Abstract
In this study, we present the performance of interdigitated capacitive-type gas sensor devices based on polypyrrole/copper phthalocyanine (PPy/CuPc) nanocomposite, for sensing of CO2 gas at room temperature. In this framework, the PPy/CuPc nanocomposites have been synthesized by a simple in situ chemical oxidation polymerization in the presence and absence of cationic surfactant. The synthesized samples have been analyzed using field emission scanning electron microscopy and Fourier-transform infrared spectroscopy in order to study surface morphology and nanocomposite formation. The interaction of developed gas sensors with CO2 was registered by the change in capacitance. Under exposure to CO2 gas in the range of 0.1–1% at room temperature, all fabricated sensor show reversible, reproducible, and linear response. Particularly, the PPy/CuPc nanocomposite containing highly interconnected network of PPy nanofibers exhibits higher response and sensitivity toward CO2 gas compared to particle-like PPy/CuPc nanocomposite and pure PPy. The long-term stability of gas sensor devices have also been examined in particular concentration of CO2 gas within 30 days by monitoring the change in capacitance response. The sensing performance of the capacitive-type gas sensor based on the PPy/CuPc nanocomposite may be of interest for CO2 detection in future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, various gases causes severe environmental problems in our planet in definite concentrations, including toxic gases (e.g., carbon monoxide, hydrogen sulfide, ammonia) and greenhouse gases such as methane and carbon dioxide. Carbone dioxide (CO2) is one of the key components in life and the most important substance in global warming that has a substantial impact on the greenhouse effect [1, 2]. Not only in terms of global warming but also in terms of food industry and human health, the CO2 gas sensors are of particular relevance [3, 4]. For example, measuring CO2 levels in breath allows for evaluation of systemic metabolism, which provides doctors and patients with a non-invasive method to diagnose cardiovascular diseases [5].
Various approaches have been explored for detecting and monitoring the different levels of CO2 and its subsequent release to the ambient environment. Commonly, the electrical responses have been used for sensing CO2 gas in the literature [6, 7]. For example, solid electrolyte sensors have been used for determining CO2 gas by the voltage difference between the target gas and the alkaline carbonate coated on working electrode. However, the structure of these type sensors is complicated and the alkaline carbonate is substantially affected by water vapor [8]. An attempt has been made to detect carbon dioxide (CO2) using an in situ-loaded Bi2O3–polypyrrole nanocomposite sensor. The prepared nanocomposites displayed significant selectivity, sensing, and stability response against CO2 gas at low concentration level [9]. Sira et al. prepared a miniature chemiresistor sensor based on a carpet-like nanostructure of polyaniline (PANI) nanothin film functionalized with poly(ethyleneimine) for detection of CO2 at room temperature. Good sensing performance was observed upon exposing the PEI–PANI device to 50–5000 ppm CO2 in presence of humidity [10]. Mojtaba et al. studied a capacitive carbon dioxide sensor operated at room temperature based on a dielectric nanocomposite comprising P-type multi-walled carbon nanotube. They found that adding a small amount of P-type multi-walled carbon nanotube in diphenylethylenediamine can dramatically improve the CO2 sensitivity [11]. Venkateswarlu et al. investigated the open metal site metal organic frameworks and surface functionalized metal organic frameworks (MOFs) as selective CO2 gas-sensing material for the work function read-out-based method at ambient temperature and different humidity levels. They found that interaction of the CO2 molecules with open metal sites or amine functional groups changes the work function of sensing layer which can serve as a signal for gas sensors [12]. Feng-Renn and coworkers prepared n-type nanocomposites on P-type Si substrate to detect carbon dioxide gas by measuring its current–voltage characteristics changes. Their results show that fabricated sensor has a shorter response time of 10 s and can be used as a high-performance CO2 sensor [13]. Boudaden et al. [14] reported a capacitive-type CO2 sensor with a highly sensitive and reversible sensing layer (a hybrid nanocomposite). They showed that the nanocomposite can detect CO2 gas in the range of interest for environmental monitoring at moderate low temperature (less than 60 °C). These short examples indicate that different materials can be used in gas-sensing devices depending on the purposes.
