Abstract
Due to the promising results of 4-{[(bis(phosphonomethyl))carbamoyl]methyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid (BPAMD) complexes in human studies, in this study, new therapeutic complex of 175Yb-BPAMD was investigated. The effect of various parameters on the labelling yield of 175Yb-BPAMD, stability tests, partition coefficient and hydroxyapatite binding of the complex were studied. Biodistribution studies (SPECT imaging and sacrifice) were performed after injection of the complex to rats and compared with the biodistribution of the 175Yb cation and other 175Yb bone-seeking agents. Totally, 175Yb-BPAMD has interesting characteristics compared to the other 175Yb bone-seeking agents and can be considered as a potential agent for bone pain palliation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skeleton is the most common organ to be affected by metastatic cancers such as breast, lung and prostate [1, 2]. Bone metastases are often clinically silent; they can lead to serious sequelae, such as pain, fractures, and hypercalcemia that generally has the high negative impact on the quality of life [3].
Among the methods for treatment of the metastatic bone pain including systemic analgesics, antitumor agents, hormones, chemotherapy, steroids, local surgery, anesthesia, and external beam radiation, a systemic bone-avid radiopharmaceutical for treatment of widespread bony metastases has potential benefit in patients who have progressive disease despite treatment [4, 5]. In general, no single method will keep the patient free of symptoms for an extended period of time, and usually a combination of systemic and local modalities may be required.
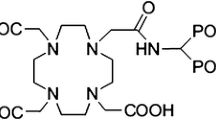
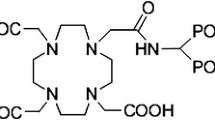
Bisphosphonates are known to show high and fast binding to apatite structures especially those of high biological activity and nowadays, various phosphonate ligands labelled with β−-emitting radionuclides have shown good efficacy for bone pain palliation [6, 7]. Some requirements and restrictions of the first generation phosphonates have encouraged the researchers to develop and evaluate the new generation of bisphosphonate such as (4 {[(bis(phosphonomethyl))carbamoyl] methyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid (BPAMD) (Fig. 1) [6, 8].
The physical characteristics of Ytterbium-175 [t 1/2 = 4.18 d, β max = 470 keV (86.5 %) and γ = 396 keV (6.4 %)] [9] as well as its medium specific activity in a moderate flux reactor make it a favorable radioisotope for bone pain palliative applications. Also, the other studies have indicated that radionuclidic impurities upon thermal neutron bombardment of natural ytterbium target should not cause any serious problem in the in vivo application of 175Yb [10] and therefore, bisphosphonate ligands labelled with 175Yb such as 175Yb-DOTMP, 175Yb-EDTMP, 175Yb-PDTMP, 175Yb-DTPMP and 175Yb-TTHMP have been developed as the potential agents for bone pain palliation therapy [9, 11].
In continuation of developing BPAMD therapeutic agents [12, 13] and due to the interesting properties of BPAMD and 175Yb, the authors tried to investigate the possible production of 175Yb-BPAMD as an agent for bone pain palliative application. In this study, radiolabeling, quality control, partition coefficient and hydroxyapatite (HA) binding assay of 175Yb-BPAMD were studied and finally the biodistribution data of the radiolabelled compound was acquired after injection of the complex to the rats using SPECT imaging and scarification.
Materials and instruments
175Yb was produced with a specific activity of approximately 1.11 GBq/mg by irradiation of natural Yb2O3 at a thermal neutron flux of 5 × 1013 n cm−2 s−1 for 5 days at Tehran Research Reactor (TRR). The Yb2O3 powder was obtained from Isotec Inc. (USA). BPAMD was purchased from ABX (Radeberg, Germany). All other chemical reagents were obtained from Sigma-Aldrich (Heidelberg, Germany). Whatman No. 2 paper was observed from Whatman (Buckinghamshire, U.K.). Radio-chromatography was performed by Whatman paper using a thin layer chromatography scanner (Bioscan AR2000, Paris, France). The activity of the samples was measured by a p-type coaxial high-purity germanium (HPGe) detector (EGPC 80-200R) coupled with a multichannel analyzer card system (GC1020-7500SL, Canberra, U. S. A.). Planar images were taken by a dual head SPECT system (DST-XL, SMV, Buc, France).
Methods
Production and quality control of 175YbCl3 solution
Ytterbium-175 was produced by neutron irradiation of 1 mg of natural Yb2O3 at Tehran Research Reactor (TRR). The irradiated target was dissolved in 1 mL of 1.0 M hydrochloric acid (HCl) to prepare a stock solution of 175YbCl3 and diluted to the appropriate volume with ultra-pure water. The mixture was filtered through a 0.22 μm biological filter and sent for use in the radiolabeling step.
