Abstract
In this work 166Ho-(4-{[(bis(phosphonomethyl))carbamoyl]methyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl) acetic acid (166Ho-BPAMD) complex was prepared successfully with sufficient radiochemical purity of >94 % and specific activity of 244 GBq/mmol at the optimized conditions. The complex demonstrated significant stability at room temperature and in human serum at least for 24 h. Hydroxyapatite (HA) binding assay demonstrated that even at the small amount of HA, >10 mg, above 98 % of the complex is bound to HA. At the pH 7.4, LogP0/w was −1.73 ± 0.02. Both planar imaging and biodistribution studies showed major accumulation of the labelled compound in the bone tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma is an aggressive plasma cell malignancy arising from plasma cells in the bone marrow with fatal consequence [1]. Nowadays, high-dose chemotherapy [2] and total body irradiation [3] are known as more intensive treatment regiments for disease control of patients are resistant to standard-dose chemotherapy as a standard treatment for multiple myeloma [4]. It has been shown that the addition of skeletal targeted radiotherapy to the patients can improve the response rate in phase I and II trials, with promising long-term survival data [5]. Radionuclides with high β− particle energies are recommended for bone marrow ablation in patients with multiple myeloma.
Over the past 30 years, bisphosphonates were established as an effective new drug class for bone pain palliation in patients with different complicated malignancies such as prostate, breast and lung cancer [6]. These molecules have a strong affinity for binding to hydroxyapatite (HA) which is the main component of the inorganic matrix of bone (69 %). As a result, bisphosphonates can be used as special agents for selective bone delivery to treat bone-related pathologies.
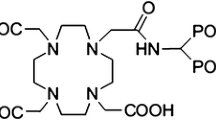
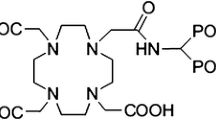
Some requirements and restrictions of the first generation phosphonates have encouraged the researcher to evaluate recently synthesized ligands with improved properties [7]. (4-{[(bis(phosphonomethyl))carbamoyl]methyl}-7,10-bis(carboxymethyl)-1,4,7,10 tetraazacyclododec-1-yl)acetic acid (BPAMD) (Fig. 1), as a new macrocyclic diphosphonate, in labeling with 68Ga, in first human studies showed promising results such as very high target-to-soft-tissue ratios and ultrafast clearance [8]. The DOTA-based bisphosphonate ligand BPAMD may also be suitable for complexation with therapeutic radionuclides such as 166Ho.
Nowadays, radiopharmaceuticals with radionuclides such as 32P, 89Sr, 186Re, 188Re, 153Sm and 177Lu are developed for treatment of painful metastases. Among the therapeutic radionuclides, 166Ho due to the high energy of the β− particles [1.85 MeV (51 %) and 1.77 MeV (48 %)] [9] is an excellent radionuclide for bone marrow ablation. Also, it’s physical half-life (26.8 h) is short enough to permit delivery of high-dose chemotherapy and reinfusion of cryopreserved peripheral blood stem cells within 6–10 days [10]. A low abundance (6.6 %) of 81-keV photons of 166Ho is convenient for SPECT imaging to determine the biodistribution and radiation absorbed dose in the organs.
Due to these special characteristics, various therapeutic possible 166Ho bone-seeking agents have been reported such as 166Ho-DOTMP [10], 166Ho-TTHMP [11], 166Ho-EDTMP [12] and 166Ho-APDDMP [13]. In addition to these complexes, 166Ho-DOTP seems to be an effective agent for bone marrow ablation in multiple myeloma patients [14]. However, the search for the development of new 166Ho-bisphisphonate ligands with higher stability, better pharmacokinetics and lower unwanted tissue uptakes (liver and GI) is still ongoing.
In this research, due to the interesting properties of BPAMD and 166Ho, the idea of developing a possible therapeutic bone-avid agent for bone-marrow ablation in multiple myeloma patients, 166Ho‐BPAMD, was investigated. Radiolabeling, quality control, partition coefficient, HA binding assay and biodistribution studies using planar and scarification, after injection of the complex to Syrian mice are reported.
