Abstract
This paper describes the pollution level in sediment core collected from Sunairi Point of Karachi coastal area Pakistan. The sediment was characterized in terms of particle size, pH, total organic carbon, calcium carbonate, metals and mineralogical composition of sediments. The metals were determined using proton induced X-ray emission. It appeared from the results of grain size that sediment of core was mainly sandy in nature and neutral pH was observed throughout the core. The calcium carbonate in the core sediment seemed to be dependent on the pH outline of the sediment. The results of heavy metals in the sediment core indicated a significant upward enrichment in heavy metals with the highest concentrations found in the upper most layer sediments, may be due to anthropogenic activities, which could be the cause of upward enrichment in heavy metals. The pollution load index values, although showed no pollution, but showing an increasing trend from bottom to top in core, may be due to the influence of anthropogenic sources. The average geo-accumulation index values for sediment core showed that sediments were moderately polluted with Co and Pb. The results of sediment quality guidelines of United States Environmental Protection Agency, indicated, sediment of core was moderately polluted with Ni and Zn. It was concluded from the guidelines that heavy metals in sediment may cause danger to aquatic life. It was also inferred from the study that Sunairi Point area of Karachi coast is facing moderately metals pollution which increased with time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal pollution of the marine environment has received significant attention because of its transfer to man through the food chains in quantities that can be harmful [1, 2]. The trace metal elements are contributed to marine sediments by a variety of sources, including chemical and physical weathering of rocks, volcanic activity, industrial and agricultural runoff [3]. As these metals are not permanently fixed with sediments so can be released back to the water column by changes in environmental conditions, rendering sea water contamination which may severely stress marine ecosystem, and ultimately may limit access to the coastal resources [4,5,6,7]. The heavy metals though occur at extremely low concentrations in seawater but may be accumulated in marine organisms and contents of the metals in their body tissue can be many times higher than the levels in seawater [8]. Hence, sediments are regarded as preferable monitoring tools since metals concentration is higher than seawater and show less variation in time and space, allowing more consistent assessment of spatial and temporal contamination [9, 10]. Much attention has been currently paid to the chemical composition of marine sediment because of the biological significance of heavy metals and their transfer to human being through food chain [1, 4, 11]. Metals are introduced to the marine environment by domestic and industrial activities as anthropogenic pollutants. Much of this input ultimately accumulates in the estuarine zone and continental shelf, which are important sinks for suspended matter and associated land-derived contaminants. Among the components of the marine environment, sediments play a critical role in the distribution and circulation of toxic compounds.
Like many other coastal zones of the world, heavy metal contamination in Karachi coastal sediments due to the indiscriminate discharge of untreated effluents of big industrial estates and raw domestic sewage of Karachi Metropolitan through Layari and Malir Rivers poses a serious threat to marine environment [12,13,14,15,16,17]. The main objective of present study to assess heavy metal contamination in sediment core collected from Sunairi Point Karachi coastal area Pakistan through different sediment quality assessment tools and to explore the pollution nature of using multivariate and cluster exploration.
Materials and methods
Study area
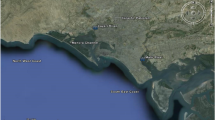
The study area is comprised of 135 km long Karachi coast, named after Matropolitan Karachi which is a capital of Sindh province. The Karachi city is bounded by latitude 24° 48′ N and longitude 66° 59′ E and is positioned on the Arabian Sea a part of the Indian Ocean [13]. The Karachi, being the largest industrial and closely populated city of Pakistan, generates over 300 million gallons per day of sewage and industrial effluents of more than six thousands industrial units, and the indiscriminate discharge of untreated industrial run-offs and raw domestic sewage into coastal waters through Layari and Malir Rivers may pose serious threat to the ecosystem and human being through food chain pollution [18]. The Manora channel and southeast coast receive industrial and domestic wastes through Layari and Malir Rivers, respectively (Fig. 1). The Layari River entails through the densely populated areas of Karachi and acts as a drain of highly polluted industrial and domestic wastewaters which finally falls into Manora channel besides the addition of wastewater produced by Karachi shipyard [19]. Similarly, the Malir River carries the industrial and domestic wastewaters of eastern areas of the Karachi and discharges the waters into southeast coast [20, 21]. The Malir River collects additional industrial pollution as compared to the Layari River [22]. Karachi Coastal belt can be distributed into northwest and southeast coastal areas. The earlier region is comparatively has no major industrial drain enters in this area. Sunairi Point is found in this region. Mubarak village is also located along the coastline of Arabian Sea almost 7 km away from Sunairi Point in District West of Karachi. This village is 30 km away from city center with population more than 10,000 people. Mubarak village is a second largest fisherman locality (with fish farming) of Karachi coast since 200 years. The seawater and sediment quality of the Karachi coast is, however, affected by domestic and small industries effluents with low and high tide phenomena’s [13].