The sensing materials have key importance in research and development of new innovative gas sensors and thus for innovations of gas sensor technology [15]. Currently, commercially available sensors for CO2 detection are based on sensitive metal oxide layers [16]. These type sensors, however, inevitably require an operating temperature higher than 100 °C (high energy consumption). Therefore, there is a need for an alternative approach. The gas-sensing devices based on organic materials have particular characteristics such as high sensitivity, short response time, and room temperature operating ability due to their porous nature [17, 18]. In this study, we have developed an easy processing and low-cost method to fabricate a nanocomposite based on organic materials, polypyrrole (PPy) and copper phthalocyanine (CuPc), as a capacitive-type gas sensor for CO2 detection. The sensing behavior of the nanocomposite casted on interdigitated electrodes (IDEs) were studied at deferent concentrations of CO2 gas ranging from 0.1 to 1%. To the best of our knowledge, this is the first attempt made to study the PPy–CuPc nanocomposite as capacitive-type CO2 sensor. The obtained results indicate that the sensor device would be of interest for future studying as CO2 gas detection.
2 Experimental
2.1 Reagents and materials
Pyrrole (Py) monomer, copper phthalocyanine (CuPc), cetyltrimethylammonium bromide (CTAB), and ammonium persulfate (APS) were analytically pure grade and received from Merck Millipore. Pyrrole monomer was purified through distillation and stored in refrigerator before use. Other reagents were used as received. Also, doubly distilled water (DW) was used throughout the synthesis procedure.
2.2 Synthesis of PPy and PPy/CuPc nanocomposite
The Polypyrrole was chemically synthesized from pyrrole monomer by one-step reaction of the chemical oxidative polymerization using APS in an acidic media. In a typical procedure, Py (0.16M) was added into a 40 mL 1M HCl solution. The solution was stirred for 30 min in order to obtain uniform homogenization. Then, 20 mL solution of APS (0.04M, molar ratio to Py 1:4) was added into the above solution in one portion and left undisturbed for at least 24 h. The resulting product was collected and washed with amounts of DW and ethanol several times, and finally dried at 60 °C overnight.
The PPy/CuPc nanocomposite were synthesized by in situ chemical oxidative polymerization with/without cationic surfactant (CTAB) at 0–5 °C as follows. In a typical synthesis process, CTAB (0.01M) is dispersed in 60 mL 1M HCl solution. After being magnetically stirred 1 h, Py (0.16M) was added into the above solution and keep stirring for 30 min. To this mixture, the amount of CuPc with special molar percentage (respect to monomer) was added. The reaction mixture subjected to vigorously stirring for 2 h in order to achieve a homogeneous mixture, and then cooled down to 0–5 °C in an ice bath. Then, 20 mL of pre-cooled APS (0.04M) solution was added into the reaction medium to initiate the oxidative polymerization. The reactant solution was kept in the refrigerator at temperature below 5 °C for 24 h without any disturbance. The resulting black precipitate was then filtered and washed with DW and methanol repeatedly, and subsequently placed in oven to dry at 60 °C. For comparison, another PPy/CuPc nanocomposite was synthesized by a similar method without CTAB. A schematic diagram of the synthesis process for all prepared samples is illustrated in Fig. 1.
2.3 Construction of gas sensor devices
A screen-printed interdigitated electrode (SP-IDE) for capacitance measurement was designed by our group and printed to a polyethylene terephthalate (PET) by Tak-Screen Tech. (Tehran, Iran). The electrode fingers are 200 µm in width with 200 µm spacing between adjacent fingers.
