Abstract
A research program has been established between Bratislava and Prague groups to study natural and anthropogenic radionuclides in the environment using both Accelerator Mass Spectrometry (AMS) and radiometric methods. The first studies have focused on 14C activity variations in the atmosphere and biosphere with the aim to evaluate an impact of Czech and Slovak Nuclear Power Plants (NPP) on the environment, and on the development of AMS technique for investigation of actinides (mainly uranium isotopes) in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accelerator Mass Spectrometry (AMS) represents a revolution in analytical technologies for measurement of long-lived radionuclides when instead of waiting for decay products of radioactive nuclides (e.g. alpha-, beta-, or gamma-rays), they are analyzed directly mass-spectrometrically as they would be stable [1, 2]. For some long-lived radionuclides this change has improved the sensitivity of analysis by more than five orders of magnitude [3, 4]. These recent developments which also include other mass spectrometry methods (e.g. ICPMS [5]) have had great impact on applications of radionuclides as tracers of environmental processes which because of the lack of suitable samples (e.g. when too big samples were required), or because of limited analytical sensitivities, could not be realized in the past [2, 6]. In this way, new investigations in all environmental compartments, e.g. in the atmosphere, biosphere and hydrosphere, as well as a coupling of interactions in the geosphere could be carried out [7]. The AMS opened new investigations in isotope oceanography [8, 9], in climate change studies [10], in space science studies [11], and environmental research [12]. The recent developments in the AMS technologies may also be illustrated by another change in the philosophy of analysis represented by a transfer from a bulk sample analysis to specific compound analysis of stable and radioactive isotopes, very often using coupled analytical systems, (e.g. coupling of GC/MS with AMS) which will open new applications in environmental and biomedical sciences [13].
Although the AMS technology was originally developed for radiocarbon measurements, later applications included almost all long-lived radionuclides [1–5]. In this respect, specifically new isotopic tracers such as for example 236U have attracted interests in terrestrial and marine environment investigations [5, 8, 12], and naturally they have been part of the Prague–Bratislava collaboration project. Important part of these new developments included preparation of targets for ion sources of tandem accelerators with the aim to obtain maximum ion yields. In the analysis of 236U, the separation precision, minimization of contamination, and sample handling are the state of art of every AMS laboratory. The natural isotopic ratio 236U/238U ranges from 10−10 to 10−14, which causes natural 236U practically undetectable by conventional techniques [14]. On the other hand, the anthropogenic ratios could be as high as 10−3, what brings many challenges to sample preparation with natural isotopic ratios. The majority of published methods of AMS analysis deals with sample preparation as the crucial issue and the 236U is also the case; because of its abundance every even small advantage in measurement could highly improve the final result. Measurements of low isotopic ratios of 236U/238U demand improvements in AMS techniques, which could also be seen in applications of new target matrices. Many current methods of sample preparation for 236U/238U isotopic ratio determinations terminate with the conversion of uranium into oxides. The isobaric interferences of more abundant 235U17O− and 234U18O− ions may, however, require improvements in the target technologies. Recently, the development of fluoride target matrix has been presented, which could increase the ion source yields. In the same time, using monoisotopic fluorine significantly decreases the isobaric interferences. This matrix has already been used in AMS measurements of other radionuclides, however, more studies are necessary to allow and optimize this method for routine measurements [15–17].
Environmental radionuclide studies around the Czech and Slovak Nuclear Power Plants (NPP) have been of interest of both Bratislava and Prague groups for many years [18–21]. Radiocarbon has been of specific interest because of its long half-life (5,730 years) and its radioecological significance, because it may have highest contribution to radiation doses of public from operation of NPPs [22]. Actinides (mainly uranium and plutonium isotopes) on the other hand play an important role in the case of nuclear accidents, or long-term storage of radioactive wastes [5, 8, 23].