The radionuclidic purity of the solution was tested by gamma-ray spectroscopy on an HPGe detector basing on the major photons of 175Yb. The radiochemical purity of the 175YbCl3 was checked using two solvent systems for instant thin layer chromatography (ITLC) (A: 10 mM DTPA pH 4 and B: ammonium acetate 10 %: methanol [1:1]).
Preparation and quality control of 175Yb-BPAMD
In order to yield maximum labelling, the effect of various parameters on the labelling yield of 175Yb-BPAMD including ligand concentration, pH, temperature and reaction time were studied.
A stock solution of BPAMD was prepared by the dissolution of 1 mg of the ligand in 1 mL pure water. Distinct values of BPAMD (150–350 nmol) were added to the 10-mL conical vials including approximately equal amounts (300 MBq) of 175YbCl3. The temperature and pH of the mixture were exchanged from 70 to 100 °C and 3 to 7, respectively, while the radiochemical purity of the mixture was checked by instant thin layer chromatography (ITLC) method. For ITLC, a 5 µL sample of the final fraction was spotted on a chromatography Whatman No. 2 paper, and developed in various mobile phase mixtures (Ammonium hydroxide:Methanol:Water (0.2:2:4; v/v/v), 0.1 M sodium citrate (pH 4) and 10 % Ammonium acetate: Methanol (1:1)).
To remove the non-complexed 175Yb from the radiolabelled compound, the mixture was passed over the strong cation exchanger (Strata-X-C 60 mg) preconditioned with 1 mL 4 M HCl and 1 mL water. The 175Yb-BPAMD complex was passed from the exchanger and collected to the separate vial, while the non-complexed Ytterbium was immobilized on the exchanger.
Stability studies
To determine the stability of the 175Yb-BPAMD, a sample of solution was kept at room temperature for 48 h and frequent analyses have been performed using Whatman No. 2 chromatography paper in NH4OH:MeOH:H2O (1:10:20) system.
The stability of 175Yb-BPAMD in human serum was determined by incubating the final solution (50 µCi, 50 µL) in the presence of the freshly collected human serum (500 µL) at 37 °C for 2 days after preparation using above mentioned chromatography system.
Hydroxyapatite binding assay
HA binding assay was performed according to the previously described procedure [14], with only a slight modification. In brief, to vials containing 5.0, 10.0, 15.0, 20.0, 25.0 and 50.0 mg of solid HA, 2 mL of saline solution of pH 7.4 was added and the mixtures were shaken for 2 h. Then, 50 µL of the radioactive preparation was added and the mixtures were shaken for 24 h at room temperature. The suspensions were centrifuged, and two aliquots of the supernatant liquid were taken from each vial and the radioactivity was measured with a well-type counter. Test experiments were performed using a similar procedure, but in the absence of HA. The percentage binding of 175Yb to HA was calculated according to HB = 1 − A/B × 100, where A is the mean radioactivity value of the supernatant sample under study and B is the mean total value of whole activity used.
Partition coefficient Determination
Partition coefficient of 175Yb-BPAMD was calculated followed by the determination of P (the ratio of specific activities of the organic and aqueous phases). A mixture of 1 mL of 1-octanol and 1 mL of isotonic acetate-buffered saline (pH = 7) containing approximately 5.55 MBq of the radiolabeled complex at 37 °C was vortexed 1 min and left 5 min. Following centrifugation at >1200 g for 5 min, the octanol and aqueous phases were sampled and counted in an automatic well-type counter. A 500 µL sample of the octanol phase from this experiment was shaken again three times with fresh buffer samples. The reported log P values are the average of the extractions from three independent measurements.
Biodistribution studies of 175YbCl3 and 175Yb-BPAMD in rat
Biodistribution studies of 175YbCl3 and 175Yb-BPAMD were investigated in rats (n = 5) until 72 h post injection. Final 175YbCl3 and the radiolabelled complex solution containing approximately 5.55 MBq (150 µL) of radioactivity were injected to rats through their tail veins. The animals were sacrificed at specified intervals (2, 4, 24, 48 and 72 h) using the animal care protocols. Five animals were sacrificed for each interval.
The specific activity of tissues (heart, lung, intestine, stomach, kidneys, spleen, liver, bladder, muscle and bone) was calculated as the percentage of injected dose per gram (%ID/g) using HPGe detector. All values were expressed as mean ± standard deviation (Mean ± SD) and the data were compared using student T test. Animal studies were performed in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations, second edition.