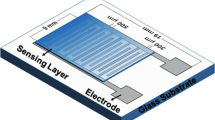
The three prepared materials were dispersed in adequate solvent to obtain homogenous mixture, which were then drop casted onto the SP-IDEs to fabricate the capacitive-type gas sensor devices. The copper wires were then attached to the electrodes as electrical contacts using soldering. A photograph of the fabricated gas sensor and schematic diagram of the interdigitated capacitor (IDC) gas sensor device with sensing layer are shown in Fig. 2a and b, respectively. For simplicity, we will refer the devices containing PPy, PPy/CuPc (synthesized in the absence of CTAB), and PPy/CuPc (synthesized in the presence of CTAB) as D1, D2, and D3, respectively.
2.4 Material characterization
The surface morphological studies of synthesized samples were obtained from the field emission scanning electron microscopy (FESEM, TESCAN MIRA III). The Fourier-transform infrared spectroscopy (FTIR) studies of PPy and PPy/CuPc was performed by recording IR spectra (AVATAR FTIR spectrometer, Thermo Fisher Scientific) from 4000 to 400 cm−1.
2.5 Gas-sensing setup
The gas-sensing analysis of the prepared devices was conducted using a home-made setup system consisted of a test chamber, a LCR meter (LCR-916, GW Instek, Goodwill Instrument Company Ltd., Taiwan), a laptop and the attachments. To evaluate the CO2 sensing performance of the devices, we measured the capacitance response using a LCR meter which is operated at 100 kHz frequency and controlled by a laptop, permitting automated data collection. The CO2 gas was injected into the measurement chamber using a microsyringe in the desired concentration, ranging from 0.1 to 1%, and exhausted by opening the chamber cover. All measurements were carried out at room temperature (27 °C) and the relative humidity of ambient air was about 15%. Before the measurement of sensing characteristics, we allowed the sensors to stabilize the capacitance for an air level. A simple schematic illustration of the gas-sensing apparatus is shown in Fig. 2c.
3 Results and discussion
3.1 Surface morphology study
Field emission scanning electron microscopy (FESEM) was used to acquire morphology information of the as-prepared samples.
Figure 3a presents the FESEM images of PPy which reveals the presence of a closely packed globular morphology. Such globules are seemingly growing one over the other to form granular agglomerates (granular particulates), which is typical for PPy synthesized by conventional chemical polymerization [19]. Figure 3c identifies the morphology of PPy/CuPc nanocomposite prepared with CTAB as surfactant. It can be seen that the PPy has a morphology consisting of a highly interconnected network of nanofibers, which is to be contrasted with the granular morphology observed in the absence of CTAB (Fig. 3b). In general, cationic surfactant CTAB works as the structure directing reagent in preparation of conducting polymers [20]. The cationic surfactant, CTAB, and oxidizing agent, APS, play a key role in tailoring the resultant PPy nanostructure by formation of a self-assembly between the cations of long chain CTAB and anions of the oxidizing agent of APS, which act as a soft-templates for the formation of fibrous structure of PPy. The mechanism of determining such morphology has been discussed elsewhere in detail [21, 22].