The sediment core was recovered from Karachi coastline area, from Sunari point core, (24° 52′ 49.66″ N and 66° 41′ 11.64″ E) in northwest coastal belt. A global positioning system (GARMIN III) was used to govern the location of sampling site. Figure 2 provides the position of sampling site. The sediment core was collected with gravity corer (KC Kojak Demanmark A/S) having acrylic coring pipe (2 m long and 7 cm diameter). The coring pipe was focused into the sediment up to a depth of 45 cm from Sunairi Point. The core was slowly recovered and top of the core was properly marked. The core was held in perpendicular location in the refrigerator for two days and frozen core was then sliced at 2.5 cm increments. The divided core was stored in duly characterized plastic bags. All samples of core were then oven dried at 75–85 °C to constant weight. The dried samples were processed, sieved mechanically using 0.5 mm sieve, homogenized and powdered with a pestle and agate mortar. The crushed portion was stored in air tight plastic bottles for additional analysis.
Sediment grain size, total organic carbon and calcium carbonate
Sediment grain size was determined by applying a vibratory sieve shaker having a set of five sieves, ranging from 2000 mm to 70 μm, following a standard method [23]. Total organic carbon (TOC) and calcium carbonate of sediment were determined using method as designated [24, 25].
Metals analysis
Metal contents were determined with proton induced X-ray emission (PIXE) following method as described by [14, 26]. Accordingly, 5 mg of ground sediment sample was mixed with 10 μl of Y(NO3)3 solution, arranged in double-distilled water containing 1.16 mg of yttrium as internal standard, and mounted on a 20 μm thick Mylar. The sample covered about 1.5 cm2 area of Mylar. Afterward, the sample was dried below a lamp in desiccator. The sample was then mounted on stepper motor-controlled wheel, placed inside the investigative work place, and irradiated with 3.8 meV proton beam created through National Electrostatic Corporation tandem pelletron accelerator (model 6SDH-2). A known salt mixture containing 12 elements (Z = 11 to Z = 38) with Y (NO3)3 as internal standard was arranged and used to calibrate the system. The accuracy of calibration was checked using reference material SOIL-7 achieved from International Atomic Energy Agency (IAEA). An assessment of results is shown in Table 1. The concentrations of elements, excluding Al, K, Ca, Fe, and Mn, are closed to the values specified by IAEA. In case of elements like Al, K, Ca, and Fe, error was < 7% and for Mn < 19%. However, the value of Mg differs with IAEA standard by a factor of 2, which may be owing to the self-absorption of low energy X-rays of Mg. The results of other elements were in agreement with IAEA standards.
The heavy metal pollution in sediment was evaluated applying a various geochemical tools, including enrichment factor (EF), degree of contamination (Cd), pollution load index (PLI), geo-accumulation index (Igeo), toxic units (TU), correlation analysis and cluster analysis (CA), which are briefly stated below.
Enrichment factor (EF)
The enrichment factor (EF) calculated from heavy metals data for marine sediment was used to determine whether heavy metals in sediment were of anthropogenic origin or not. Accordingly, the normalization of heavy metals data with a conservative element (like Al, Fe and Mn) was undertaken [27, 28]. In the present study, Al was used as reference element [14, 26]. The EF was computed applying the Eq. 1.
where Cn stands for the concentration of element n in sediment sample and background concentration which is average value of that element in shale [29]. The heavy metal enrichment factor is interpreted in accordance with the classification specified in literature [30, 31]. According to that arrangement, EF < 1 displays no enrichment, < 3 is minor; 3–5 is moderate; 5–10 is moderately severe; 10–25 is severe; 25–50 is very severe; > 50 is extremely severe.
