Abstract
This study aims to assess the activities of radioactive isotopes 234U, 235U, and 238U in earth samples collected from the Missour region in Morocco using alpha spectrometry. The samples were collected at different depths (0 cm, 10 cm, and 20 cm), and their mass, as well as the tracer mass, was accurately measured. The results showed significant variations in isotope activities depending on the depth. The activities of 234U and 238U increased with depth, while the activity of 235U showed a less pronounced increase. These observations suggest the influence of geochemical processes and soil composition on the distribution of isotopes. These findings contribute to our understanding of natural radioactivity in the region and underscore the importance of monitoring radiation levels to assess environmental and human health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Alpha decay is the process where a radioactive nucleus disintegrates by emitting an alpha-particle. Alpha-emitting radioactive isotopes of uranium, namely 234U, 235U, and 238U, hold a significant importance in various fields of science and environmental safety (Hahn 2005). Naturally occurring in the soil, these isotopes differ in their nuclear composition based on the number of neutrons in their atomic nucleus.
234U, composed of 92 protons and 142 neutrons, is a radioactive isotope that decays into thorium-230 by emitting alpha-particles. On the other hand, U-235, consisting of 92 protons and 143 neutrons, is particularly important due to its fissility, enabling nuclear fission reactions that generate energy, used in nuclear reactors and nuclear weapons. Finally, U-238, the most abundant isotope of uranium with 92 protons and 146 neutrons, can also participate in nuclear reactions, notably through neutron capture, leading to the formation of plutonium-239, a fissile material (Suppes and Storvick 2016; Moeller et al. 1980). Figure 1 illustrates the radioactive decay pathways of 235U and 238U.
These isotopes possess the unique property of emitting ionizing radiation, such as alpha-particles, beta-particles, and gamma-rays, making them versatile in various applications, ranging from nuclear energy production to radiometric dating in geology and nuclear medicine (Lloyd and Goddard 2020). However, their presence and behavior in soil also raise concerns in terms of radiological and environmental safety, prompting in-depth studies to assess risks and understand the geochemical processes governing them (Gascon and Munoz 2003; Kenna 2009). The background radiation produced from the isotopes of uranium can provided some problems like the migration of radon from soil and rocks into buildings, where it can accumulate and pose a potential health risk. Radon exposure is associated with an increased risk of lung cancer, particularly in indoor environments (Oviri et al. 2023). This introduction will shed light on the essential characteristics of these radioactive isotopes in soil, revealing their significant implications for science and society (Nash et al. 2006). Samples were collected at various depths in the Missour region of Morocco (Fig. 2). The precise measurement of radioactive isotope activities in environmental samples is of great importance for evaluating radiation levels and understanding interactions between isotopes. In this study, we applied alpha spectrometry to analyze the activities of 234U, 235U, and 238U isotopes in samples collected from the mentioned region.
2 Methodology
Samples were collected at three different depths in the Missour region, Morocco (Fig. 2). After collection, the samples were carefully processed to measure their precise mass as well as the mass of the tracer. The activities of isotopes 234U, 235U, and 238U were measured using alpha spectrometry, with particular attention to radiological safety measures.
2.1 Experimental
2.1.1 Instrumentation
The alpha-particle spectrometer utilized for measuring the massic activities of 234U and 238U radioisotopes was the Canberra Alpha Analyst model, equipped with a passivated implanted planar silicon (PIPS) detector with an active surface area of 450 mm2. Data processing was performed using the Gennie 2000 and Alpha Analyst software. Thin uranium sources for all samples were prepared via electrodeposition onto a stainless-steel disk. The uranium deposition process involved utilizing an electrodeposition cell assembly consisting of a disposable plastic vial, a cap assembly with a stainless-steel plating planchet (cathode), and a platinum electrode (anode).
2.1.2 Reagents and Tracer
All chemicals employed in this investigation were procured from Sigma-Aldrich and were of high analytical purity. The 232U tracer solution utilized was sourced from AEA Technology, QSA, with a massic activity of approximately 0.19 Bq g−1 in the diluted tracer solution.
2.1.3 Acid Leaching
To assess the moisture content, soil samples were initially weighed using an analytical balance and subsequently subjected to a 105 °C drying process lasting 24 h. Following cooling, the samples were reweighed, and the dried sample quantity was then placed in a Teflon beaker for dissolution. The addition of the 232U tracer to the sample enabled the determination of the chemical yield (Gascon and Munoz 2003). Next, a volume of 50 ml of aqua regia was introduced to the sample. The Teflon beakers were covered with Teflon watch glasses, and the sample was gently evaporated for 4 h. Once complete dissolution was achieved, the Teflon watch glass was removed, and the solution was evaporated nearly to dryness. To dissolve the silicates, 50 mL of concentrated HF was added, and the ashy sample was carefully evaporated to dryness on a hot plate, maintaining a temperature of 220 °C throughout the solution evaporation process. The resulting residue was then prepared for the chemical separation of uranium (Kenna 2009).