3.2 FTIR analysis
The bare PPy and PPy/CuPc nanocomposite were characterized by FTIR transmittance spectra, as shown in Fig. 4. The FTIR peaks in Fig. 4 are in good consistency with those reported in the literature [23,24,25]. The fundamental characteristic peaks located at 1554 and 1467 cm− 1, due to symmetric and antisymmetric ring-stretching modes, can be assigned to C=C and C–C stretching vibrations of pyrrole ring [26]. The peaks near 1320 and 1046 cm− 1 can be attributed to C–N stretching and C–H deformation vibrations, respectively [27, 28]. The peaks related to stretching vibrations of doped polypyrrole occurred at near 1199 and 923 cm− 1 [29]. Furthermore, the peaks at 782 and 675 cm− 1 can be assigned as out-of-plane C–H bending vibration [30]. In the FTIR spectra of the PPy/CuPc nanocomposite, in addition to characteristic peaks of PPy, the following typical peaks of CuPc can also be observed: the Cu–N stretching vibration at 901 cm− 1 [31], the C–C stretching vibration at 1120 cm− 1, in-plane C–N–C bending at 1168 cm− 1, in-plane pyrrole ring stretching at 1417 cm− 1, and pyrrole stretching at 1506 cm− 1 [32] which point out that CuPc was incorporated in the PPy. The peaks at 505 and 571 cm− 1 can be assigned to vibrations in the benzene ring in interaction with the pyrrole ring [33]. The presence of two peaks at 2849 and 2920 cm− 1 can be attributed to stretching modes of methyl and methylene groups in the long alkyl chains of CTAB indicating the presence of a little amount of the surfactant in the synthesized PPy/CuPc nanocomposite [28]. Some characteristic peaks of the CuPc cannot be identified, probably due to overlapping with PPy peaks. This could be a reason for observed stronger peaks in the PPy/CuPc nanocomposite compared to bare PPy, as seen in the FTIR spectra (Fig. 4). It should be noted that there is no significant difference in the FTIR spectra of PPy/CuPc nanocomposite synthesized with/without CTAB except the characteristic peaks of CTAB.
3.3 CO2 sensing characteristics
The sensing characteristics of the fabricated devices were evaluated by monitoring the variation of capacitance upon exposure to CO2 gas. Figure 5b shows a typical real-time response of the gas sensor devices after exposure to CO2 in the concentration range of 0.1–1% at room temperature. This figure demonstrates the real-time capacitance changes of the devices under exposure to target gas. As it can be noticed, the all prepared sensor devices show similar behavior after the injection of CO2 gas. The capacitance increases and reaches a steady state (stable value) after introducing the gas to the devices. When CO2 was removed, the capacitance decreases gradually with time and returns to the background (base line) capacitance. In addition, the change in capacitance increases as increasing the CO2 concentration. Consequently, the all devices have a good reversible property. As shown in Fig. 5a, the sensing response for the D2 and D3 devices containing the PPy/CuPc nanocomposite as sensitive layer is obviously better than that for pure PPy film (D1 device). Therefore, compared with pure PPy, the response of the PPy/CuPc nanocomposites has been significantly improved. It implies that the introduction of CuPc can greatly enhance the sensing response of the device due to the combination of unique merits of individual components and the excellent synergetic advantages of nanocomposite. Additionally, the sensing response for the PPy/CuPc nanocomposite containing the nanofibrous structure of polypyrrole is higher than that of the similar nanocomposite with granular morphology.
This result suggests that the nanofiber structure of PPy in the nanocomposite further improves the sensing performance. The Network structure of the PPy/CuPc offer very high surface-to-volume ratio along with porous region in it. Such nanostructure facilitate the adsorption process that helps to accumulate gas molecules inside nanocomposite. This phenomenon alters device capacitance significantly leading to improve the sensing performance [34].
To demonstrate that the nanofibrous structure of the PPy/CuPc offer very high surface-to-volume ratio than similar nanocomposite, we evaluated the specific surface area of both nanocomposite using BET (Brunauer–Emmett–Teller) analysis, as shown in Fig. 6. The results show that the BET surface area of the nanofibrous structure of the nanocomposite (21.15 m2g− 1) was almost 38% higher than granular structure (15.37 m2g− 1).
The gas-sensing performance of the devices for the CO2 gas were also examined through evaluating the sensitivity. The sensitivity, defined as the response magnitude, was expressed by the following equation [11]:
where C0 represents the capacitance of the sensor in air (baseline capacitance), Cgas is the capacitance of the sensor in air containing the target gas, and Cg is the gas concentration. Indeed, the Eq. 1 implies the relative change in the capacitance upon gas contact compared to baseline capacitance. The sensitivity value of gas sensor devices as a function of CO2 concentration, which is also known as calibration curve, has been presented in Fig. 7.