Degree of contamination (Cd)
The general heavy metal pollution of the sediment was assessed through contamination degree (Cd) which is the sum of the contamination factor (Cf) for all heavy metals under study, and following relationship Eq. 2 defines the contamination degree [32].
and
where Ce is concentration of the element in sediment and Cb is the background value of the element in average shale [33]. According to [34], the contamination degree is divided into four classes, such as Cd < 7 low levels of contamination, 7 ≤ Cd < 14 moderate level of contamination, 14 ≤ Cd < 28 considerable levels of contamination, and Cd ≥ 28 very high levels of contamination.
Pollution load index (PLI)
Similarly, the pollution load index (PLI) is an additional main parameter for evaluating metal accumulation in marine sediment over a longer period of time [35] and following association was used to calculate PLI by Eq. 3.
where CF is contamination factor and n stands for the number of metals. A PLI value > 1 displays metal contamination, whereas PLI value < 1 means no pollution.
Geo-accumulation index (Igeo)
The geo-accumulation index (Igeo), presented by [36] is commonly used to judge heavy metal contamination in sediment [32, 37]. Following relationship by Eq. 4 was applied to calculate Igeo.
where Cn represents the heavy metal concentration of the element n in sediment, Bn stands for background value of element n in an average shale [33] and 1.5 is the background matrix correction factor to rectify the terrigenous effects.
The geo-accumulation index classes to judge the quality of sediment are < 0 unpolluted, 1 unpolluted to moderately polluted, 2 moderately polluted, 3 moderately to highly polluted, 4 highly polluted, 5 highly to very highly polluted, and 6 very highly polluted.
Toxic units (TU)
Toxic units (TU) represent the ratio between the concentration of a metal in sediment and its toxicity. The potential acute toxicity of contaminants in sediment was estimated as the sum of the toxic units (TU) of metals defined as the ratio of determined concentration to probable effect level (PEL) value [38].
Statistical analysis
Multivariate statistical analyses including Pearson’s correlation coefficient matrix and cluster analysis (CA) were determined using statistical software SPSS to recognize the relationship of metals and geochemical parameters.
Correlation analysis
Correlation analysis of data was performed using the Pearson coefficient at 5% significance level in order to identify relationship among metals in the sediment [39]. High correlation coefficients between different metals show common sources, mutual dependence and identical behavior. The absence of strong correlation among the metals suggests that the distribution of metals in sediment is not controlled by a single factor [40].
Cluster analysis (CA)
Cluster analysis (CA) was applied to categorize elements of different sources on their similarities using dendrograms and to recognize different homogeneous classes of variables with similar properties [41]. Statistical software SPSS version 7 was applied for cluster analysis.
Results and discussion
Grain size analysis of sediment core was showed in Fig. 3 and results revealed that sediment of core was predominantly sandy in nature, with slightly increasing tendency of silt. Top 12.5 cm of the core was comprised of 70% sand and 30% silt, while in the rest of the core sand and silt were in the range of 62–70% and 30–38%, respectively. The clay was completely missing in the entire core. It concluded from results that Sunairi Point area was exposed to strong currents which brought larger pulses of sand which overwhelmed overall composition of sediments of the area [42].
The pH is an important factor which affects heavy metal chemistry in marine environments as the accumulation and adsorption of metals in the sediments is controlled, directly or indirectly, by pH through either a change in redox state and/or speciation [43]. The higher pH values promote adsorption and low pH can actually prevent the retention of metals by sediments [44, 45]. The pH depicts little variation in the ranges of 7.2–7.5 as indicated in Fig. 4. The pH of sub-samples reflects that there is no major industrial effluents drain which joins the sea in this area [13].
The accumulation of heavy metals in the sediments is also a function of calcium carbonate contents. The variation of calcium carbonate in marine sediments correlates with abundance of benthic calcareous shelled protozoa [46, 47]. The calcium carbonate ranged between 18.60 and 19.95% with average values of 19.20%, in core. The calcium carbonate contents were steadily increased from bottom to top in sediment core (Fig. 5). The higher values of calcium carbonate contents at the surface layers of core was due to the accumulation of shell fragments which were abundantly found in the area. This is in agreement with the findings of other research works [48, 49].