Experimental
Materials and instruments
Production of 166Ho was performed at a Research Reactor using 165Ho(n,γ)166Ho nuclear reaction. Natural holmium nitrate with purity of >99.99 % was obtained from ISOTEC Inc. BPAMD was purchased from ABX (Radeberg, Germany). All other chemical reagents were purchased from Sigma-Aldrich Chemical Co. U.K. Whatman No. 2 paper was obtained from Whatman (UK). Radio-chromatography was performed by Whatman paper using a thin layer chromatography scanner, Bioscan AR2000, Paris, France. The activity of the samples was measured by a p-type coaxial high-purity germanium (HPGe) detector (model: EGPC 80-200R) coupled with a multichannel analyzer card system. Calculations were based on the 80.6 keV peak for 166Ho. All values were expressed as mean ± standard deviation (Mean ± SD) and the data were compared using Student t test. Animal studies were performed in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations, second edition.
Production and quality control of 166HoCl3 solution
166Ho was produced by neutron irradiation of 100 µg of natural 165Ho(NO3)3 (165Ho, 99.99 % from ISOTEC Inc.) according to reported procedures [15] at a research reactor at a thermal neutron flux of 5 × 1013 n cm−2 s−1. Specific activity of the produced 166Ho was 7.5 GBq/mg after 2 days of irradiation. The irradiated target was dissolved in 200 μl of 1.0 M HCl, to prepare 166HoCl3 and diluted to the appropriate volume with ultra-pure water, to produce a stock solution. The mixture was filtered through a 0.22 μm biological filter and sent for use in the radiolabeling step. The radionuclidic purity of the solution was tested for the presence of other radionuclides using beta spectroscopy as well as HPGe spectroscopy for the detection of various interfering beta and gamma emitting radionuclides. The radiochemical purity of the 166HoCl3 was checked using two solvent systems for ITLC (A: 10 mM DTPA pH 4 and B: ammonium acetate 10 %: methanol (1:1)).
Preparation of quality control of 166Ho-BPAMD
In order to obtain maximum complexation yields, several experiments were carried out and the effect of various parameters on the labeling yield of 166Ho-BPAMD including ligand concentration, pH, temperature and reaction time were studied.
Briefly, 1 mg of BPAMD was dissolved in 1 ml pure water and the aqueous solution was used for labelling studies. Typically, equal amounts (74 MBq) of 166HoCl3 (in 0.2 M HCl) were added to the 10-ml conical vials and dried under a flow of nitrogen. Distinct values of the complex ligands (100–400 nmol) were added to the Holmium-containing vials. By varying pH (2–12) and temperature (50–100 °C), the effect of these parameters was investigated based on ITLC results. The optimal procedure for the preparation of 166Ho-BPAMD complex with a high labeling yield was obtained.
For radio thin layer chromatography (RTLC), a 5 μl sample of the final fraction was spotted on a chromatography Whatman No. 2 paper, and developed in various mobile phase mixtures.
Stability studies
The stability of the complex was checked according to the conventional ITLC method. A sample of 166Ho-BPAMD (approximately, 18.5 MBq) was kept at room temperature for 24 h while being checked by ITLC at time intervals in order to check stability in final product using chromatography system. For serum stability studies, 18.5 MBq of labelled complex was added to 500 μl of freshly collected human serum and the resulting mixture was incubated at 37 °C for 48 h, aliquots (5-μl) were analyzed by ITLC.
Hydroxyapatite binding assay
HA binding assay was performed according to the procedure described previously [16], with only a slight modification. In brief, to vials containing 5.0, 10.0, 15.0, 20.0, 25.0 and 50.0 mg of solid HA, 2 ml of saline solution of pH 7.4 were added and the mixtures were shaken for 3 h. Then, 100 μl of the radioactive preparation was added and the mixtures were shaken for 24 h at room temperature. The suspensions were centrifuged, and two aliquots of the supernatant liquid were taken from each vial and the radioactivity was measured with a well-type counter. Test experiments were performed using a similar procedure, but in the absence of HA. The percentage binding of 166Ho to HA was calculated according to HB = 1−A/B × 100, where A is the mean radioactivity value of the supernatant sample under study and B is the mean total value of whole activity used.
Determination of partition coefficient
Partition coefficient of 166Ho-BPAMD was calculated followed by the determination of P (the ratio of specific activities of the organic and aqueous phases). A mixture of 1 ml of 1-octanol and 1 ml of isotonic acetate-buffered saline (pH 7) containing approximately 3.7 MBq of the radiolabeled complex at 37 °C was vortexed 1 min and left 5 min. Following centrifugation at >1,200 g for 5 min, the octanol and aqueous phases were sampled and counted in an automatic well-type counter. A 500 μl sample of the octanol phase from this experiment was shaken again two to three times with fresh buffer samples. The reported log P values are the average of the second and third extractions from three to four independent measurements.