The residues were dissolved in 10 mL of 8 M HNO3. Uranium was separated from other radionuclides using a liquid–liquid extraction technique employing the organic solvent tributyl phosphate (TBP), xylene, and deionized water (Oviri et al. 2023). Following separation, uranium was electrodeposited onto stainless-steel disks measuring 19.6 mm in diameter, utilizing the electroplating technique. Figure 3 provides a comprehensive illustration of the electroplating system, displaying the constituent components, connections, electrodes, and electrical circuits comprising the entire setup (Johnston et al. 1991). This graphical representation facilitates a clearer understanding of the implementation and operation of the electroplating process.
After the uranium solution was evaporated to a few milliliters, 1 ml of 0.3 Mol/L Na2SO4, several drops of concentrated NH3, and 4 ml of deionized water were added to prepare the electrolysis cell. The medium's pH was adjusted by adding concentrated NH3 and 1% H2SO4. Electrodeposition was conducted at 1 A for 1 h. Subsequently, the disk was removed from the cell, rinsed sequentially with 1% NH3, ethanol, and acetone. The electrolyte-coated disk was then dried for 5 min at 200 °C and immediately measured in an alpha-particle spectrometer. The alpha sources were counted over a period of 1–2 days (Suárez-Navarro et al. 2023).
2.2 Spectrometric Analysis and Calculation of 238U and 234U Massic Activities
The regions corresponding to the 238U and 234U peaks, spanning energy intervals of 4000–4200 keV and 4600–4780 keV in the spectrum, were identified and subsequently adjusted for background interference. The ambient background was subtracted for the uranium peaks by measuring a pristine stainless-steel disk under identical conditions. The specific activities and associated uncertainties of 238U and 234U in the soil samples were calculated using the following Eqs. (1–3):
• A232 (Bq/kg) is the specific activity of the tracer.
• m232 (kg) is the weight of tracer solution added to the sample.
• t2 (s) is the duration of the sample spectrum count.
• t1 (s) is the duration of the background noise count.
• Ni (cps) is the number of raw counts attributed to isotope “i” in the spiked sample.
• Bi (cps) is the background noise measured on the area of the spectrum attributed to the isotope “i”.
• Ii is the emission rate relative to the peak considered for the radionuclide “i”.
• m is the mass (kg) of sample.
3 Results
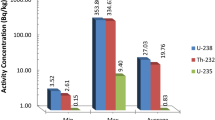
Figure 4 provides a detailed graphical representation of the spectra corresponding to the surface-level sample (0 cm) containing uranium isotopes, namely 234U, 235U, and 238U, with respective activities of 18.00 ± 1.40 Bq/kg, 0.42 ± 0.14 Bq/kg, and 12.63 ± 1.06 Bq/kg. Each of these spectra reveals distinct features that allow for the differentiation of these isotopes based on their specific radiological signatures. The analysis of these spectra is crucial for precise identification and reliable quantification of these isotopes in various scientific, industrial, and environmental applications, contributing to our overall understanding of radioactivity and its implications.
Furthermore, Fig. 5 also presents spectra corresponding to the same isotopes but at a depth of 10 cm, with respective activities of 17.78 ± 2.22 Bq/kg, 0.70 ± 0.29 Bq/kg, and 13.45 ± 1.79 Bq/kg for 234U, 235U, and 238U, respectively.
Finally, Fig. 6 reveals the spectra obtained at a depth of 20 cm, using alpha spectroscopy for the same isotopes (234U, 235U, and 238U), with respective activities of 19.66 ± 2.75 Bq/kg, 0.88 ± 0.4 Bq/kg, and 11.95 ± 1.89 Bq/kg. These graphical representations are crucial for studying the distribution and variability of these isotopes in the soil at different depths, which has significant implications in various research and application domains.
The fourth peak present in all three figures corresponds to the tracer peak used in this study. The role of the tracer is of fundamental importance in monitoring and characterizing processes involving uranium isotopes (234U, 235U, and 238U) at various soil depths. As a specific radioactive element, the tracer is intentionally introduced into the sample or the studied medium to facilitate the traceability of its movements, reactions, or transformations.
In our study, the selected tracer is 232U. This decision was made due to its ability to mimic the behaviors of uranium isotopes present in the soil while being more easily traceable and measurable. In a controlled manner, this tracer was incorporated into the soil samples at each of the examined depths, namely 0 cm, 10 cm, and 20 cm.
The use of this specific tracer is essential for monitoring and quantifying the mechanisms of dispersion, migration, and retention of uranium isotopes across different soil layers. These data are crucial for a thorough understanding of underlying geochemical and environmental processes, as well as for the assessment of potential radiological risks associated with the presence of uranium isotopes in the soil.
In this section, we present Table 1, which summarizes all the information related to each sample. This table synthesizes data on the depth of each sample and the measured activities of the corresponding uranium isotopes at each level. It provides a convenient overview of the results of your study.
