Abstract
An approach for quantitative detection of organic vapors is demonstrated. Herein, an interdigited electrode was fabricated by drop casting of graphite loaded poly(phenyl sulfone) (PPSU/Gr) film on a conductive substrate. The sensor is experimentally tested on several organic solvents including alkanes, acetates, aromatic hydrocarbons and chloroform via electrical resistance measurements and inverse gas chromatography (IGC) method. Relationships between the sensor response (as obtained by electrical resistance) and solvent properties such as polarity, acidity and partition coefficient (as obtained from IGC) are established. Moreover, the surface dispersive free energy of PPSU and PPSU/Gr were calculated according to both Dorris-Gray and Schultz equations, it is found that with the addition of graphite to PPSU the surface dispersive free energy of PPSU at 25 °C decreased from 28 to 10 mJ m−2 (from Dorris Gray approach) and from 25 to 17 mJ m−2 (from Schultz approach), suggesting higher interfacial area for exposed gases at PPSU/Gr sensor. Furthermore, the PPSU/Gr modified electrode exhibited high sensitivity towards chlorinated hydrocarbons. Importantly, the PPSU/Gr electrode showed good mechanical stability, low response time, and is costly-effective and disposable, rendering it a promising sensor for gases detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic solvents represent a major air pollutant [1] These air pollutants have a significant influence on atmosphere chemistry, health and climate change. For example, toluene is neurotoxic while chloroform is carcinogenic [1] Therefore, detection of volatile organic substances, i.e. compounds that evaporate easily at ambient conditions, is necessary from environmental and health concerns. Traditional monitoring of gases is performed using gas chromatographs (GC) which are expensive to operate and require skilled personnel. Gas sensors have been widely used to detect and quantify organic vapors [2, 3]. Typically, these sensors are made of a sensing film coated on a substrate. A multitude of coating materials for volatile organic compounds including inorganic substances, organic structures and polymer layers.[4] Due to the large variation in polymer structures and properties, they have recently attracted great interest as recognition element for gas sensors [5,6,7]. However, the long-term stability and selectivity are still challenging. [6] Moreover, the design of sensor devices often relies on sophisticated procedures. Therefore, developing simple, robust and highly sensitive sensor is needed.
Polysulfone (PSU) is a polymer that exhibits an exceptional hydrolytic stability in extreme pH conditions, good electrical and adhesion properties, exceptional resistance to environmental stress and a flame retarder agent [8, 9]. Hence, PSU may provide a promising material for measurements in aqueous and gaseous media at extreme conditions. Due to the excellent properties of PSU, it has been used in electronic and medical industry. For instance, PSU has been widely used in last years in membrane separation technology and sensors field [10, 11]. PSU was also successively used in resistive humidity and gas sensors [12, 13]. They are also susceptible to incorporate biological molecules and thus are used in enzymatic and immunologic biosensors [14, 15].
Poly(phenyl sulfone) (PPSU), which is a member of the PSU family, is sold as a trademark, Radel®R. Chemical structure of PPSU is given in Fig. 1. PPSU is an amorphous high-tech thermoplastic polymer having better chemical, blow and temperature resistivity than PSU and polyetherimide [16]. PPSU shows resistance to steam autoclaving without loss of dimensional stability or physical properties. Therefore, PPSU has been used in the medical industry such as surgical tools. Due to its temperature resistance and useful dielectric properties, PPSU has been used in electronics [16, 17]. In this context, information about the chemical behavior and resistance of PPSU against solvent vapors is needed.
Moreover, the intrinsic conductivity of polymers can be increased by adding conductive fillers as carbon black or graphite in the polymer matrix. At a certain filler content (known as percolation threshold) a transition from insulation to conduction occurs [18]. Graphite in particular is inexpensive material and has covalent character due to the covalent bonding of carbon atoms in the structure. The electrical electricity in graphite is originated from the electron delocalization resulting from the unbound external electron of each carbon atom in its structure. [19] Benefiting from the high conductivity and possible synergistic effects of carbon and its allotropes, numerous chemosensors based on the various hydrophobic polymers doped with graphite and other conductive fillers have been investigated [20,21,22,23,24,25,26]. The working principle of these gas sensors is based on the percolation of gases in the polymer composite, changing its electrical resistance and possibly cause swelling up. [27] Traditional gas analyzing techniques such as gas chromatography are complex and time consuming. Gas sensors are on the other hand portable and thus can be alternative tool specially in off-laboratory applications.