Biodistribution of 166HoCl3 and radiolabeled complex in Syrian mice
The distribution of 166HoCl3 and radiolabeled complex (2, 4, 24 and 48 h) among tissues was determined in Syrian mice. The total amount of radioactivity injected into each animal was measured by counting the 1-ml syringe before and after injection in a dose calibrator with fixed geometry.
The animals were sacrificed using the animal care protocols at selected times after injection. Blood samples were rapidly taken from the rodent aorta after scarification. The tissues (heart, lung, intestine, skin, stomach, kidneys, spleen, liver, muscle and thigh bone) were weighed and rinsed with normal saline and their specific activities were determined with an HPGe detector equipped with a sample holder device as a percent of injected dose per gram of tissues. It should notify that the thigh bone have more marrow than skull bone.
Imaging of Syrian mice
Images were taken from normal wide-type mice 24 and 48 h after administration of the radiolabeled complex by a dual-head SPECT system. The mouse-to-high energy septa distance was 12 cm. The useful field of view (UFOV) was 540 × 400 mm2.
Results and discussion
Production and quality control of 166HoCl3 solution
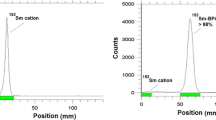
The 166Ho radionuclide in specific activity of 7.5 GBq/mg and radionuclidic purity of >99.9 was prepared for radiolabeling. The radioisotope was diluted and evaporated to obtain the desired pH and volume followed by sterile filtering. Radiochemical impurities in the 166Ho sample used in the radiolabeling step were checked by two solvent systems (Fig. 2); A, a mixture of 10 mM DTPA solution (pH 5) as the mobile phase on Whantman No. 2 paper, the free holmium cation in 166Ho3+ form, was chelated with the polydentate eluting leading to the migration of the cation in 166Ho-DTPA form to higher Rf (Rf 0.9), any other ionic species would lead to the observation of new radiopeaks, especially at the origin. B, a mixture of 10 % ammonium acetate:methanol (1:1) was used as another solvent system on the Whatman No. 2 paper, 166Ho3+ remains at the origin using this system while other ionic species would migrate to higher Rfs.
Labeling optimization studies
In order to obtain maximum complexation yield, several experiments were carried out by varying different reaction parameters such as ligand concentration, pH, and reaction time.
Labeling yield increased with increasing BPAMD concentration and reached above 94 % by adding 175 μl (303 nmol) of the ligand. It was observed that at the temperature range of 90–100 °C, the maximum complexation yield would be achievable. The effect of variation of pH on complexation was also studied by varying the pH of the reaction mixture from 2 to 12 using 1 M HCl or 2 M NaOH solution. Maximum yield was observed at pH 5–7 while decreased beyond this range. The reaction mixture was incubated at 100 °C temperature for different time periods and 45 min incubation was found to be adequate to yield maximum complexation.
Based on the obtained results, the optimal procedure for the preparation of 166Ho-BPAMD complex with a high labeling yield is as follows.
1 milligram of BPAMD was dissolved in 1 ml pure water and the aqueous solution was used for labelling studies. 74 MBq of 166HoCl3 were added to the 10-ml conical vials and dried under a flow of nitrogen. 175 μl (303 nmol) of the stock solution was added to the holmium-containing vials and the pH was adjusted to 6. The mixture was incubated for 45 min at 100 °C.
Different chromatographic systems were used for the detection of the radiolabeled compound from the free cation. The best ITLC mobile phase was considered by Whatman No. 2 paper using NH4OH:MeOH:H2O (0.2:2:4). Using this mixture, free cation remains at the origin of the paper as a single peak, while the radiolabeled compound migrates to higher Rf (0.7–0.8) (Fig. 3). ITLC studies approved the production of a single radiolabeled compound.
Stability tests
The chemical stability of 166Ho-BPAMD was high enough to perform further studies. Incubation of labelled complex in freshly prepared human serum for 24 h at 37 °C showed no loss of 166Ho from the complex. The radiochemical purity of the complex remained >94 % for 24 h under physiologic conditions.
