Abstract
In this study, 177Lu-(4-{[(bis(phosphonomethyl))carbamoyl]methyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl) acetic acid (177Lu-BPAMD) was successfully prepared. The quality control, partition coefficient, hydroxyapatite binding assay and stability of the complex were determined. For better comparison, biodistribution patterns of 177Lu-BPAMD and 177Lu-EDTMP complexes were compared in same animal model. 177Lu-BPAMD was prepared with high radiochemical purity (>93 %) and specific activity of 534 GBq/mmol at the optimal conditions. Comparative study between 177Lu-BPAMD and 177Lu-EDTMP indicated higher bone uptake and lesser accumulation in the other organs for 177Lu-BPAMD. 177Lu-BPAMD can be considered as a promising agent for bone pain palliation in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal metastases are major clinical concerns observed in the vast number of primary cancers that commonly associated with disabling pain, spinal cord compression, hypercalcemia and pathologic fracture [1–3]. Radiation therapy can be considered as a successful procedure for palliation of painful bone metastasis with very few side effects [4]. In patients who have progressive disease despite treatment, a systemic bone-avid radiopharmaceutical for treatment of widespread bony metastases has potential benefit [5]. For this purpose, various phosphonate ligands labeled with β−-emitting radionuclides have shown good efficacy [6, 7].
Bisphosphonates have been known as potent inhibitors of osteoclast-mediated bone resorption and effective agents in the treatment of several bone diseases [3]. Ethylenediaminetetramethylene phosphonic acid (EDTMP) is actually the most common phonates for palliative care.
Unfortunately, EDTMP complexes have shown low in vivo stability and thus dissociate which result in liver accumulation and high toxicity [8, 9]. In order to prevent complex dissociation, high amounts of EDTMP should be injected (>1.5 mg/kg body weight) [10]. Besides, phosphonate groups in the ligand are involved in the complexation with metal ions and no free phosphonates will be available for binding to apatite [11].
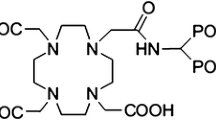
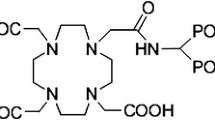
These requirements and restrictions of the first generation phosphonates, like EDTMP, have encouraged the researcher to evaluate recently synthesized ligands with improved properties [11]. (4-{[(bis(phosphonomethyl))carbamoyl]methyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid (BPAMD) (Fig. 1), as a new macrocyclic diphosphonate, in labeling with 68Ga, in first human studies showed promising results such as very high target-to-soft-tissue ratios and ultrafast clearance [12]. It has been shown that the BPAMD complex, as a new generation of DOTA bisphosphonate, can solve the mentioned limitations of the first generation phosphonates either in diagnostic or in therapeutic applications [11].
Radiopharmaceuticals with radionuclides such as 32P, 89Sr, 186Re, 188Re, 153Sm and 177Lu are developed for treatment of painful metastases. Among the therapeutic radionuclides, 177Lu with favourable physical characteristics [t 1/2 = 6.73 days, E βmax = 497 MeV, Eγ = 112 keV (6.4 %), 208 keV (11 %)], is one of the most promising radionuclides for this purpose and therefore, 177Lu-EDTMP has been used as an effective and safe radiopharmaceutical for palliation of metastatic bone pain [13].
Due to the interesting properties of BPAMD and 177Lu, 177Lu-BPAMD was introduced as a promising therapeutic compound after the only reported clinical study by Fellner et al. [14], but there is no published data in details on the preclinical studies of this compound until now. In this study, in continuation of developing BPAMD bone-seeking agents [11, 15], the radiolabeling, quality control, partition coefficient, hydroxyapatite (HA) binding assay, biodistribution studies and preliminary dosimetric evaluation of 177Lu-BPAMD are reported and compared with 177Lu-EDTMP as the only clinically used 177Lu bone-seeking agent.
Experimental
Production of 177Lu was performed at a research reactor using 176Lu (n,γ)177Lu nuclear reaction. 176Lutetium with purity of 52 % was obtained from ISOTEC Inc. BPAMD was purchased from ABX (Radeberg, Germany). All other chemical reagents were obtained from Sigma–Aldrich (Heidelberg, Germany). Whatman No. 2 paper was observed from Whatman (Buckinghamshire, UK). Radio-chromatography was performed by Whatman paper using a thin layer chromatography scanner (Bioscan AR2000, Paris, France). The activity of the samples was measured by a p-type coaxial high-purity germanium (HPGe) detector (EGPC 80-200R) coupled with a multichannel analyzer card system (GC1020-7500SL, Canberra, USA). Calculations were carried out based on the 112 keV peak for 177Lu. Planar images were taken by a dual head SPECT system (DST-XL, SMV, Buc, France). All values were expressed as mean ± standard deviation (Mean ± SD) and the data were compared using student T test. Animal studies were performed in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations, second edition.