Herein, a solvent vapor detection sensor based on the PPSU/Gr conductive layer is presented. Via a simple procedure, graphite powder was mixed with PPSU polymer in chloroform, forming the conductive layer. This layer was then drop casting method onto a conductive interdigited electrode. A resistive sensor device was constructed to explore the sensing properties of PPSU/Gr against different solvent vapors such as alkanes, acetates, aromatic hydrocarbons and chloroform. The sensor performance was examined by electric resistance measurements and inverse gas chromatography (IGC). The net retention volumes, which are related to the interactions between solute and solvents, were determined by IGC. The values of the retention volumes were used to establish a relationship between the resistance response of the sensor device and the distribution coefficient of proportional to net retention volume of solvents on the electrode. Moreover, the sensor showed excellent mechanical stability, sensitivity and low response time.
Experimental
Materials and instrumentations
PPSU polymer, Radel®R (Tg = 220 °C) was purchased from Amoco Performance Products and was purified before use to remove impurities. Graphite powder was obtained from Fluka. To prepare homogeneous and uniform conductive film, graphite powder was sieved from about 100 μm graphite particles and the obtained smaller graphite particles of less than 45 μm are used. Studied solvents were of analytical grade and obtained from Merck. Studied samples were alkanes as octane (O), nonane (N) and decane (D); acetates as isoamyl acetate (IAA), isobutyl acetate (IBA); aromatic hydrocarbons as propyl benzene (nPB), toluene (T), iso-propyl benzene (IPB), chlorobenzene (CB) and chlorinated hydrocarbon as chloroform (Ch). They were used after purification with molecular sieves. The glass wool was treated with dimethyl dichlorosilane to close the two ends of stainless-steel column (dimensions:1 m in length, 3.2 mm o.d.) containing PPSU/Gr covered chromosrob-W (Merck AG.Inc., acid washed and treated with dimethyldichlorosilane, pore size is 80/100 mesh) was received from Alltech Associates, Inc. and the mobile phase was high purity helium with a flowing rate in the range of 25–28 cm3 min–1.
The solvent vapor responses of the PPSU/Gr sensor device were measured at room temperature using HIOKI 3522–50 LCR meter with a four-point probe configuration. The retention times of the solvents on the PPSU/Gr loaded on chromosorb column were determined using HP (5890-Series II) gas chromatography. The thickness of PPSU/Gr film on the electrode was determined by Veeco Dektak XT Bruker Mechanical Profilometer. SEM analysis was examined on a Zeiss EVO LS 10 scanning electron microscope.
Designing sensor device and electrical resistance measurements
Conventional epoxy-based copper circuit board, which was made with epoxy substrate, was used to prepare the low-cost, comb-like interdigited electrode. Firstly, the epoxy-based copper circuit board electrode was chemical etched methode with HCl:H2O2 solution and then the copper lines were coated with silver in silver cyanide bath. The prepared electrodes were washed with distilled water and acetone and dried before using (Fig. 2).
To load the PPSU/Gr conductive layer on the hand-made, comb-like interdigited electrode (diameter = 0.8 cm), first, the required amount of PPSU polymer (0,6 g) was dissolved in chloroform and the solution was stirred to solve the whole polymer. Afterwards, graphite powder (1 g) having smaller particles (< 45 µm size) was poured into the PPSU-chloroform solution and stirred to obtain a uniform, homogeneous PPSU/Gr solution (graphite content: 60% in weight). Then, 20 µL of the PPSU/Gr solution was deposited onto the electrode under 2.0 V constant voltage, see Fig. 3. The thickness of the PPSU/Gr film was measured as 8,6 µm by mechanical profilometer. For obtaining a stable baseline resistance of PPSU/Gr sensor, the solvent vapors were evaporated at room temperature.
The PPSU/Gr loaded sensor device was sealed in the glass test chamber and was connected to the LCR meter and. Afterwards, 1 μl solvent was injected into the vessel, rapidly stirred to obtain a homogeneous steam, then the resistance of the sensor device towards solvent vapor was recorded continuously with the LCR meter. To remove atmospheric contaminants and remaining solvent vapors, compressed nitrogen gas was passed through the test vessel before and after measurements. The experimental set up was schemed in Fig. 3.