From one hand, we observe a similar pattern, and the sensitivity of all devices increases as the CO2 concentration increases from 0.1 to 1%. On the other hand, it can be seen that the addition of CuPc to polypyrrole further improves the sensitivity of both D2 and D3 devices to CO2 gas in which the D3 device, containing polypyrrole nanofibers, quite interestingly exhibited stronger response. For example, at 0.5% CO2 concentration, the sensitivity of the PPy/CuPc nanocomposite network was nearly 15 times higher than granular PPy, and almost 2 times higher than the particle-like PPy/CuPc aggregate. Nevertheless, in all cases, according to standard calibration curve, the sensitivity is almost linearly proportional to the concentrations of the CO2 gas indicating the absence of saturation phenomena at tested range. Such observed linear trends may be of interest in view of possible applications. Considering from calibration curve, it is expected that the CO2 gas can be detected at further high concentration although we have not yet examined the sensing characteristics at higher than 1% CO2. According to the calibration curves’ data, the limit of detection (LOD) values for prepared sensors is computed and tabulated in Table 1.
For possible practical applications, the reproducible response of a gas sensor is important component to be considered. Therefore, the repeatability of sensor response for all devices upon cyclic exposure, for example to 0.5% CO2 gas, was examined and results are shown in Fig. 8. The response amplitudes of all devices have nearly been retained over five cycles of exposure to CO2 and recovery, exhibiting good repeatable and stable characteristic during alternate injection/vent, with an insignificant deviation.
To testify long-term stability, we also checked out the performance of the fabricated gas sensors to a fixed concentration (0.5%) of CO2 gas continuously for a period of 30 days. The stability performance of gas sensor devices has been shown in Fig. 9.
The D1 device was nearly stable for the first 11 days; however, its sensitivity drops about 19.7% after a period of 30 days compared to initial sensitivity. It is found that the sensitivity of D2 and D3 devices did not change to a noticeable extent for the first 16 days. Interestingly, compared to 10.3% sensitivity decrease of D3 device (PPy/CuPc nanofiber composite), the sensitivity of D2 device (particle-like PPy/CuPc nanocomposite) decreased by only 3% after 30 days of continuous operation. In spite of that, the response of both nanocomposites is more considerable than the corresponding sensor based on only PPy during periodic exposure to the target gas. Table 2 presents a comparative study of the present work with other literatures.
3.4 Gas-sensing mechanism
The exact gas-sensing mechanism of the fabricated IDC gas sensors is not completely understood at present, and it is a matter of conjecture. However, the possible sensing mechanisms will qualitatively be discussed based on the obtained results.
Generally, the gas sensor responses arise from interaction between target gas and the sensing material. The approach adopted here is based on considerations that the applied alternate electric field between the interdigitated electrodes will cause polarizing the medium (sensing layer). When the gas sensor device is exposed to CO2 gas, the CO2 molecules undergo physical or chemical adsorption in the surface of sensing layer. This adsorption can cause changes in the dielectric properties which can then be measured as a change in the capacitance. But which type of adsorption? It should be pointed out that chemical adsorption (Chemisorption) requires relatively higher energy. Since the recovery time of the sensors response was fast and the gas-sensing tests were carried out at room temperature, the chemisorption is limited. Thus, overall, the response of the IDE capacitive-type gas sensors relies on the physisorption of CO2 molecules in the sensing layers (PPy and PPy/CuPc) leading to a response due to the change of the dielectric properties. Besides, the use of an interdigitated design maximizes the area available to the gas interface and offer significant improvement in capacitive sensing especially for the porous sensing layer [40]. A schematic diagram of the sensing mechanism is illustrated in Fig. 10.