Hydroxyapatite binding assay
HA assay demonstrated high capacity binding for 166Ho-BPAMD to HA. Even at 5 mg amount of HA, more than 91 % binding was observed. At the further amounts of HA, >10 mg approximately, binding to HA reaches above 98 % (Fig. 4).
Partition coefficient of 166Ho-BPAMD
The partition coefficient (P0/w) was calculated by dividing the counts in the octanol phase by those in the buffer, and the results expressed as LogP0/w. The average value from three to four independent measurements for 166Ho-BPAMD was −1.73 ± 0.02 which highlight the strong hydrophilic nature of the complex as expected from the chemical formula (Fig. 1). The water solubility of the radiocomplex leads to less unnecessary uptakes in tissues including liver and fat and faster kidney wash-out.
Biodistribution of 166HoCl3 and radiolabeled complex in Syrian mice
Biodistribution study was performed for free Ho3+. As shown in Fig. 5, for 166Ho cation, the biodistribution was mainly accumulated in the liver, kidney and bone.
The distribution of injected dose in mice organs up to 48 h after injection of 166Ho-BPAMD (100 μCi/100 μl) solution was determined. The radiolabeled compound biodistribution is also demonstrated in Fig. 6.
The free cation is mainly soluble in water and it can be excreted via urinary tract. Low blood radioactivity content demonstrates the rapid removal of 166Ho from the circulation after injection. Since the metallic 166Ho is transferred in plasma in protein-bond form, the liver radioactivity uptake of the cation is high and the maximum uptake is seen in 4 h (about 2.5 %). The liver radioactivity uptake of the cation is comparable to other radio-lanthanides such as Yb, Sm and Tb [17]. Muscle and skin do not demonstrate significant 166Ho uptake was in accordance with other cations accumulation. A bone uptake is observed for 166Ho which remains almost constant until 24 h (1 %).
Based on the results obtained from the biodistribution study of the labelled complex, it was clearly concluded that the major portion of the injected activity of the complex was transferred from blood circulation into bones. The significant excretion of the radioactivity is observed for kidneys as anticipated due to the water solubility of the compound, the major route of excretion for the labeled compound is through the urinary tract. The liver however plays no significant role in metabolism (<0.4 %) and also lower GI (intestines, colon) uptake is observed. The removal of 166Ho-BPAMD from the circulation after injection occurs very fast.
The importance of an ideal bone-avid radiopharmaceutical, especially with therapeutic applications, relays on the accumulation of the complex in bone compared to the critical organs such as liver and kidneys since the radiation imposed to these organs as well as secondary irradiation to the neighboring organs are important in developing a therapeutic agent. Therefore, for such radiopharmaceuticals, clearance from blood, accumulation in bone and the ratio of accumulated activity in bone: critical organs are parameters which should be considered. The ratios of bone/non-target organs for the radiolabeled complex are presented in Table 1.
For better comparison, a comparative study of vital organs uptake for 166Ho-BPAMD and 166HoCl3 was performed. As it can be seen in Figs. 5 and 6, the biodistribution data demonstrate different kinetic pattern for both species.
Low blood radioactivity content for both species demonstrates the rapid removal of 166Ho and labelled complex from the circulation after injection, however, 166Ho-BPAMD has lesser amount in all time intervals and it shows the labelled complex has ultrafast clearance from the blood.
Since both species are water soluble and excreted from the kidneys, the activity of the kidneys for 166Ho and labelled compound is rather high, especially in early hours. The activity of kidneys, for both of 166Ho-BPAMD complex and free cation, decreases slightly after injection until 48 h.
For 166Ho-BPAMD, the major radioactivity is accumulated in bones as expected for bone-avid agent. Bone activity for 166Ho cation is rather low all time intervals after injection.
166Ho cation is accumulated in the liver in the first 4 h post injection (2.5 %), while the accumulation of 166Ho-BPAMD in the liver is negligible all time after injection (<0.4 %).
Imaging of Syrian mice
Planar images were taken from normal wide-type mice 24 and 48 h after administration of the radiolabeled complex (Fig. 7). Imaging in the Syrian mice showed a distinct accumulation of the radiotracer in the skeletal region 24 and 48 h after injection.
