Ishikawa Prefecture) spiked with 0.57 pg 242Pu were placed into a 250 mL Teflon beaker. This sediment reference sample was pre-ignited at high temperature (1000 °C) to prepare Pu containing refractory material. The overall sample preparation procedure was as illustrated in Fig. 1, and the obtained results were as listed in Table 2. For the Pu isotopes, the average activities of 239Pu, 240Pu, and 240+239Pu were 0.561 ± 0.017, 0.446 ± 0.001, and 1.008 ± 0.018 mBq g−1, which agreed well with the respective reference values of 0.569 ± 0.061, 0.437 ± 0.029, and 1.006 ± 0.068 mBq g−1. The average atom ratio of 240Pu/239Pu was measured as 0.217 ± 0.007, which also agreed well with the reference value of 0.209 ± 0.009. The average activity of 237Np was measured to be 0.268 ± 0.022 μBq g−1, which also agreed well with the reference value of 0.268 ± 0.031 μBq g−1, and the average atom ratio of 237Np/239Pu was measured as 0.043 ± 0.005, also in good agreement with the reference value of 0.044 ± 0.003. Therefore, it was concluded that the developed method can be used to accurately determine 237Np and Pu isotopes in environmental samples and nuclear power plant decommissioning samples containing high refractory materials.
For the SF-ICP-MS measurement, the corresponding parameters were set according to Huang et al. [12], and the instrumental sensitivity was about 3 × 107 cps ppb−1. Based on the counting rates of 237Np and Pu isotopes and chemical recovery of 242Pu (85%), the limits of detection (LODs) of 237Np, 239Pu, and 240Pu were calculated for 1.5 g steel + concrete mixed samples to be 0.25 fg g−1 (6.5 × 10–6 mBq g−1), 0.16 fg g−1 (3.7 × 10–4 mBq g−1), 0.14 fg g−1 (1.2 × 10–3 mBq g−1), respectively [34]. The LODs of Pu isotopes were lower than that reported by Wang et al.[17] due to the high sensitivity of the present measurement system. The LOD of 237Np was also corrected for chemical fractionation, and it was the same level as the 0.23 fg g−1 LOD obtained by Huang et al. [12]. These LODs were sufficiently low enough to analyze Np and Pu isotopes for steel and concrete samples during decommission and decontamination of a nuclear reactor.
The whole analytical process can be achieved within 1 day for 24 samples with the support of a 24—hole vacuum box system as shown in Fig. 1, including 0.75 h for digestion and transfer of the samples, 1.5 h for CaF2/LaF3 co-precipitation and filtration, 4 h for chromatography and sample separation, and 3.5 h for SF-ICP-MS sample preparation and measurement. Therefore, it was concluded that this method can analyze great numbers of steel and concrete samples rapidly after a nuclear accident or during the decommission and decontamination of a nuclear reactor.
Conclusions
In this study, a novel, simple, and rapid method was developed for the simultaneous analysis of 237Np and Pu isotopes in steel and concrete samples. NH4HF2 in combination with conc. HCl and conc. HNO3 was chosen for the total digestion of the steel and concrete samples. Then, CaF2/LaF3 co-precipitation was used to remove matrix elements from the samples, and NH4HF2 was also used to get CaF2/LaF3 co-precipitation. Afterwards, an anion exchange resin (AG MP-1 M) column was employed to separate and purify Np and Pu. Finally, the 237Np and Pu isotopes were determined by the SF-ICP-MS measurement system equipped with an APEX-Q/ACM sample introduction system. The method avoids the use of toxic HF, and can be completed within 1 day. The decontamination factor of U was estimated to be 1.1 × 106. However, it should be noted that the average yield ratios of 237Np/ 242Pu were 0.922 ± 0.025 and 0.948 ± 0.006 for the analysis of steel samples and steel + concrete mixed samples, respectively, indicating that about 5 − 8% more 237Np was lost compared with 242Pu tracer because of the high iron amount, therefore, the results of Np need to be corrected according to the average yield ratio of 237Np/ 242Pu. The accuracy of this developed method for the Pu and Np determination was validated by analyzing a mixed sample of steel + sediment with known amounts of 237Np and Pu isotopes that had been treated at high temperature (1000℃), and the determined 239Pu, 240Pu and 237Np concentrations agreed well with the reference values. In addition, the low LODs obtained for 237Np, 239Pu, and 240Pu of 0.25, 0.16, and 0.14 fg g−1, respectively, will allow for effective detection of low amounts of Pu and Np in steel and concrete samples.