Production and quality control of 177LuCl3 solution
In order to provide a suitable target, 1 mg of enriched 176Lu2O3 (176Lu, 52 % from ISOTEC Inc.) was weighed on a calibrated balance and dissolved in 1 mL of 0.1 M HNO3. Then a polystyrene tube was filled with the 100 µL of the above solution (containing 100 µg of the enriched 176Lu2O3) and located in the oven at 80 °C. The tube was placed in an aluminum can, sealed and irradiated in a research reactor at a thermal neutron flux of 5 × 1013 n cm−2 s−1 for 5 days. A stock solution of 177LuCl3 was produced by dissolving the irradiated target in 200 μL of 1.0 M HCl and diluted to the appropriate volume with ultra-pure water. The mixture was filtered through a 0.22 μm biological filter and sent for use in the radiolabeling step. The radionuclidic purity of the solution was performed utilizing beta spectroscopy as well as HPGe spectroscopy for the detection of various interfering beta and gamma emitting radionuclides. The radiochemical purity of the 177LuCl3 was checked by instant thin layer chromatography method (ITLC) using two solvent systems for ITLC [A: 10 mM DTPA pH 4 and B: ammonium acetate 10 %: methanol (1:1)].
Preparation and quality control of 177Lu-BPAMD
A stock solution of BPAMD was prepared by the dissolution of 1 mg of the ligand in 1 mL pure water. Approximately equal amounts (185 MBq) of 177LuCl3 were added to the 10-mL conical vials and dried under a flow of nitrogen. Distinct values of the complex ligands (100–400 nmol) were added to the lutetium-containing vials. By varying pH (2–12) and temperature (50–100 °C), the effect of these parameters was investigated based on ITLC results. For radio thin layer chromatography, a 5 µL sample of the final fraction was spotted on a chromatography Whatman No. 2 paper, and developed in various mobile phase mixtures [ammonium hydroxide:methanol:water (0.2:2:4; v/v/v), 0.1 M sodium citrate (pH 4) and 10 % ammonium acetate:methanol (1:1)].
Preparation and quality control of 177Lu-EDTMP
177Lu-EDTMP was prepared according to the previously reported procedure [16]. Briefly, a stock solution of EDTMP was prepared by dissolving in 1 M NaOH and diluted to the appropriate volume with ultrapure water, in order to produce a solution of 50 mg/mL. An appropriate amount of the 177LuCl3 solution containing the required activity was added to the specified amount of EDTMP solution (the amount of EDTMP was selected to have Lu/EDTMP molar ratio of 1:10). The complex solution was then kept at room temperature for 60 min, while the pH was adjusted to 7 with 0.05 M phosphate buffer. The radiochemical purity was determined using Whatman No. 2 chromatography paper and NH4OH:methanol:water (0.2:2:4; v/v/v) mixture.
Stability studies of 177Lu-BPAMD and 177Lu-EDTMP
The stability of the complexes were checked by the conventional ITLC method. A sample of both 177Lu-BPAMD and 177Lu-EDTMP (approximately, 37 MBq) were kept at room temperature for 168 h while being checked by ITLC at the specified time intervals (2, 4, 24, 48, 72 and 168 h). For serum stability studies, 37 MBq of labelled complexes were added to 500 µL of freshly collected human serum and the resulting mixtures were incubated at 37 °C for 72 h, aliquots (5-µL) were analyzed by ITLC method at 2, 4, 24, 48 and 72 h after preparation.
Hydroxyapatite binding assay of 177Lu-BPAMD and 177Lu-EDTMP
HA binding assays were performed according to the procedure described previously [17], with only a slight modification. In brief, to vials containing, 2.5, 5.0, 10.0, 15.0, 20.0, 25.0 and 50.0 mg of solid HA, 2 mL of saline solution of pH 7.4 were added and the mixtures were shaken for 2 h. Then, 50 µL of the radioactive preparations was added and the mixtures were shaken for 24 h at room temperature. The suspensions were centrifuged, and two aliquots of the supernatant liquid were taken from each vial and the radioactivity was measured with a well-type counter. Test experiments were performed using a similar procedure, but in the absence of HA. The percentage binding of 177Lu to HA was calculated according to HB = 1 − A/B × 100, where A is the mean radioactivity value of the supernatant sample under study and B is the mean total value of whole activity used.