Conclusion
The radiolabeled 166Ho complex was prepared in high radiochemical purity (>94 %, ITLC) and specific activity of 244 GBq/mmol. Labeling and quality control took 45 min. The complex demonstrated significant stability at room temperature and in human serum at least for 24 h. HA binding assay demonstrated that even at the small amount of HA, >10 mg, above 98 % of the complex is bond to HA. At the pH 7.4, LogP0/w was −1.73 ± 0.02. The final preparation was administered to Syrian mice and biodistribution of the complex was checked until 48 h post injection. Both planar imaging and biodistribution studies showed major accumulation of the labelled compound in the bone tissue. All tissues approximately have insignificant uptake in comparison with bone tissue. The results show that 166Ho-BPAMD has interesting characteristics as an agent for bone marrow ablation, however further biological studies in other mammals are still needed.
References
Child AH (2003) High dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875
Alexanian R, Barlogie B (1990) New treatment strategies for multiple myeloma. Am J Hematol 35:194–198
Rostom AY, O’Cathail SM, Folkes A (1984) Systemic irradiation in multiple myeloma: a report on nineteen cases. Br J Haematol 58:423–431
Cavo M, Gobbi M, Tura S (1981) Peptichemio in multiple myeloma, (preliminary results). Haematologica 66:208–215
Giralt S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R, Alexanian R, Weber D, Maloney D, Holmberg L et al (2003) 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase I/II trials. Blood 102:2684–2691
Palma E, Correia JDG, Campello MPC, Santos I (2011) Bisphosphonates as radionuclide carriers for imaging or systemic therapy. Mol BioSyst 7:2950–2966
Fellnera M, Biesalski B, Bausbacher N, Kubícek V, Hermann P, Rösch F, Thews O (2012) 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol 39:993–999
Fellner M, Baum RP, Kubícek V, Hermann P, Lukeš I, Prasad V et al (2010) PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: first human study. Eur J Nucl Med Mol Imaging 37:834
Calhoun JM, Cessna JT, Coursey BM, Hoppes DD, Schima FJ, Unterweger MP (1992) Standardization of holmium-166 by the CIEMAT/NIST liquid scintillation efficiency-tracing method. Radioact Radiochem. 2:38–45
Breitz HB, Wendt RE III, Stabin MS, Shen S, Erwin WD, Rajendran JG, Eary JF, Durack L, Delpassand E, Martin W et al (2006) 166Ho-DOTMP radiation-absorbed dose estimation for skeletal targeted radiotherapy. J Nucl Med 47:534–542
Yousefnia H, Zolghadri S, Jalilian AR, Tajik M, Ghannadi-Maragheh M (2014) Preliminary dosimetric evaluation of 166Ho-TTHMP for human based on biodistribution data in rats. Appl Radiat Isot 94:260–265
Pedraza-López M, Ferro-Flores G, de Murphy CA, Tendilla JI, Villanueva-Sánchez O (2004) Preparation of 166Dy/166Ho-EDTMP: a potential in vivo generator system for bone marrow ablation. Nucl Med Commun 25:615–621
Zeevaart JR, Jarvis NV, Louw WK, Jackson GE (2001) Metal-ion speciation in blood plasma incorporating the tetraphosphonate, N, N-dimethylenephosphonate-1-hydroxy-4-aminopropilydenediphosphonate (APDDMP), in therapeutic radiopharmaceuticals. J Inorg Biochem 83:57–65
Marques F, Gano L, Campello MP, Lacerda S, Santos I (2007) Biological evaluation of 153Sm and 166Ho complexes with tetraazamacrocycles containing methylcarboxylate and/or methylphosphonate pendant arms. Radiochim Acta 95:335–341
IAEA-TECDOC-1340 (2003) Manual for reactor produced radioisotopes. ISBN 92-0-101103-2, ISSN 1011-4289, IAEA, Austria
Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M et al (2002) Synthesis, characterization and bio-distribution of bisphosphonates 153Sm complexes: correlation with molecular modeling interaction studies. Nucl Med Biol 29:329–338
Du XL, Zhang TL, Yuan L, Zhao YY, Li RC, Wang K, Yan SC, Zhang L, Sun H, Qian ZM (2002) Complexation of ytterbium to human transferrin and its uptake by K562 cells. Eur J Biochem 269:6082–6090
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yousefnia, H., Amraei, N., Hosntalab, M. et al. Preparation and biological evaluation of 166Ho-BPAMD as a potential therapeutic bone-seeking agent. J Radioanal Nucl Chem 304, 1285–1291 (2015). https://doi.org/10.1007/s10967-014-3924-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3924-1