Two main methods of 14C monitoring around NPPs have been developed in the past. The most sensitive and precise one is a direct monitoring of atmospheric 14CO2 together with measurement of CO2 mixing ratio.Footnote 1 Utilising 14CO2 activitiesFootnote 2 and CO2 concentration data, the molar activity concentration can be calculated (i.e. 14CO2 quantity per molarFootnote 3 unit of the air). Such robust parameter is connected only with quantity of 14CO2 molecules in molar unit of air and cannot be influenced by Suess effect caused by dilution of 14C abundance in carbon isotopic mixture as a result of local/regional fossil CO2 emissions [21, 24]. In other words, CO2 molar activity concentration cannot be locally influenced by releases of fossil CO2, but only by emissions of 14CO2. A disadvantage of the (direct) molar activity concentration monitoring is higher operation cost, and necessity of special sampling and monitoring instruments [25, 26].
A biota based monitoring is cheaper and can be applied on a bigger scales, the resulting parameter is, however, only the 14C activity (i.e. abundance of 14C in carbon isotopic mixture). Such parameter can be influenced by local/regional Suess effect, which causes a dilution of 14C in carbon isotopic mixture by fossil CO2 emissions. This monitoring method requires therefore a reference site with a local Suess effect similar to that of a NPP site. When a suitable monitoring is applied, periods of biomass accumulation should be contemplated carefully. Biota samples, growing during spring/autumn, could represent well varying 14C levels as a result of different microclimatic conditions.
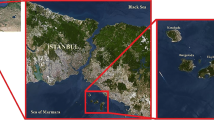
Recent developments in accelerator technologies and their applications in environmental sciences have been an inspiration for creation of a Centre for Nuclear and Accelerator Technologies (CENTA) at the Comenius University in Bratislava. The centre consists of a tandem accelerator designed for nuclear reaction studies and for ion beam analysis of environmental and material samples. A special interest has been devoted to AMS studies of long-lived radionuclides in environmental and life sciences, including evaluation of 14C levels around NPPs. A Prague–Bratislava program has been established with the aim to use the AMS technology for investigations of radionuclide variations in the environment. This paper is focusing on radiocarbon variation studies around the Czech (Temelín—2 × 1000 MWel and Dukovany—4 × 440 MWel) and Slovak (Jaslovské Bohunice—4 × 440 MWel) PWR based NPPs (Fig. 1) using radiometric and AMS techniques. We compare radiocarbon concentrations measured in atmospheric carbon dioxide with those in biota samples with the aim to study long-term contributions of NPPs on 14C levels in the environment. The development of a new method of target preparation for analysis of uranium isotopes in the environment by AMS is also presented.
Experimental
Radiocarbon (sampling and measurement)
Bratislava group
Long-term monitoring of 14C in atmospheric carbon dioxide has been carried out monthly in Žlkovce, a monitoring station located close to the Jaslovské Bohunice NPP [29, 30], and at the Bratislava university campus [31]. The method is based on bubbling the air through NaOH solution, precipitation of BaCO3 in the laboratory, liberation of CO2 by H3PO4, and finally preparation of CH4 (over a ruthenium catalyst) as a counting gas of proportional counters. A detailed description of sampling, preparation of gases and counting methods has already been described [32, 33].
Core sampling of tree rings from a lime tree (Tillia cordata) was also carried out in Žlkovce. Tree rings after separation under a microscope were dried, chemically cleaned (acid-basic-acid), combusted to CO2, and finally graphite targets were prepared for AMS measurements, which were carried out in the VERA laboratory of the Vienna University [34, 35].
Prague group
Monitoring of atmospheric 14CO2 is performed in the Czech Republic at two sites: Prague–Bulovka and Košetice. The site in Prague is near the border of the urban agglomeration of Prague, in the vicinity of a heavily used motorway; therefore, a local load from fossil fuel combustion can be expected there. The Košetice site is situated in the area of the Meteorological Observatory (a part of the Czech Hydrometeorological Institute). The surroundings of the Košetice site have an agricultural-forestry character, without significant local sources of fossil CO2. Monthly CO2 samples from both sites were collected by bubbling air through a 0.7 M NaOH solution in 1.9 L flasks (the final amount of carbonates did not exceed 30 % of the saturation capacity of the solution). Subsequently, samples were processed in laboratory by acid carbonate decomposition using H3PO4.