4 Conclusion
In summary, we developed interdigitated capacitive-type (IDC) gas sensor devices based on the PPy and PPy/CuPc nanocomposites and investigated their sensing properties toward exposure to different concentrations of CO2 gas ranging from 0.1 to 1% at room temperature. The two types of PPy/CuPc nanocomposites were successfully synthesized in the presence and absence of cationic surfactant CTAB, in which the PPy has two different morphologies: particle-like and highly interconnected network of nanofibers. An accordance to capacitance changes of devices after exposing them to different concentrations of CO2 gas at room temperature, we found that the PPy/CuPc nanocomposite network exhibits better response than particle-like PPy/CuPc nanocomposite and pure PPy. The sensitivity of the sensors was observed to be increasing linearly with CO2 concentration, in which we noticed that the sensitivity of PPy/CuPc nanocomposite network is higher than that of particle-like PPy/CuPc nanocomposite and much higher than that of pure PPy. The better sensing performance of PPy/CuPc nanocomposite network to CO2 gas can be attributed to fast and rapid diffusion of the gas into the sensing layer according to very porous structure of the sensing layer, interconnected network of the PPy nanofibers, higher surface-to-volume ratio of the nanofibrillar structure of PPy, and a long with synergistic effect of the properties of the individual materials (CuPc and PPy) in the nanocomposite. Moreover, monitoring the sensing performance of devices reveals that the PPy/CuPc nanocomposites are considerably stable despite a slight decrease in sensitivity.
Based on the presented results here, it is anticipated that this research can be extended to synthesis of other conducting polymer and phthalocyanine nanocomposites to provide opportunities for fabricating low-cost, reliable, sensitive, and innovative gas sensors. In this respect, future research will be involved in further improving and optimizing fabrication conditions and addressing problems so that the nanocomposites could be incorporated into practical gas-sensing devices.
References
G. Gerlach, U. Guth, Carbon Dioxide Sensing (Wiley Online Library, 2015)
T.C. Doan, R. Ramaneti, J. Baggerman, J.F. van der Bent, A.T. Marcelis, H.D. Tong, C.J. van Rijn, Sens. Actuators B 168, 123 (2012)
E.P. Cummins, A.C. Selfridge, P.H. Sporn, J.I. Sznajder, C.T. Taylor, Cell. Mol. Life Sci. 71, 831 (2014)
S. Neethirajan, D. Jayas, S. Sadistap, Food Bioprocess Technol. 2, 115 (2009)
D. Zhao, D. Miller, X. Xian, F. Tsow, E.S. Forzani, Sens. Actuators B 195, 171 (2014)
C.-J. Chiang, K.-T. Tsai, Y.-H. Lee, H.-W. Lin, Y.-L. Yang, C.-C. Shih, C.-Y. Lin, H.-A. Jeng, Y.-H. Weng, Y.-Y. Cheng, Microelectron. Eng. 111, 409 (2013)
H. Liu, J. Kameoka, D.A. Czaplewski, H. Craighead, Nano Lett. 4, 671 (2004)
S. Matsubara, S. Kaneko, S. Morimoto, S. Shimizu, T. Ishihara, Y. Takita, Sens. Actuators B 65, 128 (2000)
A.R. Choudhary, S.A. Waghuley, Iran. Polym. J. 28(11), 933 (2019)