Like grain size, heavy metal elements have relationship with organic carbon in sediments [14, 50]. It is normally supposed that higher the organic carbon contents the higher the accumulation of heavy metals in the sediments [51,52,53,54]. The decomposition of organic material produces organic ligands which may impact distribution of metals in seawater and sediments [55, 56]. Figure 6 validated total organic carbon variation with depth in core sediment. The total organic carbon in sediment core was found in the range of 1.08–2.63%. In most of core the vertical distribution was irregular with lower concentration in the bottom sediments and higher concentrations at surface sediments. The higher variation in organic matter in upper portion of core sediments is normally due to local contamination by shipping oil, i.e., petroleum and its components thrown out this area by waves and the remaining to the biogenic sources (including, land plants, phytoplankton, animals, bacteria, and macroalgae) or in the early stages of diagenesis in recent marine sediments. The presence of silt sediments in this area also support the deposition of organic debris [57].
Mineralogical composition
For mineralogical analysis six samples of core from different depths were used to determine mineralogical composition of the sediment using X-ray spectrometry. The powdered sediment sample was mounted on a glass slide following a procedure given in the literature [58]. Figure 7 shows the mineralogical composition of the sediment core. Minerals included quartz (SiO2), calcite (CaCO3), albite (NaAlSi3O8), muscovite KAl2(AlSi3O10) (F,OH)2, hematite (Fe2O3), and thuringite Fe3(Si,Al)2O5(OH)4. In core sediment, quartz was the most abundant mineral (63.0–70.64), followed by calcite (12.32–21.97) albite (5.37–10.36), muscovite (4.17–7.18), thuringite (3.82–5.75) and hematite (0–0.61%). The high contents of quartz in core sediment may owe to the fact that strong sea currents and wind caused erosion of the shoreline which resulted in relatively higher contents of quartz [59]. The distribution of minerals in core sediments are mainly dependent upon to discharges of rivers run off into sea and the wave action [60].
Heavy metals distribution in sediment core
The distribution of metals with depth in sediment core is shown in Fig. 8a–c. The mean metal concentrations with their ranges were (µg/g): 3372 (2880–3830) for Al, 3108 (2657–3659) for Ca, 30 (25–35) for Co, 24 (19–29) for Cr, 26 (21–31) for Cu, 1922 (1431–2219) for Fe, 3327 (2968–3569) for K, 2997 (2479–3488) for Mg, 39 (33–43) for Mn, 34 (20–42) for Ni, 32 (18–41) for Pb, 142 (108–172) for Ti, 45 (39–50) for V and 132 (115–141) for Zn.
In core vertical distribution pattern of metals like Al, Mg, Ca and K appeared to be fluctuating, but with increasing tendency from bottom to top. Fe showed an irregular pattern from bottom to a depth of 32.5 cm followed by a gradual increase up to the surface (Fig. 8a). The variation of Pb showed gradual increasing tendency from bottom to surface, whereas Cr, Ni and V showed fluctuating pattern in the entire length of core (Fig. 8b). The distribution pattern of Mn, Cu, Co and Zn remained consistent throughout the core, whereas Ti showed an increasing trend from bottom to top (Fig. 8c). Higher concentrations of abundant elements (Mg, Al, Ca, and K) and heavy metals (Cu, Mn, Ni, Pb and Zn) in the entire sediment core reflect significant input increasing in upper portion of core, but found below than background values in upper crust [33]. The values of abundant and heavy metals were found lower in Sunairi Point area (North west region) than the earlier studies of other areas of Karachi coast (from South east coast area, Korangi Creek, Ghizri Creek etc. areas) [13, 14, 26]. The findings of comparisons with earlier studies are highly supported regarding heavy metal pollution in sediment cores as evident of no major industrial drain enters in this region and located away from Karachi industrial zone.
Metal elements correlation
Pearson’s correlation coefficient matrices of heavy metals in sediment core was prepared to see relationship between the metals and to trace their origin (Table 2). The results showed that Mg had significant positive correlation with Ca, Cr, Ti and Al was positively correlated with Fe, Pb and Ti and positive correlation of Ca with K, Cr, Pb and Ti as evident in Table 3 demonstrated that the metals were of the same origin. A negative correlation among the metals, however demonstrates that the input of these metals is not controlled by a single factor but rather by a combination of geochemical support phases and their mixed association [41].