Partition coefficient determination of 177Lu-BPAMD
Partition coefficient of 177Lu-BPAMD was calculated followed by the determination of P (the ratio of specific activities of the organic and aqueous phases). A mixture of 1 mL of 1-octanol and 1 mL of isotonic acetate-buffered saline (pH 7) containing approximately 7.4 MBq of the radiolabeled complex at 37 °C was vortexed 1 min and left 5 min. Following centrifugation at >1200×g for 5 min, the octanol and aqueous phases were sampled and counted in an automatic well-type counter. A 500 µL sample of the octanol phase from this experiment was shaken again three times with fresh buffer samples. The reported logP values are the average of the extractions from three independent measurements.
Biodistribution of 177LuCl3 and the radiolabeled complexes in Syrian mice
The distribution of 177LuCl3 and the radiolabeled complex among tissues was determined in male Syrian mice [with around 18 weeks old and mean weight of 35.61 g]. For this purpose, a distinct volume of the 177LuCl3 stock solution was evaporated under a flow of nitrogen gas and diluted to the appropriate volume by the addition of 0.9 % NaCl. 100 μL of the final solutions of 177LuCl3 and the radiolabeled complexes (177Lu-BPAMD and 177Lu-EDTMP) with approximately 3.7 MBq radioactivity was injected intravenously to the mice through their tail vein. The total amount of radioactivity injected into each animal was measured by counting the 1-mL syringe before and after injection in a dose calibrator with fixed geometry.
The animals were sacrificed at selected times after injection (2, 4, 24, 48, 72 and 168 h) using the animal care protocols. Blood samples were rapidly taken from the mice after scarification. The tissues (bladder, kidney, liver, spleen, lung, stomach, pancreas, intestine, colon, femur, skull, heart, brain, muscle, thyroid, adrenal, skin and fat) were weighed and rinsed with normal saline and their activities were determined with a p-type coaxial HPGe detector coupled with a multichannel analyser according to Eq. 1 [18]:
where ε is the efficiency at photopeak energy, γ is the emission probability of the gamma line corresponding to the peak energy, t s is the live time of the sample spectrum collection in seconds, m is the mass (kg) of the measured sample, k 1, k 2, k 3, k 4, and k 5, are the correction factors for the nuclide decay from the time the sample is collected to start the measurement, the nuclide decay during counting period, self-attenuation in the measured sample, pulses loss due to random summing and the coincidence, respectively. N is the corrected net peak area of the corresponding photopeak given as:
where N s is the net peak area in the sample spectrum, N b is the corresponding net peak area in the background spectrum and t b is the live time of the background spectrum collection in seconds.
The percentage of injected dose per gram (%ID/g) for different organs was calculated by dividing the activity amount of each tissue (A) to the decay-corrected injected activity and the mass of each organ. Five mice were sacrificed for each time interval. All values were expressed as mean ± SD and the data were compared using Student’s T test.
Imaging studies of 177Lu-BPAMD
7.4 MBq of 177Lu-BPAMD was injected intravenously into a Syrian rat through their tail vein. The rat was anesthetized using xylazine–ketamine mixture. Planar images were taken at 2 and 168 h after administration of the radiolabeled complex by a dual-head SPECT system. The rat-to-high energy septa distance was 12 cm. The useful field of view was 540 × 400 mm2.
Results
Production and quality control of 177LuCl3 solution
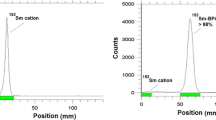
177Lu was prepared with specific activity of 90–100 GBq/mg and radionuclidic purity of >99.9 % (Fig. 2). Radiochemical purity of the 177LuCl3 solution was checked using Whatman No. 2 paper and in two solvent systems (Fig. 3). In a mixture of 10 mM DTPA solution (pH 5), free 177Lu3+ cation is converted to more lipophilic 177Lu-DTPA form and migrates to higher R f (R f 0.8). In the case of 10 % ammonium acetate:methanol (1:1), 177Lu3+ remains at the origin while other ionic species would migrate to higher R fs.