Imaging studies of 175Yb-BPAMD
3.7 MBq of 175Yb-BPAMD was injected intravenously into a rat through their tail vein. The rat was anesthetized using xylazine-ketamine mixture. Planar images were taken at 2 and 48 h after administration of the radiolabelled complex by a dual-head SPECT system. The rat-to-high energy septa distance was 12 cm. The useful field of view (UFOV) was 540 × 400 mm.
Results and discussion
Quality control of 175YbCl3
The radionuclidic purity of 175Yb produced was >96 % as obtained from the analysis of the gamma-ray spectrum (Fig. 2).
Also, radiochemical purity of 175YbCl3 was determined by ITLC method using two solvent systems (Fig. 3). At the mixture of 10 mM DTPA solution as the mobile phase, the free Ytterbium cation in the 175Yb3+ form, was chelated with the polydentate eluting leading to the migration of the cation in 175Yb-DTPA form to higher R f (R f 0.7); any other ionic species would lead to the observation of new radiopeaks, especially at the origin (R f 0.0–0.1). At the mixture of 10 % ammonium acetate: methanol (1:1) as another solvent system, 175Yb3+ remains at the origin while other ionic species would migrate to higher Rfs. Species such as YbCl4 − might be present due to possibility of coordination of more anions to central Yb as a transition metal espcially at lower pHs.
Preparation and quality control of 175Yb-BPAMD
In order to obtain maximum labelling yield, several experiments were carried out by the variation of different reaction parameters.
The results indicated the growth of labelling yield with increasing the amount of BPAMD and reached above 97 % by adding 225 µL (389 nmol) of the ligand. It was observed that at the temperature range of 90–100 °C, the maximum complexation yield would be achievable. The effect of pH on the labelling yield was also studied by varying the pH of the reaction mixture from 3 to 7 using HEPES. Maximum yield was observed at pH 5–6 while decreased beyond this range. The reaction mixture was incubated at 100 °C temperature for different time periods and 45 min incubation was found to be adequate to yield maximum complexation.
Different chromatographic systems were used for the detection of the radiolabelled compound from the free cation. The best ITLC mobile phase was considered by Whatman No. 2 paper using NH4OH:MeOH:H2O (0.2:2:4). In this mixture, free cation remains at the origin of the paper as a single peak, while the radiolabelled compound migrates to higher R f (0.7–0.8) (Fig. 4).
Stability tests
The study of the stability tests in room temperature and in human serum in 37 °C was performed until 48 h post preparation showing the radiochemical purity of greater than 96 %.
Hydroxyapatite binding assay of 175Yb-BPAMD
HA assay demonstrated high capacity binding for 175Yb-BPAMD to HA. Even at 5 mg amount of HA, more than 84 % binding was observed. At further amounts of HA (>15 mg), approximately, all radiolabelled complex was bound to HA (Fig. 5).
Partition Coefficient Determination of 175Yb-BPAMD
The partition coefficient (P o/w) was calculated by dividing the counts in the octanol phase by those in the buffer, and the results expressed as log P o/w. The average value from three independent measurements for 175Yb-BPAMD was −1.67 ± 0.03 witch highlight the strong hydrophilic nature of the complex.
Biodistribution of 175YbCl3 and the Radiolabelled Complexes in Rats
The percentage of injected dose per gram in rat organs were determined up to 72 h post injection of 175YbCl3 and 175Yb-BPAMD (Figs. 6, 7). In the case of 175YbCl3, the maximum amount of the retained activity accumulates in the liver. The spleen, kidney and bone are the other organs with significant accumulation. Also, the obtained results, it is clearly concluded that the major portion of the injected activity of the complex is transferred from the blood circulation into the bone.
When metastases are widespread or when new sites continue to appear, systemic radiotherapy is the first choice. Recently, various useful radiopharmaceuticals for systemic treatment of bone pain in bone metastases using different beta emitter radionuclides have been reported. 175Yb seems to be an attractive therapeutic radioisotope because of its suitable physical properties.
Therefore, radiolabelled complexes such as 175Yb-DOTMP, 175Yb-TTHMP, 175Yb-EDTMP, 175Yb-PDTMP, and 175Yb-DTPMP have been produced and shown good accumulation in bone tissue. In this research study, BPAMD as a new generation of bisphosphonate ligand was labelled with 175Yb. The complex is prepared in 45 min with radiochemical purity of >97 %.
As it can be seen in Fig. 7, the main part of the remained activity of the complex was transferred from blood circulation into the bones. Low accumulation is indicated in the liver and also gastrointestinal system (intestines, colon). The main way of the excretion for the labelled compound is through the urinary tract due to the water solubility of the compound.