When a carbon/polymer composite is exposed to solvent vapors, the electrical resistance response of the polymeric composite is generally increased due to the breakage of conductive pathways as a result of swelling of the polymer. [24, 27, 28]
The relationship between the electrical resistivity of a sensor device to the solvent (Rr) and the electrical resistivity of the sensor exposure to solvent (before, Ro and after, Rt) is given as following;
The electrical resistivity of the polymer composite based on sensor to solvent vapors is related with the solvent partition coefficient on the polymer composite. The solvent partition coefficient on the polymer composite (Kp) is given as:
In Eq. 2, the vapor concentration in the polymer phase is defined as Cp, while Cv is the vapor concentration in the gas phase. [21, 29]
In the IGC technique, the solvent partition coefficient on the polymer is related to the net retention volume (VN) of a solvent on the column which is filled with polymer. This relationship is defined as follows: [29, 30]
The weight of the solute (i.e. polymer-graphite composite) in the column is w. The net retention volume of solvent vapors on the polymer-graphite loaded on chromosorb column, VN is determined from retention times of solvents as follows [31]:
Surface properties of solid materials is related to the dispersive surface free energy, γSd and is a specific interaction parameter that defines surface ability to behave as electron acceptor or electron donor. Dorris-Gray and Schultz equations are useful to calculate the dispersive surface free energy of the solid stationary phase from IGC measurements. [32,33,34,35,36] According to Dorris-Gray equation, γSd is defined as;
In Eq. 5, the adsorption energy of one methylene group, \(\Delta {G}_{\left[C{H}_{2}\right]}\) is given by;
The dispersive free energy changing with temperature for polyethylene is as follows:
where \(\gamma [C{H}_{2}]\) is dispersive surface free energy of methylene groups.
According to Schultz equation, γSd is given by:
In Eq. 8, the dispersive free energy of the studied n-alkane is \({\gamma }_{l}^{d}\), and the cross-sectional area of the n-alkane molecule is \(a\). The \(a({\gamma }_{l}^{d}{)}^{0.5}\) data for studied n-alkane molecules are presented in Table 1.
Results and discussion
Effect of solvent properties on the sensor response: establishing solvent property-sensor response relationships
Time dependent electrical resistance changes of the PPSU/Gr composite sensor upon exposure to the solvents at saturated vapor pressure are given in Fig. 4. Some curves are not displayed in Fig. 4 for clarity.
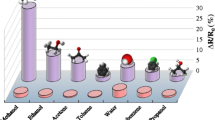
The equilibrium responses of PPSU/Gr composite sensor to solvent vapors at saturated vapor pressures of studied solvents are presented in Fig. 5. The saturated vapor pressures of the solvents at 25 °C were taken from previous reports. [37,38,39,40,41]
As shown in Fig. 4 and 5, the higher responses were obtained for chlorinated aliphatic and aromatic hydrocarbons. Although CB has a significantly low vapor pressure than that of Ch, they exhibited similar sensing response. The main difference between Ch and CB is the faster sorption–desorption behavior of Ch. Aromatic hydrocarbons showed high responses as well but with low sorption–desorption rates. On the other hand, the aliphatic esters have lower responses and sorption–desorption rates. Aliphatic hydrocarbons exhibit the lowest responses but relatively faster sorption–desorption rates. Generally, although the saturated vapor pressure of solvents is increasing, the PPSU/Gr sensor response is decreasing in aliphatic hydrocarbons and is increasing in aromatic hydrocarbons, aliphatic esters and chloroform. Increasing the volume of solvents in the test chamber containing PPSU/Gr sensor device resulted in improved sensor response in the following order: CH > CB > T > nPB > IBA > IAA > D > N > O > IPB.
The net retention volumes of solvents on PPSU/Gr adsorbed on chromosorb were calculated from net retention times using Eq. 3 and the results are presented in Table 1. The plots of ln(VN) versus 1/T were given in Fig. 6 and the linear straight line was extrapolated to 25 °C to obtain VN and Kp values at 25 °C.
Moreover, the relationship between the response of the PPSU/Gr sensor and the distribution coefficient of solvents is depicted in Fig. 7.