In the period of 2007–2008 samples of Urtica dioica were collected in the surroundings of Dukovany NPP–EDU and Temelín NPP–ETE (18 samples, without a preferred wind direction). Stinging nettle or common nettle is an herbaceous perennial flowering plant having an exiguous annual biomass supply and a thin root system. Vegetation period of this plant in the Central Europe is between the end of March and beginning of November. Distances of sampling points were in the interval of 0.7–1.2 km, where maximal 14C activity surplus can be expected. Reference samples (rEDU and rETE, 11 samples, 10–20 km from a given NPP) were collected in localities with estimated densities of roads and traffic loads similar to those in the vicinity of NPPs. Biota samples were washed with 10 % HCl and distilled water, dried at 105 °C, homogenized, and combusted to produce CO2.
Samples of resulting CO2 (both from exposed NaOH solution and from combustion of biota samples) were after purification processed by benzene synthesis [20, 21]. 14C activity was measured by liquid scintillation spectrometer Quantulus 1220 in 3–mL low-background Teflon vials.
Oxalic acid NIST standard reference material SRM 4990-C was used for calibration by both the Bratislava and Prague groups. Resulting 14C data are reported as Δ14C (in ‰), following Stuiver-Polach convention [27].
Uranium targets chemistry
In the analysis of 236U/238U mass ratios in environmental samples, the final sample preparation step has been usually a conversion of uranium into its oxides. Another possibility could be a conversion of uranium dioxide into anhydrous uranium tetrafluoride, which may therefore offer a higher isobaric suppression for molecular ions, together with a higher accuracy and sensitivity of uranium isotope analysis.
For the preparation of uranium tetrafluoride, uranyl nitrate, hydrofluoric and hydrochloric acid, ammonium hydrogen difluoride, and hydrazine hydrate solution (24–26 %) of p. a. commercial preparation were used. The preparation of uranium tetrafluoride was carried out following the published procedures [17, 36, 37].
Results and discussion
Radiocarbon around Czech NPPs
Time behaviour of atmospheric 14CO2 activity can be characterized by an interannual decrease since the beginning of 1990s, which is caused by global Suess effect [38, 39]. Seasonal fluctuations with minima during cold parts of the year are amplified by local and regional Suess effect, what is evident from the time series of atmospheric 14CO2 in Prague–Bulovka (Fig. 2). The observed 14CO2 activities approximate to reference values from clean-air high mountain station Jungfraujoch during summer months [39].
Basic statistical parameters of 14C monitoring in the vicinity of NPPs and in reference localities utilising nettle plant (Urtica dioica) samples (EDU, ETE, rEDU, rETE) are reported in Table 1. The results from each type of area were compared utilizing t test (unpaired, probability of first kind of observation error 5 %).
The observed difference of 5.4 ‰ in Δ14C (ETE−rETE) for nettle sampling is in a good agreement with observed difference of 4.8 ‰ of Δ14C (significant for 5 % probability of the first kind of observation error) obtained on the base of previous sampling campaign based on leaves of deciduous trees collection in years 2002–2005. Application of nettle plants as a sampling material also seems to reduce variations of 14C activities on half in comparison with tree leaves sampled during our previous campaign [40].
The observed variations in 14C activities for each type of locality are due to fluctuations in local Suess effect, which can be well visible during winter months (e.g., heating of houses, increased occurrence of atmospheric inversions). Another source of variations can be due to a relatively short time interval of biomass accumulation in tree leaves (about 4–5 weeks in April and May), when the activity of atmospheric 14CO2 changes relatively quickly (Fig. 2). The biomass accumulation in tree leaves also depends on local microclimatic conditions (atmospheric precipitations, soil moisture, and sunlight exposure), which can cause shifts in the period of atmospheric 14CO2 intake by tree leaves [40–42].