Cluster analysis of metals in sediment core
The Hierarchical Cluster Analysis (HCA) is the most commonly used method to identify the metal elements according to their similarities as metal elements falling in the same group indicate their association with each other [61]. Figure 9 showed the cluster analysis of metal elements in sediment core. It appeared from the results that group-I contained Cu, Co, Pb, Ni, V, Ti, Zn, Cr and Mn, group-II comprised of Mg, Ca, Al and K while Fe and Ca in group-III indicated their association with each other. The results of cluster analysis suggested that most of metals were strongly associated with each other and had same source adding heavy metals into the sediments.
Heavy metal contamination in sediment core was also assessed using parameters like enrichment factor (EF), degree of contamination factor, pollution load index (PLI), geo-accumulation index (Igeo) and toxic units (TU). These parameters were computed following standard procedures as discussed earlier sections.
Enrichment factor (EF)
The mean enrichment factor values for heavy metals in sediment core is given in Table 3. The sediment of core was extreme to severely enriched with Co and Zn (EF > 50) and very severely enriched with Pb, Ni and Cu (25 < EF < 50) indicated the influences of anthropogenic as well as natural sources (human, tourist activities, fish farming, shipping, boat painting and making factories, urbanizations, plastic waste, land using, weathering of rocks and waste water might be one of the most significant causes of various metals like Pb, Co Ni, etc.) [62, 63]. The sediment was severely enriched with V and Cr (10 < EF < 25) and minorly enriched with Ca, K, Fe, Mn and Ti (< 3) in sediment.
Degree of contamination (Cd) and pollution load index (PLI)
Table 4 shows degree of contamination and pollution load index for metals in sediment core. In accordance with classification after [34], An upward increasing trend in the values of degree of contamination was observed in core, which implied that heavy metal contamination falls in the category of moderate pollution (7 ≤ Cd < 14). The pollution load index (PLI) values for metals were also increased from bottom to surface in the core indicating increased anthropogenic activities with passage of time.
Geo-accumulation index (I geo )
The mean geo-accumulation index (Igeo) values for metals in sediment core is given in Table 5. The results showed that sediments of core were moderately polluted with Co, Pb and unpolluted for metals such as Mg, Al, Ca, K, Cr, Cu, Fe, Mn, Ni, Ti, V and Zn.
Toxic units (TU)
The potential acute toxicity of contaminants in sediment core was estimated from sum of the toxic units (TU) which are defined as the ratio of the determined concentration to probable effect level (PEL) [38, 62] and Table 6 shows toxic units (TU) values for heavy metals in core.
Heavy metal pollution in sediment core based on sediment quality guidelines (SQGs)
Sediments can also be classified as non-polluted or moderately polluted based on sediment quality guidelines of United States Environmental Protection Agency (USEPA) [64] as given in Table 7. According to the guidelines, sediment of core was not polluted with Pb, Cr and Cu, but moderately polluted with Ni and Zn, which may cause danger to aquatic life.
Conclusions
-
The results grain size analysis showed that sediment of Sunairi Point was predominantly sandy in nature.
-
The calcium carbonate in the sediment core appeared to be dependent on the pH profile and ranged between 18.60 and 19.95%.
-
The distribution of heavy metals in sediment core indicated a significant upward enrichment in heavy metals with the highest concentrations found in the upper most layer sediments, may be due to anthropogenic activities, which could be the cause of upward enrichment in heavy metals.
-
The pollution load index values, although showed no pollution, but found increasing trend from bottom to top in core and indicated increase in PLI in recent sediment may be due to the influences of anthropogenic sources.
-
The results of mineralogical composition indicated, quartz was the most abundant mineral (63.0–70.64), followed by calcite (12.32–21.97), albite (5.37–10.36), muscovite (4.17–7.18), thuringite (3.82–5.75) and hematite (0- 0. 61%) The high contents of quartz in core sediment may owe to the fact that strong sea currents and wind caused erosion of the shoreline.
-
According to the sediment quality guidelines of United States Environmental Protection Agency (USEPA), sediment of core was moderately polluted with Ni and Zn. It was concluded from the guidelines that mentioned metals in sediment may cause danger to aquatic life.
-
It was inferred from the study that Sunairi Point area of Karachi coast is facing moderately metals pollution which increased with time.