A corelation between the sensor responses with the partition coefficients of all studied solvents is complex. However, it is useful to make a relationship within the same class of solvents. The responses and distribution coefficient of alkanes which are nonsolvents for PPSU [40] are increasing with increasing the alkyl chain length of the alkane. The responses of aliphatic esters which are moderately poor solvent for PPSU [42] are increasing while the distribution coefficients are decreasing with increasing molecular weight of aliphatic esters (see Fig. 6 and 7). The results suggest that the responses are not only dependent on the type of the solvent but also on their molar volumes. The vapor pressure was not determining factor to describe the sensor response because enough time was allowed to reach to equilibrium response.
Electrostatic interactions between polymer and solvent may result in higher swelling behavior of the polymeric film. This increases the gap between the graphite particles and thus causes an increased electrical resistance of the solvent to the polymer-graphite composite. [19, 43] This interpretation is supported with relation between chloroform/chlorobenzene and PPSU/Gr composite sensor. PPSU has a nucleophilic character due to a lone electron pair on sulfone groups and ether connections. Chloroform molecule has electrophilicity and chlorobenzene has hydrogen atoms with electron lack on the benzyl group due to the electronegative chlorine atoms. The chloroform and chlorobenzene responses of PPSU/Gr sensor device are higher which can be attributed to swelling of the polymer and increased gap between graphite particles.
Moreover, the dispersive surface free energy, \({\gamma }_{S}^{D}\) of PPSU and PPSU/Gr at 25 °C were calculated according to Dorris Gray (Eq. 5) and Schultz (Eq. 8) approaches. By comparing the surface dispersive free energy, \({\gamma }_{S}^{D}\)of PPSU and PPSU/Gr at 25 °C, we found that \({\gamma }_{S}^{D}\) is lowered from 28.5 mJ m−2 to 9.9 mJ m−2 (calculated according to Dorris Gray approach) and from 24.8 mJ m−2 to 17.2 mJ m−2 (calculated according to Schultz approach) by adding graphite into PPSU. This suggests that graphite acts as a surface dispersive free energy reducing agent in the PPSU matrix.
Concentration dependency of response: calibration curve
As shown from the above results, PPSU/Gr composite film exhibits higher sensitivity against chlorinated hydrocarbons. Therefore, we investigated the use of the PPSU-based sensor to detect this important class of solvents. The relationship between the concentration of good solvents and responses of PPSU/Gr composite sensor was demonstrated for chloroform (Fig. 8).
A linear correlation (Rr% = 98.76 V – 3.9454, R2 = 0.979) was obtained between the volume of exposed chloroform (between Psat/8 and Psat) and response. This linear range (calibration curve) which is obtained at the PPSU/Gr composite electrode render this sensor promising candidate for detection of chlorinated hydrocarbons. Polyaniline (PA) and expanded graphene oxide (EGO) composite materials are exposed with various concentrations of vapours of different volatile organic compounds (VOCs) such as acetone, chloroform and carbon tetrachloride and compared with the pristine polymer, PA. The oxidising VOCs like acetone on exposure to pristine polymer and PA/EGO composite is found to be decrease in resistivity by hydrogen bonding with the redox cites of PA. When it is compared the chloroform sensitivity of PPSU/Gr composit with PA and PA/EGO composite, the chloroform sensitivity is found to be more in PPSU/Gr composite like PA [44].
Scanning electron microscopy (SEM) was used to investigate the surface morphology of the PPSU/Gr film on electrode. The SEM images of PPSU/Gr film on electrode were shown in Fig. 9.
As shown from the SEM images, the graphite particles are covered by PPSU and the film surface contains random distributed PPSU covered graphite particles (Fig. 9(a)). However, in spite of the random distribution of the graphite flakes in PPSU film, randomly distributed spherical zones appeared on the electrode surface. The PPSU and Gr particles have been used as the polymer matrix and conductive filler material respectively. Gr addition to PPSU matrix is improved the sensing capability and rapid response of the electrode.
Conclusions
Application of the solvent vapor concentration dependent electrical resistance measurement method on the PPSU/Gr composite film was examined and analysed. The results showed that PPSU/Gr composite film electrode is promising for sensor applications especially for environmental monitoring of air pullutants such as chlorinated hydrocarbons. The modified electrode showed good linear response towards the determination of chlorinated hydrocarbons. The addition of graphite (Gr) to PPSU polymer has reduced the surface energy of PPSU, while improving the sensitivity of PPSU sensor. The graphite-modified PPSU thin film exhibited improved sensitivity towards all tested gases, particularly towards chlorinated gases with fast response and recovery times. The reversibility of sensitive behaviour provides a potential of such composites in emerging sensing applications.