Radiocarbon around Slovak NPPs
Another possible sampling material for 14C monitoring around NPPs is represented by tree rings. Such type of sampling enables to reconstruct 14C chronology in a given locality, usually for several decades. To reach one-year time resolution, an analytical method based on AMS measurement (in which a sufficient sample of carbon quantity is only a few milligrams) is necessary. Results of 14C activity in tree rings sequence lime tree (Tillia cordata) collected at the Žlkovce monitoring station are presented in Fig. 3. Another advantage of the AMS based method of 14C activity determination is a smaller uncertainty of measurements (reduced by about a factor of two).
Due to wood biomass accumulation from May to September, a small decrease in the comparison with maximum activities of atmospheric 14CO2 (during the summer period) can be expected in tree ring samples [24], what is also documented in Fig. 3. Nevertheless, possible local variations of 14C activities caused by microclimatic differences (a time shift in biomass ingrowth) can be reduced for tree rings compared to leaves and other biota samples.
The atmospheric 14C record in the Žlkovce monitoring station (Fig. 3) indicates large variations. The deep winter minima are due to the local Suess effect, while during a year several 14C emissions from the Bohunice NPP can be identified. However, the total impact of the Bohunice NPP on 14C levels in Žlkovce, as represented both by the atmospheric 14CO2 and the tree-ring record, is negligible. The observed 14C levels are comparable with the high-altitude clean air station at Jungfraujoch, as well as with the surface air station at Košetice (Fig. 2).
The 14C tree-ring record is well averaging atmospheric 14C concentrations in CO2, documenting its suitability for long-term monitoring of 14C levels around NPPs.
Development of uranium targets for AMS
In the analysis of 236U/238U mass ratios in environmental samples using AMS, the final sample preparation step is usually a conversion of uranium into its oxides. Thereafter, with such oxide target matrix, the UO− molecular ions are used for the beam production. Recently, Wang et al. [17] studied preparation of fluoride matrix in the 236U/238U ratio determination, and showed a feasible method of conversion of uranium dioxide into anhydrous uranium tetrafluoride. In comparison with uranium oxide matrices, uranium fluoride targets contain no oxygen and hydrogen, and may therefore offer a higher isobaric suppression for molecular ions together with a higher accuracy and sensitivity of uranium isotope analysis. However, the preparation of anhydrous UF4 targets is more complicated than the preparation of uranium oxide targets, and the preparation procedure is associated with hazardous evaporation of concentrated hydrofluoric acid.
When reproducing the procedure of anhydrous uranium tetrafluoride preparation published by Wang et al. [17], the sample mass scan of AMS measurement clearly showed several peaks related to oxide molecular ions (Fig. 4). The ion current from only one UF2 − sample was in average higher by about 50 % than the UO− current from the uranium oxide samples. The targets were completely sputtered away, and the estimated ionization yields of UO− and UF2 − were of the order of 10−3. However, the improved procedure of the UF4 targets production could provide even higher ionization yields.
In order to prepare pure anhydrous uranium tetrafluoride, two more preparation procedures have been added into this research. Yeamans [36] published a simpler preparation procedure consisting of a contacting uranium dioxide with ammonium bifluoride, and a consequent combustion at the temperature around 400 °C. Sahoo and Satapathy [37] published probably the most suitable procedure among the others, because the entering uranium compounds could be in the +VI oxidation state. In this publication, the uranyl compound is mixed with hydrazine bifluoride and combusted at 400 °C. The uranium reduction takes place during the temperature increase. Samples of anhydrous uranium tetrafluoride were prepared using all three methods and then measured by X-ray powder diffraction. In every spectrum the uranium oxide diffraction lines of low intensities can be easily identified, and in addition, the diffraction patterns of uranium dioxide showed also the lines of metaschoepite ((UO2)4O(OH)6(H2O)5)) in which uranium is partially oxidised to higher oxidation state. In order to improve the purity of uranium tetrafluoride samples, a new uranium dioxide sample will be prepared and the influence of reducing atmosphere during the combustion will be also studied.
Conclusions
The main results presented in this paper may be summarized as follows:
-
(i)
Two biota-sampling campaigns around the NPPs in the Czech Republic (2002–2005, leaves of deciduous trees; 2007–2008, nettle plants) indicate an excess of 14C levels close to the border of statistical significance. The 14C levels in nettle samples collected in the vicinity of the Temelín NPP were about 5.4 ‰ above the average reference background value. Recently tree-ring samples around the Czech NPPs have also been collected, which are at present under analysis. We are also considering a regular 14C activity monitoring in atmospheric 14CO2 around NPPs.