References
GESAMP (2001) IMO/FAO/UNESCO/WHO/WMO/IAEA/ UNDP joint group of experts on the scientific aspects of marine pollution (GESAMP): Reports and studies no. 71, protecting the oceans from land-based activities, land base sources and activities affecting the quality and uses of the marine, coastal and associated freshwater environment
Goldberg ED (1992) Marine metal pollutants: a small set. Mar Pollut Bull 14:45–47
Szefer P, Szefer K, Hallera GJ, Glasby GP, Pempkowsak J, Kaliszan R (1996) Heavy metal pollution in surficial sediment from the Southern Baltic Sea of Poland. J Environ Sci Health Part A 33:2723–2754
Krishna K, Mohan KR, Murthy NN (2011) A multivariate statistical approach for monitoring of heavy metals in sediment: A case study from wailpalli watershed, Nalgonda District, Andhra Pradesh. India Environ Earth Sci 3(2):103–113
Ramirez M, Serena M, Frache R, Correa J (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine. Chile Mar Pollut Bull 50:62–72
Cardoso A, Boaventura G, Silva E, Brod J (2001) Metal distribution in sediment from the Ribiera bay, Rio de Janeiro—Brazil. J Braz Chem Soc 12(6):767–774
Jones B, Turki A (1997) Distribution and speciation of heavy metals in surficial sediment from the Tees Estuary, north-east England. Mar Pollut Bull 34:768–779
Al-Saad HT, Mostafa YZ, Al-Imarah FJ (1997) Distribution of trace metals in tissues of fish from Shatt Al-Arab Estuary, Iraq. Mesoamerican Reef 11:15–25
Beiras R, Bellas J, Fernandez N, Lorenzo JI, Cobela-Garcia A (2003) Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula) metal concentrations in seawater, sediment and mussels (Mytilus galloprovincialis) verus embryo-larval bioassays using Paracentrotus lividus and Ciona intestinalis. Mar Environ Res 56:531–553
Caccia VG, Millero FJ, Palanques A (2003) The distribution of trace metals in Florida Bay sediment Mar. Pollut Bull 46:1420–1433
Aprile FM, Bouvy M (2008) Distribution and enrichment of heavy metals in sediment at the Tapacura River Basin, North eastern Brazil. Braz J Aquat Sci Tech 12(1):1–8
Nergis Y, Sharif M, Farooq Choudhry A, Hussain A, Butt AJ (2012) Impact of industrial and sewage effluents on Karachi coastal water and sediment quality, middle-east. J Sci Res 11(10):1443–1454
Mashiatulla A, Chaudhary MZ, Ahmad N, Qureshi RM, Javed T, Ghaffar A, Khan MS (2011) Metal pollution assessment in sediment of Karachi Coast. Pak Nucl 48(3):223–230
Chaudhary MZ, Ahmad N, Mashiatullah A, Ahmad N, Ghaffar A (2013) Geochemical assessment of metal concentrations in sediment core of Korangi Creek along Karachi Coast. Pak Environ Monit Assess 185:6677–6691
Hasnie FR, Qureshi NA (2002) Quantification of pollution load in wastewater channels of village Rehri draining in to Korangi Creek. Pak Steel Res J 3:47–54
Saleem M, Kazi GH (1998) Concentration and distribution of heavy metals (lead, cadmium, copper, nickel, zinc) in Karachi shore and off shore sediment. Pak J Mar Sci 7:71–79
Kumar SP, Edward JKP (2009) Assessment of metal concentration in the sediment cores of Manakudy estuary, south west coast of India. Indian J Mar Sci 38(2):235–248
Mashiatullah A, Qureshi RM, Ahmad N, Khalid F, Javed T (2009) Physico-chemical and biological water quality of Karachi coastal water. Nucl 46(1–2):53–59
Siddique A, Mumtaz M, Zaigham NA, Mallick KA, Saied S, Zahir E, Khwaja HA (2009) Heavy metal toxicity levels in the coastal sediment of the Arabian Sea along the urban Karachi (Pakistan) region. Baseline/ Mar Pollut Bull 58:1406–1419
Zaigham NA (2004) Unauthorized squatter settlements are one of major sources for polluting surface and subsurface waters in Karachi, Proceedings of the WSSD workshop on human settlement and environment (Pakistan’s response to its obligations under the WSSD plan of implementation), Islamabad, December 14–15, pp 100–112
Rizvi SHN, Saleem M, Baquer J (1988) Steel mill effluents: influence on the Bakran Creek environment. In: Thompson MF, Tirmizi NM (Eds). Proceedings of marine science of the Arabian Sea. American Institutes of Biological Sciences, Washington, DC, pp. 549–569.