Preparation and quality control of 177Lu-BPAMD
In order to obtain maximum labelling yield, several experiments were carried out by the variation of different reaction parameters. The results indicated the growth of labelling yield with increasing the amount of BPAMD and reached above 93 % by adding 200 µL (346 nmol) of the ligand. It was observed that at the temperature range of 90–100 °C, the maximum complexation yield would be achievable. The effect of pH on the labelling yield was also studied by varying the pH of the reaction mixture from 2 to 12 using 1 M HCl or 2 M NaOH solution. Maximum yield was observed at pH 5–7 while decreased beyond this range. The reaction mixture was incubated at 100 °C temperature for different time periods and 60 min incubation was found to be adequate to yield maximum complexation. The variation of labelling yield with the ligand concentration of BPAMD, temperature and time has been shown in Figs. 4, 5 and 6, respectively.
Different chromatographic systems were used for the detection of the radiolabeled compound from the free cation. The best ITLC mobile phase was considered by Whatman No. 2 paper using NH4OH:MeOH:H2O (0.2:2:4). In this mixture, free cation remains at the origin of the paper as a single peak, while the radiolabeled compound migrates to higher R f (0.7–0.8) (Fig. 7).
Preparation and quality control of 177Lu-EDTMP
Several experiments were carried out by the variation of different reaction parameters to obtain optimized labelling conditions. The variation of labelling yield with the ligand concentration of EDTMP and time has been shown in Figs. 4 and 6, respectively. The ITLC results using Whatman No. 2 paper and NH4OH:MeOH:H2O (0.2:2:4) as the mobile phase indicated a single radiopeak at R f of 0.9 which is attributed to 177Lu-EDTMP complex. Radiochemical purity of more than 99 % was observed at the selective conditions (Lu/EDTMP molar ratio of 1:10, pH 7, at room temperature and incubation time of 60 min).
Stability studies of 177Lu-BPAMD and 177Lu-EDTMP
The stability of the complexes was investigated in room temperature and in human serum at 37 °C. The radiochemical purity of 177Lu-BPAMD and 177Lu-EDTMP remained >92 and >98 % after 168 h at room temperature. Incubation of labelled complexes in freshly prepared human serum at 37 °C for 72 h showed approximately no loss of 177Lu from the complexes.
Hydroxyapatite binding assay of 177Lu-BPAMD and 177Lu-EDTMP
HA assay demonstrated high capacity binding for 177Lu-BPAMD to HA. Even at 2.5 mg amount of HA, more than 92 % binding was observed. At further amounts of HA, approximately, all radiolabeled complex was bound to HA. For 177Lu-EDTMP, at 10 mg amount of HA, more than 93 % binding was observed (Fig. 8).
Partition coefficient determination of 177Lu-BPAMD
The partition coefficient (P o/w) was calculated by dividing the counts in the octanol phase by those in the buffer, and the results expressed as LogP o/w. The average value from three independent measurements for 177Lu-BPAMD was −1.93 ± 0.04 witch highlight the strong hydrophilic nature of the complex.
Biodistribution of 177LuCl3 and the radiolabeled complexes in Syrian mice
The percentage of injected dose per gram in mice organs up to 48 h after injection of 177LuCl3 (3.7 MBq/100 µL) solution was determined (Fig. 9). According to the results, most of the activity is accumulated in the liver and spleen.
Biodistribution of 177Lu-BPAMD and 177Lu-EDTMP after intravenously injection of 3.7 MBq into Syrian mice is demonstrated in Figs. 10 and 11, respectively. Based on the obtained results, it is clearly concluded that the major portion of the injected activity of the complexes are transferred from the blood circulation into the bones.
Imaging studies of 177Lu-BPAMD
Planar images were taken from normal rat 2 and 168 h after administration of the radiolabeled complex (Fig. 12). Imaging of the Syrian rat showed a distinct accumulation of the radiotracer in the skeletal region 2 and 168 h after injection.
Discussion
Recently, various bone-seeking agents utilizing different beta emitter radionuclides have been reported for bone pain palliation. Among these complexes, 153Sm-EDTMP is actually the most common administered bone targeting agent for palliative care. Although, the results of using this complex in clinical studies indicated significant pain relief in metastatic patients but high amounts of 152Sm in the complex can result in lower therapeutic efficiency. As a consequence, new compounds such as 177Lu-EDTMP with improved characteristics were developed [19].