References
Szczurek A, Szecówka PM, Licznerski BW (1999) Application of sensor array and neural networks for quantification of organic solvent vapours in air. Sens Actuators B Chem 58:427–432
Lin C, Xu W, Yao Q, Wang X (2018) Nanotechnology on Toxic Gas Detection and Treatment. In: Novel Nanomaterials for Biomedical, Environmental and Energy Applications. Elsevier, pp 275–297
Feng S, Farha F, Li Q, Wan Y, Xu Y, Zhang T, Ning H (2019) Review on Smart Gas Sensing Technology. Sensors 19:3760–3782
Constantinoiu I, Viespe C (2019) Detection of volatile organic compounds using surface acousticwave sensor based on nanoparticles incorporated in polymer. Coatings 9:373–382
Dong XM, Fu RW, Zhang MQ, Zhang B, Li JR, Rong MZ (2003) A novel sensor for organic solvent vapors based on conductive amorphous polymer composites: Carbon black/poly(butyl methacrylate). Polym Bull 50:99–106
Stahl U, Voigt A, Dirschka M, Barié N, Richter C, Waldbaur A, Gruhl F, Rapp B, Rapp M, Länge K (2017) Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors. Sensors 17:2529
Bai H, Shi G (2007) Gas Sensors Based on Conducting Polymers. Sensors 7:267–307
Tickin A, Weiser KE (1996) Polymeric Materials Encyclopedia
Olabisi O (1997) Handbook of Thermoplastics, 1st edn. Marcel Dekker, New York
Ciobanu M, Marin L, Cozan V, Bruma M (2009) Aromatic polysulfones used in sensor applications. Rev Adv Mater Sci 22:89–96
Adhikari B, Majumdar S (2004) Polymers in sensor applications. Progress in Polymer Science (Oxford) 29:699–766
Xin Y, Wang S (1994) An investigation of sulfonated polysulfone humidity-sensitive materials. Sens Actuators, A 40:147–149
Telipan G, Ignat M, Cozan V (2006) Use of the polysulfone polymer in NO x detection. J Optoelectron Adv Mater 8:582–584
Bryjak J, Bryjak M, Noworyta A (1996) Membrane reactor with soluble forms of penicillin acylase. Enzyme Microb Technol 19:196–201
Prieto-Simón B, Fàbregas E (2006) New redox mediator-modified polysulfone composite films for the development of dehydrogenase-based biosensors. Biosens Bioelectron 22:131–137
Arbós P, Wirth M, Arangoa MA, Gabor F, Irache JM (2002) Gantrez® AN as a new polymer for the preparation of ligand-nanoparticle conjugates. J Control Release 83:321–330
Norrman K, Wang Y, Stamate E, Zhang W (2019) Controlling surface properties of electrospun polyphenylsulfone using plasma treatment and X-ray photoelectron spectroscopy. Heliyon 5:e01943
Zhang MQ, Xu JR, Zeng HM, Huo Q, Zhang ZY, Yun FC, Friedrich K (1995) Fractal approach to the critical filler volume fraction of an electrically conductive polymer composite. J Mater Sci 30:4226–4232
Mukhopadhyay P, Gupta RK (2013) Graphite, Graphene, and Their Polymer Nanocomposites. CRC Press, Boca Raton
Luo Y, Li Z, Lan W (2007) Response behavior of an epoxy resin/amine curing agent/carbon black composite film to various solvents. Mater Sci Eng B: Solid-State Mater Adv Technol 139:105–113
Arshak K, Moore E, Cavanagh L, Cunniffe C, Clifford S (2005) Examining the use of oxide particles to enhance the sensitivity of polymer\carbon black nanocomposite gas sensors. Prog Solid State Chem 33:199–205
Grate J, Abraham M (1991) Solubility interactions and the selection of sorbent coating materials for chemical sensors and sensor arrays. Sens Actuators B 3:85–111
Phillips C, Al-Ahmadi A, Potts SJ, Claypole T, Deganello D (2017) The effect of graphite and carbon black ratios on conductive ink performance. J Mater Sci 52:9520–9530
Chen SG, Hu XL, Hu J, Zhang MQ, Rong MZ, Zheng Q (2006) Relationships between organic vapor adsorption behaviors and gas sensitivity of carbon black filled waterborne polyurethane composites. Sens Actuators B Chem 119:110–117