Bano F (1999) A study of physical and major chemical constituents of Malir river (within Karachi) to determine the extent of pollution, PhD thesis, Karachi University, pp 1–4
Folk RL, Ward WC (1957) Brazos river bar: a study in the significance of grain size parameters. J Sediment Petrol 27(1):3–27
Allison LE (1965) Organic carbon. In: Black DD, Evans JL, White LE, Clark FE (eds) Methods of soil analysis. American Society of Agronomy Wisconsin, pp 1372–1378
Loring DH, Rantala RTT (1992) Manual for the geochemical analyses of marine sediment and suspended particulate matter. Earth-Sci Rev 32:235–283
Mashiatullah A, Chaudhary MZ, Ahmad N, Ahmad N, Javed T, Ghaffar A (2015) Geochemical assessment of metal pollution and ecotoxicology in sediment cores along Karachi coast Pakistan. Environ Monit Assess 187:249–264
Baptista Neto JA, Smith BJ, McAllister JJ (2000) Heavy metal concentrations in surface sediment in a nearshore environment, Jurujuba Sound. Southeast Brazil Environ Pollut 109(1):1–9
Mucha AP, Vasconcelos MTSD, Bordalo AA (2003) Macrobenthic community in the Doura estuary: Relations with trace metals and natural sediment characteristics. Environ Pollut 121(2):169–180
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72(2):175–192
Ergin M, Saydam C, Basturk O, Erdem E, Yoruk R (1991) Heavy metal concentrations in surface sediment from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the north eastern Sea of Marmara. Chem Geol 91(3):269–285
Birch G (2003) A scheme for assessing human impacts on costal aquatic environments using sediment. In: Woodcoffee CD, Furness RA (eds) Costal GIS. Wollongong University papers in Center for Maritime Policy, 14, Australia
Lina F, Nayak GN, Ilangovan D (2012) Geochemical assessment of metal concentrations in mangrove sediment along Mumbai coast India. World Acad Sci Eng Technol 61:258–263
Wedepohl KH (1995) The composition of continental crust. Geochim Cosmochim Acta 59:1217–1233
Hakanson L (1980) Ecological risk index for aquatic pollution control: a sedimentological approach. Water Res 14(5):975–1001
Tomilson DC, Wilson DJ, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol Mar Res 33(1–4):566–575
Muller G (1969) Index of geo-accumulation in sediment of the Rhine River. Geol J 2(3):108–118
Karbassi AR, Nabi-Bidhendi GR, Bayati I (2005) Environmental geochemistry of heavy metals in a sediment core off Bushehr, ersian Gulf. Iran J Environ Health Sci Eng 2:255–260
Pederson F, Bjorestad E, Anderson HV, Kjolholt J, Poll C (1998) Characterization of sediment from Copenhagen harbour by use of biotests. Water Sci Technol 37(6–7):233–240
Zar JH (1996) Bio statistical Analysis, 3rd edn. Prentice-Hall, NJ, USA
Jain CK, Singhal DC, Sharma MK (2005) Metal pollution assessment of sediment and water in the river- Hindon, Indian Environ. Monit Assess 105:193–207
Aitchison J (1986) The statistical analysis of compositional data. Chapman and Hall, London-New York, p 416
Veerasingam S, Venkatachalapathy R, Ramkumar T (2014) Distribution of clay minerals in marine sediment off Chennai, Bay of Bengal, India: indicators of sediment sources and transport processes. Int J Sediment Res 29:11–23
McKay JL, Pedersen TF, Mucci A (2007) Sedimentary redox conditions in continental margin sediment (NE Pacific): influence on the accumulation of redox-sensitive trace metals. Chem Geol 238(3–4):180–196
Gambrell RP (1994) Trace and toxic metals in Wetlands: a review. J Environ Qual 23:883–891
Schlinder PW (1991) The regulation of heavy metal concentrations in natural aquatic systems. In: Vernet J-P (ed) heavy metals in the environment 1. Elsevier, Amsterdam, pp 95–124
Martin WR, Sayles FL (1996) CaCO3 dissolution in sediments of the Ceara Rise, western equatorial pacific. Geochim Cosmochim Acta 60:243–263