Nowadays, many radiopharmaceuticals are developed for treatment of painful metastases. The most important point which should be considered in developing radiopharmaceuticals as bone pain palliative agents is the absorbed dose delivered in the bone marrow. Whereas the radionuclides with low β− particle energies are recommended for bone pain palliation, those with higher energies are used for bone marrow ablation [15].
Among the radionuclides of 166Ho (Eβ max = 1.85 MeV), 153Sm (Eβ max = 0.810 MeV), 186Re (Eβ max = 1.07 MeV) and 175Yb (Eβ max = 470 keV [86.5 %]), the last one has the lowest beta energy. Because of the lower beta particle energy of 175Yb rather than other mentioned radionuclides, for a given absorbed dose to the bone surface, lower dose would be delivered to the bone marrow in the case of 175Yb-BPAMD usage. Having the higher beta energies of 166Ho and 153Sm is the reason for their bone marrow ablation applications in the patients with multiple myeloma [16–19].
On the other hand, the half-life of the radionuclides as a noticeable parameter should be considered. It can affect the required irradiation and complex preparation times. Also, the radiopharmaceutical transportation to the farther hospitals is an important parameter for radionuclides with shorter half-life especially for countries with limited production centers. 175Yb with half-life of 4.18 d can solve this limitation compared to 153Sm (t 1/2 = 1.93 d) and Ho (t 1/2 = 26.8 h). However, the physical properties of 177Lu [t 1/2 = 6.73 d, Eβ max = 497 MeV] are comparable with 175Yb, but the calculation results show that especial activity of the 175Yb would be about 3 times higher than 177Lu after irradiation of natural ytterbium and lutetium in the same flux and time. Generally, these characteristics introduce 175Yb as a good candidate for bone pain palliative therapy.
The comparison between 175Yb and 175Yb-BPAMD demonstrate different biokinetic pattern for both species. Low blood radioactivity content demonstrates that rapid removal of 175Yb from the circulation after injection. In fact, because of the water solubility of the Yb cation, the free Yb can be excreted via urinary tract. The liver has significant 175Yb uptake related to metal transfer in plasma in protein-bond form. The maximum liver radioactivity uptake is seen in 24 and 48 h (about 2.3 %) that is comparable to other radio-lanthanides. Also, spleen and bone indicate considerable uptake. The muscle, thyroid and intestine do not demonstrate considerable uptake after injection of 175YbCl3.
For an ideal therapeutic radiopharmaceutical, the receiving dose to the critical non-target organs is a very important point that should be considered. The ratios of bone/non-target organs for 175Yb-BPAMD are shown in Table 1. The target to non-target organ ratios for 175Yb-BPAMD are compared with 175Yb-alendronate (175Yb-ALN) and 175Yb-pamindronate (175Yb-PAM) as the two recently synthesized 175Yb bone-seeking agents [20] in Table 2.
The bone to liver, spleen and kidney ratios for 175Yb-BPAMD is greater than these ratios for other 175Yb bone-seeking agents. This means for a given dose to the bone tissue as the target organ, other critical organs such as liver, spleen and kidney would receive lesser dose in the case of 175Yb-BPAMD.
Imaging studies of 175Yb-BPAMD
Planar images were taken from normal rat 2 and 48 h after administration of the radiollabeled complex (Fig. 8). Imaging of the rat showed a distinct accumulation of the radiotracer in the skeletal region 2 and 48 h after injection. The images proved that the activity accumulate to the bone very fast and retain in the bone for long time.
Conclusions
In this study, 175Yb-BPAMD was prepared with high radiochemical purity (>97 %, ITLC) in 45 min. The complex demonstrated significant stability at room temperature and in human serum at least for 48 h. HA binding assay demonstrated at the amount of more than 15 mg, approximately, all radiolabelled complex was bind to HA. At the pH 7, log P o/w was −1.67 ± 0.03. The final preparation was administered to rats and biodistribution of the complex was checked until 72 h post injection. Both SPECT and scarification showed major accumulation of the radiolabelled compound in the bone tissue. All tissues approximately have insignificant uptake in comparison with bone tissue. The comparison of the bone to the critical organs ratios for 175Yb-BPAMD and other 175Yb bone-seeking agents showed greater amounts for 175Yb-BPAMD at early times after injection. Totally, it can be concluded that 175Yb-BPAMD has interesting characteristics as an agent for bone pain palliation, however further biological studies in other mammals are still needed.