4 Discussion
Data analysis reveals significant variations in the activities of isotopes 234U, 235U, and 238U with depth in the Missour region. The observed increases in the activities of U-234 and U-238 with depth could be attributed to geochemical processes and soil composition. The activity of U-235 also shows an increase, but to a lesser extent. These results suggest the importance of considering the natural distribution of radioactive isotopes in samples.
The results mentioned in the table appear to indicate relatively low activities for isotopes 234U, 235U, and 238U in the collected samples. These activities are typically expressed in Becquerel’s per kilogram (Bq/kg). Based on the values provided in the table, it seems that the measured activities are within a reasonable range and do not pose a major issue for the environment (UNSCEAR 2008).
However, it is important to note that assessing the environmental impact of radioactive activities depends on several factors, such as soil composition, local geology, background natural radiation levels, and ecosystem characteristics. Radiological safety standards and thresholds also vary from one country to another.
To assess the potential impact of the measured radioactive activity levels on the environment, we compared these values to the standards and regulatory thresholds established by competent radiation protection authorities. These standards are specifically designed to ensure the safety of both humans and the environment. Our analyses revealed that the observed radiation levels are below the recommended thresholds.
Please note that the values of the activities of radioactive isotopes 234U, 235U, and 238U can vary significantly depending on geology, geographical location, and other factors.
Here are some examples of activity ranges for these isotopes:
U-234: Typically ranging from 0.1 to 10 Bq/kg in terrestrial soils.
U-235: Typically ranging from 0.01 to 1 Bq/kg in terrestrial soils.
U-238: Typically ranging from 1 to 100 Bq/kg in terrestrial soils.
When the measured values remain below regulatory thresholds and do not exceed levels considered safe for human health and the environment, the results suggest that radioactive activities in the studied region likely do not pose a significant risk to the environment.
5 Conclusion
The application of alpha spectrometry allowed for the determination of the activities of the radioactive isotopes 234U, 235U, and 238U in earth samples collected in the Missour region of Morocco. These results provide crucial information about natural radioactivity in the studied area and underscore the importance of monitoring radiation levels to assess potential risks to human health and the environment. This study also contributes to the understanding of geochemical processes influencing the distribution of radioactive isotopes in the region.
References
Expósito-Suárez VM, Suárez-Navarro JA, Aguado-Herreros CM, Sanz MB, Suárez-Navarro MJ, Caro A (2023) Increasing the recovery and selectivity of 238U, 235U, and 234U extraction with tri-n-butyl phosphate in mine tailing samples with a high copper content. Anal Chim Acta 1259:341183
Gascon JL, Munoz A (2003) Optimization of the parameters affecting the solid state detector efficiency in alpha-spectrometry. J Radioanal Nucl Chem 257(2):371–374
Hahn FF (2005) Encyclopedia of Toxicology (Second Edition). Elsevier, s.l.
Johnston PN, Moroney JR, Burns PA (1991) Preparation of radionuclide” sources” for coincident high-resolution spectrometry with low-energy photons and electrons or alpha-particles. Appl Radiat Isot 42:245–249
Kenna TC (2009) Using sequential extraction techniques to assess the partitioning of plutonium and neptunium-237 from multiple sources in sediments from Ob River (Siberia). J Environ Radioact 100:547–557
Lloyd C, Goddard B (2020) Impacts of gamma ray emissions of materials containing 232U on safety, security, and safeguards. Nucl Eng Des 370:110905
Moeller T, Bailar JC, Kleinberg J, Guss CO, Castellion ME (1980) Clyde Metz. Academic Press, Chemistry, pp 157–196
Nash KL, Madic C, Mathur JN, Lacquement J (2006) Actinide separation science and technology. In: Morss LR, Edelstein NM, Fuger J (eds) The Chemistry of Actinide and Transactinide Elements. Springer, Dordrecht
Oviri MO, Fredrick OU, Ochuko A (2023) Environmental risk assessment of background radiation, natural radioactivity and toxic elements in rocks and soils of Nkalagu quarry Southeastern Nigeria. J Hazard Mater 10:100288
Suppes GJ, Storvick TS (2016) Chapter 8 - The Future in Nuclear Power. Academic Press, Sustainable Power Technologies and Infrastructure, pp 247–343
UNSCEAR. Sources and Effects of Ionizing Radiation. Volume 1: Sources: Report to the general assembly, scientific annexes A and B. UNSCEAR 2008 Report. United Nations sales publication E.10XI.3. United Nations, New York.2010.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elafia, Z., Didi, A., Hilal, I. et al. In-depth Study of Radioactive Isotope Profiles 234U, 235U, and 238U in Earth Samples from the Missour Region, Morocco: A Detailed Exploration of Nuclear Activity. Iran J Sci 48, 503–508 (2024). https://doi.org/10.1007/s40995-024-01587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-024-01587-y