Smith WR (2000) Calcite precipitation and trace metal partitioning in ground water and the vodose zone: remediation of strontium-90 and other divalent metals and radionuclides in arid western environments. Project ID 70206, EMSP Project Book, pp 168–170
Natesan U, Kumar MM, Depthi K (2014) Anthropogenic effects on sediment quality of Muthupet mangroves: assessing the sediment core geochemical record. Indian J Mar Sci 43(6):1045–1054
Muthuraj S, Jayaprakash M (2008) Distribution and enrichment of trace metals in marine sediment of Bay of Benagal, off Ennore, south east coast of India. Environ Geol 56:207–217
Usui T, Nagao S, Yamamoto M, Suzuki K, Kudo I, Montani S, Noda A, Minagawa M (2006) Distribution and sources of organic matter in surficial sediments on the shelf and slope off Tokachi, western North Pacific, inferred from C and N stable isotopes and C/N ratios. Mar Chem 98:241–259
Renghunath R, Sareedhara Murthy STR (1996) Carbonate and organic matter studies of the shelf sediments off Kasargod, west coast of India. Indian J Mar Sci 25:355–357
Hedges JI, Keil RG (1995) Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar Chem 49:81–115
Canfield DE (1994) Factors influencing organic carbon preservation in marine sediments. Chem Geol 114:315–329
Meyers PA, Ishiwater R (1993) Lacustrine organic geochemistry: an overview of organic matter sources and diagenesis in lake sediments. Org Geochem 20(7):867–900
Henrichs SM (1992) Early diagenesis of organic matter in marine sediments-progress and perplexity. Mar Chem 39:119–149
Kemp ALW, Johnston LM (1979) Diagenises of organic matter in the sediments of Lakes Ontario, Erie, and Huron. J Great Lakes Res 5:1–10
Venkatachalapathy R, Veerasinga S, Basavaiah N, Ramkumar T, Deenadayalan K (2011) Environmental magnetic and petroleum hydrocarbons records in sediment cores from the north east coast of Tamilnadu, Bay of Bengal. India Mar Pollut Bull 62:681–690
Brown G, Brindly GW (1984) X-ray diffraction procedures for clay mineral identification: In: Brindly GW, Brown G (eds) Crystal structure of clay minerals and their identification, pp 305–360
Veerasingam S, Venkatachalapathy R, Ramkumar T (2014) Distribution of clay minerals in marine sediment off Chennai, Bay of Bengal, India: Indicators of sediment sources and transport processes. Int J Sedim Res 29:11–23
Ekwere SJ (1992) Geochemical studies of sediments in Qua Iboe estuary associated creeks, south eastern Nigeria. Trop J Appl Sci 2:91–95
Veerasingam S, Venkatachalapathy R, Ramkumar T (2012) Heavy metals and ecological risk assessment in marine sediment of Chennai. India Carpathian J Earth Environ Sci 7(2):111–124
Singh JK, Kumar P, Kumar R (2020) Ecological risk assessment of heavy metal contamination in mangrove forest sediment of Gulf of Khambhat region, West Coast of India, SN Applied Sciences
Nour HES (2019) Distribution ecological risk, and source analysis of heavy metal in recent beach sediments of Sharm El – Sheikh, Egypt. Environ Monit Assess 191:546
Perin G, Bonardi M, Fabris R, Simoncini B, Manente S, Tosi L, Scotto S (1997) Heavy metal pollution in central Venice Lagoon bottom sediments, evaluation of the metal bioavailability by geochemical speciation procedure. Environ Technol 18:593–604
Acknowledgements
The authors are highly thankful to Head IAD, Director Technology and Director General PINSTECH for providing financial support to conduct this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, M.Z., Ahmad, N., Mashiatullah, A. et al. Metal contamination in Sunairi Point sediment core along Karachi coast, Pakistan. J Radioanal Nucl Chem 328, 605–615 (2021). https://doi.org/10.1007/s10967-021-07666-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07666-3