References
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Vigna L, Matheoud R, Ridone S, Arginelli D, Della Monica P, Rudoni M et al (2004) Characterization of the [(153)Sm]Sm-EDTMP pharmacokinetics and estimation of radiation absorbed dose on an individual basis. J Nucl Med 45:1358–1365
Pandit-Taskar N, Batraki M, Divgi CR (2001) Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 42:895–906
Serafini AN (1993) Therapy of metastatic bone pain. J Nucl Med 34:1031–1036
Eary JF, Collins C, Stabin M, Vernon C, Petersdorf S, Baker M et al (2012) Samarium-153-EDTMP biodistribution and dosimetry estimation. Nucl Med Biol 39:993–999
Fellner M, Biesalski B, Bausbacher N, Kubícek V, Hermann P, Rösch F et al (2012) (68)Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol 39:993–999
Rajendran JG, Eary JF, Bensinger W, Durack LD, Vernon C, Fritzberg A (2002) Highdose 166Ho-DOTMP in myeloablative treatment of multiple myeloma: pharmacokinetics, biodistribution, and absorbed dose estimation. J Nucl Med 43:1383–1390
Yousefnia H, Amraei N, Hosntalab M, Zolghadri S, Bahrami-Samani A (2015) Preparation and biological evaluation of 166Ho-BPAMD as a potential therapeutic bone-seeking agent. J Radioanal Nucl Chem 304:1285–1291
Firestone R (1996) In: Shirley VS (ed) Table of isotopes, 8th edn. Wiley, New York
Safarzadeha L, Ghannadi-Maraghehb M, Anvaric A, Aghamiric SMR, Shirvani-Aranib S, Bahrami-Samani A (2012) Production, radiolabeling and biodistribution studies of 175Yb-DOTMP as bone pain palliation. Iran J Pharm Sci 8:135–141
Mathewa B, Chakrabortyb S, Dasb T, Sarmac HD, Banerjeeb Sh, Samuelb G et al (2004) 175Yb labeled polyaminophosphonates as potential agents forbone pain palliation. Appl Radiat Isot 60:635–642
Rabie A, Enayati R, Yousefnia H, Jalilian AR, Shamsaei M, Zolghadri S,et al. (2015) Preparation, quality control and biodistribution assessment of 153Sm-BPAMD as a novel agent for bone pain palliation therapy. Ann Nucl Med. doi:10.1007/s12149-015-1014-2
Yousefnia H, Zolghadri S, Sadeghi HR, Naderi M, Jalilian AR, Shanehsazzadeh S (2015) Preparation and biological assessment of 177Lu-BPAMD as a high potential agent for bone pain palliation therapy: comparison with 177Lu-EDTMP. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4225-z
Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M et al (2002) Synthesis, characterization and biodistribution of bisphosphonates 153Sm complexes: correlation with molecular modeling interaction studies. Nucl Med Biol 29:329–338
Bouchet LG, Bolch WE, Goddu SM, Howell RW, Rao DV (2000) Considerations in the selection of radiopharmaceuticals for palliation of bone pain from metastatic osseous lesions. J Nucl Med 41:682–687
Dispenzieri A, Wiseman GA, Lacy MQ, Hayman SR, Kumar SK, Buadi F et al (2010) A phase II study of 153Sm-EDTMP and high dose melphalan as a peripheral blood stem cell conditioning regimen in patients with multiple myeloma. Am J Hematol 85:409–413
Macfarlane DJ, Durrant S, Bartlett ML, Allison R, Morton AJ (2002) 153Sm EDTMP for bone marrow ablation prior to stem cell transplantation for haematological malignancies. Nucl Med Commun 23:1099–1106
Breitz HB, Wendt RE, Stabin MS, Shen S, Erwin WD, Rajendran JG et al (2006) 166Ho-DOTMP radiationabsorbed dose estimation for skeletal targeted radiotherapy. J Nucl Med 47:534–542
Sohaib M, Ahmad M, Jehangir M, Perveen A (2011) Ethylene diamine tetramethylene phosphonic acid labeled with various β-emitting radiometals:labeling optimization and animal biodistribution. Cancer Biother Radiopharm 26:159–164
Fakhari A, Jalilian AR, Yousefnia H, Shanehsazzadeh S, Bahrami-Samani A, Johari-Daha F et al (2015) Preparation, biological evaluation and dosimetry studies of 175Yb-Bis-phosphonates for palliative treatment of bone pain. Mol Imaging Radionucl Ther. 24:110–119
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaez-Tehrani, M., Zolghadri, S., Afarideh, H. et al. Preparation and biological evaluation of 175Yb-BPAMD as a potential agent for bone pain palliation therapy. J Radioanal Nucl Chem 309, 1183–1190 (2016). https://doi.org/10.1007/s10967-016-4734-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4734-4