-
(ii)
The 14C tree-ring record at the Žlkovce monitoring station (located close to the Bohunice NPP, Slovakia) has been well averaging atmospheric 14C concentrations in CO2, documenting its suitability for long-term monitoring of 14C levels around NPPs. The observed 14C levels were comparable with those observed at clean-air monitoring stations at Jungfraujoch (a high-altitude station in Swiss Alps) and at Košetice (a low-altitude station in Czech Republic).
-
(iii)
The development of uranium tetrafluoride targets for AMS analysis of 236U/238U mass ratio in environmental samples has shown promising results, but more work is needed to clearly demonstrate advantages of this technique.
Notes
CO2 mixing ratio means number of CO2 mol per one mole of air.
Molar units of air can also be replaced by normalized volume or weight units [25].
References
Tuniz C, Bird JR, Fink D, Herzog GF (1998) Accelerator mass spectrometry: ultrasensitive analysis for global science. CRC Press, Boca Raton
Kutschera W (2005) Intern J Mass Spectrom 242:145–160
Fifield LK (2008) In: Povinec PP (ed) Analysis of environmental radionuclides. Elsevier, Amsterdam
Jull AJT, Burr GS, Beck JW, Hodgins GWL, Biddulph DL, McHargue LR, Lange TE (2008) In: Povinec PP (ed) Analysis of environmental radionuclides. Elsevier, Amsterdam, pp 241–262
Lehto J, Hou XL (2010) Chemistry and analysis of radionuclides. Wiley, Weinheim
Povinec PP, Sanchez-Cabeza JA (eds) (2006) Radionuclides in the environment. Elsevier, Amsterdam
Povinec PP, Betti M, Jull AJT, Vojtyla P (2008) Acta Phys Slovaca 58:1–154
Povinec PP (2004) In: Livingston HD (ed) Marine radioactivity. Elsevier, Amsterdam
Povinec PP, Eriksson M, Scholten J, Betti M (2012) In: Annunziata M (ed) Handbook of radioactivity measurement. Elsevier, Amsterdam
Kutschera W (2010) Nucl Instrum Meth Phys Res B268:693–700
Jull AJT, McHargue LR, Bland PA, Greenwood RC, Revan AWR, Kim KJ, LaMotta SE, Johnson JA (2010) Meteor Planet Sci 45:1271–1283
Steier P, Dellinger F, Forstner O, Golser R, Knie K, Kutschera W, Priller A, Quinto F, Srncik M, Terrasi F, Vockenhuber C, Wallner A, Wallner G, Wild EM (2010) Nucl Instrum Meth Phys Res B268:1045–1049
Povinec PP, Litherland AE, von Reden KF (2009) Radiocarbon 51:45–78
Steier P, Bichler M, Fifield KL, Golser R, Kutschera W, Priller A, Quinto F, Richter S, Srncik M, Terrasi P, Wacker L, Wallner A, Wallner G, Wilcken KM, Wild EM (2008) Nucl Instrum Meth B 266:2246–2250
Vockenhuber C, Ahmad I, Golser R, Kutschera W, Liechtenstein V, Priller A, Steier P, Winkler S (2003) Int J Mass Spectrom 223–224:713–714
Spendlikova I, Raindl J, Němec M, Steier P, Mičolová P (2014) J Radioanal Nucl Chem 300:1151–1158
Wang X, Dong K, He M, Wu S, Jiang S (2013) Nucl Technol 182:235–241
Povinec P, Šivo A, Chudý M (1986) Nucl Instrum Meth B17:556–559
Povinec P, Šivo A, Chudý M (1986) Radiocarbon 28:668–672
Svetlik I, Fejgl M, Turek K, Michalek V, Tomaskova L (2012) J Radioanal Nucl Chem 292:689–695
Svetlik I, Povinec P, Molnár M, Váňa M, Šivo A, Bujtás T (2010) Radiocarbon 52:823–834
United Nations Scientific Committee on the Effects of Atomic Radiation (2000) Exposures from natural and man-made sources of radiation. UNSCEAR, New York