References
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488(7411):294–303
Alam F, Sarkar R, Chowdhury H (2019) Nuclear power plants in emerging economies and human resource development: a review. Energy Procedia 160:3–10
Samseth J, Banford A W, Batandjieva-Metcalf B, Cantone MC, Lietava P, Peimani H, Szilagyi A (2012) Closing and decommissioning nuclear power reactors: another look following the Fukushima accident. UNEP Year Book. 35–49.
Zhu LC, Hou XL, Qiao JX (2021) Determination of 135Cs concentration and 135Cs/137Cs ratio in waste samples from nuclear decommissioning by chemical separation and ICP-MS/MS. Talanta 221:121637
Weinreich R, Bajo S, Eikenberg J, Atchison F (2004) Determination of uranium and plutonium in shielding concrete. J Radioanal Nucl Chem 261(2):319–325
Thakur P, Mulholland GP (2012) Determination of 237Np in environmental and nuclear samples: a review of the analytical method. Appl Radiat Isot 70(8):1747–1778
De Andrade JC, Cuelbas CJ, Paula Eiras DE, S, (1998) Spectrophotometric determination of Mo(VI) in steel using a homogeneous ternary solvent system after single-phase extraction. Talanta 47(3):719–727
Tamba MGDM, Falciani R, López TD, Ceodo AG (1994) One-step microwave digestion procedure for the determination of aluminum in steels and iron ores by inductively coupled plasma atomic emission spectrometry. Analyst 119(9):2081–2085
Wang ZT, Yang GS, Zheng J, Cao LG, Yu HJ, Zhu YB, Tagami K, Uchida S (2015) Effect of ashing temperature on accurate determination of plutonium in soil samples. Anal Chem 87(11):5511–5515
Maxwell SL, Culligan B, Hutchison JB, Sudowe R, McAlister DR (2017) Rapid method to determine plutonium isotopes in steel samples. J Radioanal Nucl Chem 314(2):1103–1111
Qiao JX, Hou XL, Roos P, Miró M (2010) Rapid and simultaneous determination of neptunium and plutonium isotopes in environment samples by extraction chromatography using sequential injection analysis and ICP-MS. J Anal At Spectrom 25(11):1769–1779
Huang ZY, Ni YY, Wang H, Zheng J, Yamazaki S, Sakaguchi A, Long XG, Uchida S (2019) Simultaneous determination of ultra-trace level 237Np and Pu isotopes in soil and sediment samples by SF-ICP-MS with a single column chromatographic separation. Microchem J 148:597–604
Yang GS, Zheng J, Kim E, Zhang S, Seno H, Kowatari M, Aono T, Kowatari O (2021) Rapid analysis of 237Np and Pu isotopes in urine by single chromatographic column coupled to SF-ICP-MS or ICP-MS/MS. Anal Chim Acta 1158:338431
Matteson BS, Hanson SK, Miller JL, Oldham WJ Jr (2015) Concurrent determination of 237Np and Pu isotopes using ICP-MS: analysis of NIST environmental matrix standard reference materials 4357, 1646a, and 2702. J Environ Radioact 142:62–67
Chen Q, Dahlgaard H, Nielsen SP, Aarkrog A (2002) 242Pu as tracer for simultaneous determination of 237Np and 239, 240Pu in environment samples. J Radioanal Nucl Chem 253(3):451–458
Qiao JX, Hou XL, Roos P, Miró M (2011) High-throughput sequential injection method for simultaneous determination of plutonium and neptunium in environmental solids using macroporous anion-exchange chromatography, followed by inductively coupled plasma mass spectrometric detection. Anal Chem 83(1):374–381
Wang ZT, Wen W, Quan W, Du L, Wang P, Lin JX, Xie Y, Tan ZY (2018) Rapid determination of Pu isotopes for decommission concrete samples by inductively coupled plasma mass spectrometry. J Radioanal Nucl Chem 316(1):411–417
Maxwell SL, Culligan BK, Kelsey-Wall A, Shaw PJ (2011) Rapid determination method for determination of actinides in emergency concrete and brick samples. Anal Chim Acta 701(1):112–118