177Lu with considerable characteristics has been recognized as one of the most promising radionuclides. The significant advantage of using 177Lu is its β− particle energies which are adequately low, therefore the bone marrow suppression is minimum when it accumulates in skeletal lesions [20]. On the other hand, 177Lu can be produced with high specific activity via the indirect method by neutron irradiation of 176Yb. However, different complexes of 177Lu with bisphosphonates have been developed, but 177Lu-EDTMP demonstrated greater uptake in bone compared to the other tissue and nowadays is clinically used as an encouraging bone pain palliation therapeutic agent [21]. However, as mentioned before, some restrictions of EDTMP led to the development of the new bone-seeking complexes.
In this study, 177Lu-BPAMD was prepared as a novel bone pain palliative agent. For better evaluation of the complex, the obtained results were compared with those for 177Lu-EDTMP. The optimized conditions for the preparation of these two complexes were studied by several experiments. As shown in Fig. 4, the radiochemical purity of higher than 93 % was observed for 177Lu-BPAMD complex at the ligand concentration of 346 nmol, while for the achievement of approximately the same radiochemical purity for 177Lu-EDTMP complex more than 4500 nmol of EDTMP is required. The results showed that the increment of temperature led to the growth of radiochemical purity of 177Lu-BPAMD and reached to above 93 % at 90–100 °C. Since the maximum labelling yield of 177Lu-EDTMP was achievable at room temperature, the effect of temperature was not studied in this case. Also, the results demonstrated that for both of complexes, radiochemical purity of higher than 90 % was accessible after 1 h.
The comparison of the HA binding for two complexes showed that 177Lu-BPAMD could bind to HA more than 177Lu-EDTMP at any amount of HA. However, the binding percentages approach together at the amount of higher than 20 mg of HA. It can be concluded that 177Lu-BPAMD has more affinity to HA in vitro.
Based on the biodistribution study of 177Lu-BPAMD in the male Syrian mice, the major portion of the injected activity of the complex is accumulated into bones which increases to 9.77 % at 24 h post injection and remains approximately constant up to 168 h. Rapid removal from the blood circulation is observed after 177Lu-BPAMD injection. The radioactivity is significantly excreted from kidneys as anticipated due to the water solubility of the compound. In fact the major route of excretion for the labelled compound is through the urinary tract. Low accumulation is indicated in the liver and also gastrointestinal system (intestines, colon).
For better evaluation, biodistribution of 177LuCl3 was also studied in the male Syrian mice. The results indicate low blood radioactivity which show the rapid removal of 177Lu from the blood circulation. Significant uptake of the cation is perceived in the liver and spleen especially after 48 h of injection. Little amount of radioactivity is accumulated in the bone. The organs uptake for 177Lu-BPAMD and 177LuCl3 demonstrate completely different pattern for both species especially for vital organs which represent the high in vivo stability of the radiolabeled complex.
Since, the biodistribution of an agent may show some discrepancy between different type animals, for more accurate comparison, 177Lu-EDTMP was also produced and its biodistribution was investigated in the same type mice. Comparison between the biodistribution data for 177Lu-EDTMP and 177Lu-BPAMD demonstrate higher bone uptake after 177Lu-BPAMD injection, although similar accumulation pattern is observed for both of the complexes (Figs. 7, 8). Besides, target/non target uptake ratio for both of the complexes was calculated which indicate higher values for 177Lu-BPAMD (Tables 1, 2).
Conclusions
The radiolabeled 177Lu-BPAMD complex was prepared in high radiochemical purity (>93 %, ITLC) and specific activity of 534 GBq/mmol. Labeling and quality control took 60 min. The complex demonstrated significant stability at room temperature and in human serum at least for 72 h. HA binding assay demonstrated that at the amount of more than 5 mg, approximately, all radiolabeled complex was bound to HA. At the pH of 7.4, LogP o/w was −1.93 ± 0.04. Both planar imaging and biodistribution study showed major accumulation of the labelled compound in the bone tissue. Comparative biodistribution study between 177Lu-BPAMD and 177Lu-EDTMP indicated higher bone uptake and target/non target uptake ratio for 177Lu-BPAMD. Generally, 177Lu-BPAMD was prepared easily showing satisfactory results and can be considered as a viable alternative for EDTMP-based bone pain palliative radiopharmaceuticals in near future.