Lujaniené G, Beneš P, Štamberg K, Šapolaite J, Vopalka D, Radžiute E, Ščiglo T (2010) J Radioanal Nucl Chem 286:353–359
Rakowski AZ, Nadeau M-J, Nakamura T, Pazdur A, Pawelczyk S, Piotrowska N (2013) Nucl Instrum Meth Phys Res B 294:503–507
Molnár M, Major I, Haszpra L, Svetlik I, Svingor E, Veres M (2010) J Radioanal Nucl Chem 286:471–476
Galli I, Bartalini S, Caforio S, Calzolai G, Carraresi L, Manetti M, Taccetti F, Mando PA (2013) Radiocarbon 55:213–223
Stuiver M, Polach HA (1977) Radiocarbon 19:355–363
Svetlik I, Povinec PP, Pachnerova Brabcova K, Fejgl M, Tomaskova L, Turek K (2013) Radiocarbon 55:1546–1555
Povinec P, Šaro Š, Chudy M, Šeliga M (1968) Int J Appl Radiat Isotopes 19:877–881
Povinec PP, Šivo A, Šimon J, Holy K, Chudy M, Richtarikova M, Moravek J (2008) Appl Rad Isot 66:1686–1690
Povinec PP, Chudy M, Šivo A, Šimon J, Holy K, Richtarikova M (2009) J Environ Radioact 100:125–130
Povinec P (1972) Radiochem Radioanal Lett 9:127–135
Povinec P (1978) Nucl Instrum Methods 156:441–445
Ješkovský M, Povinec PP, Steier P, Šivo A, Richtáriková M, Golser R (2014) In: Book of abstracts. 13th International AMS Conference, Aix en Provence
Steier P, Golser R, Liechtenstein V, Kutschera W, Priller A, Vockenhuber C, Wallner A (2005) Nucl Instrum Meth Phys Res B240:445–451
Yeamans CB (2003) Dry process fluorination of uranium dioxide using ammonium bifluoride. Master Thesis, Massachusetts Institute of Technology, Boston
Sahoo B, Satapathy KC (1964) J Inorg Nucl Chem 26:1379–1380
Levin I, Kromer B (2004) Radiocarbon 46:1261–1272
Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, Steele LP, Wagenbach D, Weller R, Worthy DE (2010) Tellus B62:26–46
Svetlik I, Molnár M, Svingor E, Rinyu L, Futó I, Michalek V (2007) Regional and global aspects of radiation protection. IRPA, Brasov
Magnusson A, Stenstroem K, Skog G, Adliene D, Adlys G, Hellborg R, Ovariu A, Zakaria M, Rääf C, Mattsson S (2004) Radiocarbon 46:863–868
Roussel-Debet S, Gontier G, Siclet F, Fournier M (2006) J Environ Radioact 87:246–259
Acknowledgments
The authors acknowledge fruitful collaboration during AMS measurements with Profs. P. Steier and A. Priller of the VERA laboratory of Vienna University. This work was supported by the EU Research and Development Operational Program funded by the ERDF (projects Nos. 26240120012, 26240120026 and 26240220004), by the institutional funding of Nuclear Physics Institute AS CR (RVO61389005), by project No. 5/2008 of the SONS of the Czech Republic, by the Grant Agency of the Czech Technical University in Prague (grant No. SGS14/154/OHK4/2T/14), and by the Ministry of Industry and Trade of the Czech Republic (grant No. FR-TI3/245).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Prof. Petr Beneš.
Rights and permissions
About this article
Cite this article
Povinec, P.P., Svetlik, I., Ješkovský, M. et al. Joint Bratislava–Prague studies of radiocarbon and uranium in the environment using accelerator mass spectrometry and radiometric methods. J Radioanal Nucl Chem 304, 67–73 (2015). https://doi.org/10.1007/s10967-014-3618-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3618-8