Lee J, Kim WK (2021) PEGylated graphene oxide-based colorimetric sensor for recording temperature. J Ind Eng Chem 94:457–464
Hue N, Wu Q, Liu W, Bu X, Wu H, Wang C, Li X, Wang X (2021) Graphene oxide/graphene hybrid film with ultrahigh ammonia sensing performance. Nanotechnology 32(11):115501
Marquez A, Uribe J, Cruz R (1997) Conductivity variation induced by solvent swelling of an elastomer–carbon black–graphite composite. J Appl Polym Sci 66:2221–2232
Feller JF, Grohens Y (2004) Evolution of electrical properties of some conductive polymer composite textiles with organic solvent vapours diffusion. Sens Actuators B Chem 97:231–242
Lux F (1993) Models proposed to explain the electrical conductivity of mixtures made of conductive and insulating materials. J Mater Sci 28:285–301
Conder JR, Young CL (1979) Physicochemical measurement by gas chromatography. John Wiley & Sons
Santos JMRCA, Guthrie JT (2005) Analysis of interactions in multicomponent polymeric systems: The key-role of inverse gas chromatography. Mater Sci Eng R Reports 50:79–107
Sesigur F, Sakar D, Yazici O, Cakar F, Cankurtaran O, Karaman F (2014) Dispersive Surface Energy and Acid-Base Parameters of Tosylate Functionalized Poly(ethylene glycol) via Inverse Gas Chromatography. J Chem 2014:402325
Gumrukcu G, Garikyan S, Keser Karaoglan G, Sakar D (2013) Structural and surface characterization of newly synthesized D-π-D type Schiff base ligand:(1E, 2E)-3-[4-(dimethylamino) phenyl] prop-2-en-1-ylidene) phenylamine. J Chem 2013:298205
Karaoglan GK, Sakar D (2011) Surface characterization of a novel D-π-D type ligand by IGC at infinite dilution. Chromatographia 73:93–98
Fidan İ, Daşdan DŞ, Karaman F, Kaban Ş (2017) Surface characterization of thiazolidinone derivatives by ınverse gas chromatography. Bul Chem Commun 49:742–749
Shi B, Wang Y, Jia L (2011) Comparison of Dorris-Gray and Schultz methods for the calculation of surface dispersive free energy by inverse gas chromatography. J Chromatogr A 1218:860–862
Yaws CL (1994) Handbook of vapor pressure vol 3. C8-C28 compounds. Gulf Pub. Co, Houston, TX
Daubert TE, Danner RL (1989) Physical and thermodynamic properties of pure chemicals: data compilation
Boublik T, Fried V, Hala E (1984) The vapour pressures of pure substances. Elsevier Science Pub. Co., Inc., New York, NY, United States
Riddle FL, Fowkes FM (1990) Spectral Shifts in Acid-Base Chemistry. 1. Van der Waals Contributions to Acceptor Numbers. J Am Chem Soc 112:3259–3264
Pascal Kamdem D, Kanti Bose S, Luner P (1993) Inverse Gas Chromatography Characterization of Birch Wood Meal, Langmuir, 9(11):3039-3044
Cankurtaran O, Serin M, Cankurtaran H, Yilmaz F (2003) Secondary transitions and thermodynamic interaction parameters of poly (phenyl sulfone) by gas chromatography. Polym Bull 51:225–230
Tillman ES, Lewis NS (2003) Mechanism of enhanced sensitivity of linear poly(ethylenimine)-carbon black composite detectors to carboxylic acid vapors. Sensors and Actuators, B: Chemical 96:329–342
Konwer S, Begum A, Bordoloi S, Boruah R (2017) Expanded graphene-oxide encapsulated polyaniline composites as sensing material for volatile organic compounds. J Polym Res 24(37):1–10
Acknowledgements
This research was supported by Yildiz Technical University, Scientific Research Projects Coordination Department (Project no: 29-01-02-ODAP03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakar Dasdan, D. Poly (phenyl sulfone)/graphite composite as a robust low-cost, comb-type interdigited sensor for detection of organic solvent vapors. J Polym Res 28, 130 (2021). https://doi.org/10.1007/s10965-021-02489-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02489-y