References
Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, Frei E (2003) Holland-Frei Cancer Medicine. 6th edn. Hamilton (ON), BC Decker
IAEA-TECDOC-1549 (2007) Criteria for palliation of bone metastases—clinical applications, IAEA, Austria, Vienna
Lipton A (2004) Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol 2:205–220
Chow E, Harris K, Fan G, Tsao M, Sze WM (2007) Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 25:1423–1436
Eary JF, Collins C, Stabin M, Vernon C, Petersdorf S, Baker M, Hartnett S, Ferency S, Addison SJ, Appelbaum F, Gordon EE (1993) 153Samarium-EDTMP biodistribution and dosimetry estimation. J Nucl Med 34:1031–1036
Rajendran JG, Eary JF, Bensinger W, Durack LD, Vernon C, Fritzberg A (2002) High-dose 166Ho-DOTMP in myeloablative treatment of multiple myeloma: pharmacokinetics, biodistribution, and absorbed dose estimation. J Nucl Med 43:1383–1390
Farhanghi M, Holmes RA, Volkert WA, Logan KW, Singh A (1992) 153Samarium-EDTMP: pharmacokinetic, toxicity and pain response using an escalating dose schedule in treatment of metastatic bone cancer. J Nucl Med 33:1451–1458
Kálmán FK, Király R, Brücher E (2008) Stability constants and dissociation rates of the EDTMP complexes of samarium(III) and yttrium(III). Eur J Inorg Chem 30:4719–4727
Beyer GJ, Offord R, Künzi G, Aleksandrova Y, Ravn U, Jahn S, Barker J, Tengblad O, Lindroos M (1997) The influence of EDTMP concentration on the biodistribution of radio-lanthanides and 225Ac in tumor-bearing mice. Nucl Med Biol 24:367–372
Fellner M, Riss P, Loktionova N, Zhernosekov KP, Thews O, Geraldes CFGC, Kovacs Z, Lukeš I, Rösch F (2011) Comparison of different phosphorus-containing ligands complexing 68Ga for PET-imaging of bone metabolism. Radiochim Acta. 99:43–51
Fellnera M, Biesalski B, Bausbacher N, Kubícek V, Hermann P, Rösch F, Thews O (2012) 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol 39:993–999
Fellner M, Baum RP, Kubícek V, Hermann P, Lukeš I, Prasad V, Rösch F (2010) PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: first human study. Eur J Nucl Med Mol Imaging 37:834
Yuan J, Liu C, Liu X, Wang Y, Kuai D, Zhang G, Zaknun JJ (2013) Efficacy and safety of 177Lu-EDTMP in bone metastatic pain palliation in breast cancer and hormone refractory prostate cancer: a phase II study. Clin Nucl Med 38:88–92
Fellner M, Baum R, Kubicek V, Hermann P, Roesch F (2010) 177Lu-BPAMD-From bone imaging to therapy with a macrocycle-bisphosphonate ligand. J Nucl Med 51:1164
Yousefnia H, Amraei N, Hosntalab M, Zolghadri S, Bahrami-samani A (2015) Preparation and biological evaluation of 166Ho-BPAMD as a potential therapeutic bone-seeking agent. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3924-1
Bahrami-Samania A, Anvari A, Jalilian AR, Shirvani-Arania S, Yousefniaa H, Aghamiri MR, Ghannadi-Maragheh M (2012) Production, quality control and pharmacokinetic studies of 177Lu-EDTMP for human bone pain palliation therapy trials. Iran J Pharm Res 11:137–144
Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M, Rosado M, Fausto R (2002) Synthesis, characterization and biodistribution of bisphosphonates 153Sm complexes: correlation with molecular modeling interaction studies. Nucl Med Biol 29:329–338
IAEA-TECDOC-1401. (2004) Quantifying uncertainty in nuclear analytical measurements, IAEA, Austria, Vienna
Meckel M. (2014) Macrocyclic bisphosphonates for PET-diagnosis and endoradiotherapy of bone metastases. http://d-nb.info/1062595645/34
Deligny CL, Gelsema WJ, Tji TG, Huigen YM, Vink HA (1990) Bone seeking radiopharmaceuticals. Nucl Med Biol 17:161–791
Agarwal KK, Singla S, Arora G, Bal C (2014) 177Lu-EDTMP for palliation of pain from bone metastases in patients with prostate and breast cancer: a phase II study. Eur J Nucl Med Mol Imaging 42:79–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yousefnia, H., Zolghadri, S., Sadeghi, H.R. et al. Preparation and biological assessment of 177Lu-BPAMD as a high potential agent for bone pain palliation therapy: comparison with 177Lu-EDTMP. J Radioanal Nucl Chem 307, 1243–1251 (2016). https://doi.org/10.1007/s10967-015-4225-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4225-z