O’Hara MJ, Kellogg CM, Parker M, Morrison SS, Corbey JF, Grate JW (2017) Decomposition of diverse solid inorganic matrices with molten ammonium bifluoride salt for constituent elemental analysis. Chem Geol 466:341–351
Wang H, Ni Y, Zheng J, Huang Z, Xiao D (2019) Low-temperature fusion using HN4HSO4 and NH4HF2 for rapid determination of Pu in soil and sediment samples. Anal Chim Acta 1050:71–79
Zhang W, Hu ZC, Liu YS, Chen HH, Gao S, Gaschnig RM (2012) Total rock dissolution using ammonium bifluoride (NH4HF2) in screw-top Teflon vials: a new development in open-vessel digestion. Anal Chem 84(24):10686–10693
Zheng J (2015) Evaluation of a new sector-field ICP-MS with Jet Interface for ultra-trace determination of Pu isotopes: from femtogram to attogram levels. J Nucl Radiochem Sci 15(1):7–13
Wang ZT, Zheng J, Ni YY, Men W, Tagami K, Uchida S (2017) High-performance method for determination of Pu isotopes in soil and sediment samples by sector field-inductively coupled plasma mass spectrometry. Anal Chem 89(4):2221–2226
Kim CS, Kim CK, Martin P, Sansone U (2007) Determination of Pu isotope concentrations and isotope ratio by inductively coupled plasma mass spectrometry: a review of analytical methodology. J Anal At Spectrom 22(7):827–841
Haynes WM (2015) CRC Handbook of Chemistry and Physics, 96th edn. CRC Press, Boca Raton, FL
Tian ZH, Song LJ, Fang KW, Lin GX, Zhou HL (2019) Corrosion behavior of 304L stainless steel in a B-Li coolant for a nuclear power plant. Mater Tehnol 53(5):643–647
Baddoo NR (2008) Stainless steel in construction: A review of research, applications, challenges and opportunities. J Constr Steel Res 64(11):1199–1206
Sun C, Tan Y, He K, Zhang SH, Liang KX, Lu Q (2020) Galvanic corrosion behaviors of A508-III/304L couples in boric acid solution. Int J Electrochem Sci 15:3298–3314
Maxwell SL, Culligan B, Hutchison JB, Utsey RC, Sudowe R, McAlister DR (2017) Rapid method to determine 89/90Sr in steel samples. J Radioanal Nucl Chem 314(1):439–450
Kerry T, Banford AW, Bower W, Thompson OR, Carey T, Mosselmans JFW, Ignatyev K, Sharred CA (2018) Uranium contamination of stainless steel in nuclear processing plants. Ind Eng Chem Res 57(11):3957–3962
Vajda N, Kim CK (2011) Determination of transuranium isotopes (Pu, Np, Am) by radiometric techniques: A review of analytical methodology. Anal Chem 83(12):4688–4719
Epov VN, Benkhedda K, Evans D (2005) Determination of Pu isotopes in vegetation using a new on-line FI-ICP-DRC-MS protocol after microwave digestion. J Anal At Spectrom 20(9):990–992
Huff EA, Bowers DL (1989) The determination of impurities in plutonium metal by anion exchange and ICP/AES. Appl Spectrosc 43(2):223–226
Wang ZT, Lin JX, Li SX, Guo QJ, Huang WN, Dan GP, Tan ZY (2018) Rapid method for accurate determination of actinides (U, Th, Pu and Am) in water samples for emergency response. J Radioanal Nucl Chem 315(1):103–110
Acknowledgements
This study was supported by the JSPS KAKENHI (JP 17K00537 and JP 21H03609), the Grant of Fukushima Prefecture related to research and development in radiological sciences, and ERAN I-21-24. Shuai Zhang thanks the China Scholarship Council for offering a scholarship (201906190116) to support his Ph. D. study
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Shuai Zhang], [Guosheng Yang], [Jian Zheng]. The draft of the manuscript was written by [Shuai Zhang] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, S., Yang, G., Zheng, J. et al. A simple acid digestion using HCl–HNO3−NH4HF2 for rapid SF-ICP-MS determination of 237Np and Pu isotopes in steel and concrete samples. J Radioanal Nucl Chem 329, 1083–1090 (2021). https://doi.org/10.1007/s10967-021-07825-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07825-6