S. Srinives, T. Sarkar, R. Hernandez, A. Mulchandani, Anal. Chim. Acta 874, 54 (2015)
M. Rahimabady, C.Y. Tan, S.Y. Tan, S. Chen, L. Zhang, Y.F. Chen, M. Bolt, Sens. Actuators B 243, 596 (2017)
V. Pentyala, P. Davydovskaya, M. Ade, R. Pohle, G. Urban, Sens. Actuators B 225, 363 (2016)
F.R. Juang, W.C. Chern, B.Y. Chen, Thin Solid Films 660, 771 (2018)
J. Boudaden, A. Klumpp, H.-E. Endres, I. Eisele, Multidisciplinary Digital Publishing Institute Proceedings 1, 472 (2017)
N. Yamazoe, Sens. Actuators B 108, 2 (2005)
R. Moos, K. Sahner, M. Fleischer, U. Guth, N. Barsan, U. Weimar, Sensors 9, 4323 (2009)
V. Buzanovskii, Rev. J. Chem. 4, 33 (2014)
D.W. Hatchett, M. Josowicz, Chem. Rev. 108, 746 (2008)
L. Qie, L.-X. Yuan, W.-X. Zhang, W.-M. Chen, Y.-H. Huang, J. Electrochem. Soc. 159, A1624 (2012)
T. Wang, W. Zhong, X. Ning, Y. Wang, W. Yang, J. Colloid Interface Sci. 334, 108 (2009)
A. Wu, H. Kolla, S.K. Manohar, Macromolecules 38, 7873 (2005)
Z. Liu, X. Zhang, S. Poyraz, S.P. Surwade, S.K. Manohar, J. Am. Chem. Soc. 132, 13158 (2010)
X. Zhang, J. Zhang, W. Song, Z. Liu, J. Phys. Chem. B 110, 1158 (2006)
J. Jang, H. Yoon, Langmuir 21, 11484 (2005)
M. Mazur, J. Phys. Chem. B 113, 728 (2008)
H.P. De Oliveira, S.A. Sydlik, T.M. Swager, J. Phys. Chem. C 117, 10270 (2013)
J.L. Yagüe, S. Borrós, Plasma Processes Polym. 9, 485 (2012)
T. Dai, X. Yang, Y. Lu, Nanotechnology 17, 3028 (2006)
Y. Liao, X.-G. Li, R.B. Kaner, ACS Nano 4, 5193 (2010)
P. Song, Q. Wang, Z. Yang, Mater. Lett. 65, 430 (2011)
D. Tiwari, R. Sharma, K. Vyas, M. Boopathi, V.V. Singh, P. Pandey, Sens. Actuators B 151, 256 (2010)
S. Kumar, N. Kaur, A.K. Sharma, A. Mahajan, R. Bedi, RSC Adv. 7, 25229 (2017)
S. Sivamalar, J. Shanthi, P. Kalugasalam, Int. J. Mod. Eng. Res. 2, 3032 (2012)
C. Lu, Z. Chen, Int. J. Hydrogen Energy 35, 12561 (2010)
T. Ishihara, K. Kometani, Y. Mizuhara, Y. Takita, J. Am. Ceram. Soc. 75(3), 613 (1992)
R.R. Desai, D. Lakshminarayana, P.B. Patel, C.J. Panchal, Sens. Actuators B 107(2), 523 (2005)
C.J. Chiang, K.T. Tsai, Y.H. Lee, H.W. Lin, Y.L. Yang, C.C. Shih, K.C. Ho et al., Microelectron. Eng. 111, 409 (2013)
D.Y. Kim, H. Kang, N.J. Choi, K.H. Park, H.K. Lee, Sens. Actuators B 248, 987 (2017)
R. Wimmer-Teubenbacher, F. Sosada-Ludwikowska, B. Travieso, S. Defregger, O. Tokmak, J. Niehaus, A. Köck et al., Chemosensors 6(4), 56 (2018)
M. Babaei, N. Alizadeh, Sens. Actuators B 183, 617 (2013)
Acknowledgements
The authors wish to express their gratitude to Dr. Mahmood Kazemzad at Department of Energy, Materials, and Energy Research Center for valuable expertise. We are also thankful to Dr. Salar Pourtiemoor at Shahrood University of Technology for helpful comments and discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riyazi, S., Azim Araghi, M.E. Performance of interdigitated capacitive-type CO2 sensor based on polypyrrole/copper phthalocyanine nanocomposite. J Mater Sci: Mater Electron 31, 3539–3548 (2020). https://doi.org/10.1007/s10854-020-02902-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-02902-0