Abstract
In the present research article, a detailed study on the synthesis, characterization, and structure–property correlation study of the vinyl ester resin (AVEOCN) based on the rosin modified o-cresol-formaldehyde epoxy novolac resin has been done. The rosin was condensed with o-cresol formaldehyde novolac resin to obtain the product (AOCN). The AOCN resin was epoxidized and subsequently esterified with methacrylic acid using triphenylphosphine as a catalyst and inhibitor hydroquinone to get vinyl ester resin (AVEOCN) having acid value ~ 7 mg of KOH per gram of solid. The chemical structures were confirmed using FT-IR, 1H-NMR, 13C-NMR, and DEPT-135° spectroscopic techniques, and their number average molecular weights were evaluated using 1H-NMR spectroscopy as well as Gel Permeation Chromatographic technique (GPC). The curing dynamics of synthesized VER with lignin modeled compounds, methacrylated eugenol (ME) and methacrylated guaiacol (MG), and petroleum-based styrene as reactive diluents was studied using Differential Scanning Calorimetry (DSC). The thermal stability analysis and mechanical performance of the VER samples were done using Thermogravimetric analysis (TGA) and Universal Testing Machine (UTM), respectively. Chemical resistance tests of the above VER samples were also assessed via exposing the sample coated panels to the different chemical environments for 90 days and their % weight loss was determined. The surface morphology of exposed samples was also studied using Scanning Electron Microscopy (SEM). The results obtained were compared to VER systems based on petroleum products and epoxy resins systems and the superior performance of rosin-modified VER systems indicate that they are suited for pressing demands for coating applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vinyl ester resin is a versatile class of thermosetting polymers. These are extensively used in numerous industrial applications as matrix for composite materials, nanostructured composites, coating materials, or adhesives owing to their excellent handling characteristics, ease of cure, high strength, heat performance, and corrosion resistance which include marine, industrial, building and construction, etc. [1, 2]. Presently, concerns over the environmental issues due to the volatile organic content (VOC) emissions and diminishing petroleum resources have led to a growing quest to develop environment-friendly vinyl ester resins. Such types of vinyl ester resins can serve as an alternative to purely petroleum-based vinyl ester resins [3, 4].
The use of phenol–formaldehyde resins in varnishes is the oldest application of phenolic resins but their brittleness and dark color limit their applicability. These resins, however, initially had limited application in spirit varnishes. Modification of phenol–formaldehyde resins with gum rosin made them oil-soluble and the product could be dissolved in drying oil to make varnishes and printing ink bases that showed quick-drying property, a prerequisite for the application [5,6,7]. Rosin, a natural resin, contains abietic acid and its double bond isomers as main components. The presence of a hydrophobic skeleton in combination with hydrophilic carboxylic groups in rosin enhances its solubility and compatibility with a variety of other synthetic resins also [8,9,10,11,12]. Rosin-modified phenolic resins were first produced by BEHRENDS by the polycondensation of phenols, formaldehyde, and rosin. The most common phenols, used in the modification of rosin, are 4-tert-butylphenol and 4, 4′-diphenyl propane (bisphenol-A). A large number of rosin-modified phenolic resins can be synthesized by either varying the type of phenol or the ratio of phenol to formaldehyde [13]. Rosin-modified phenolic resins show good compatibility with alkyd resins and is widely used in alkyd coatings, printing inks, especially vehicles for gravure and offset printing [14, 15]. These show good compatibility with various animal and vegetable oils as well. The amalgamation of rosin-modified phenolic resins with any other polymer used in coating accelerates its drying, increases the resistance to water and chemicals, and improves the gloss. Okoshi et al. [16] prepared rosin modified phenolic resins for printing inks, by reacting resole type phenol–formaldehyde, animal or vegetable oil, and rosin. De Blasi et al. [17] synthesized phenolic resole modified with rosin ester obtained from the reaction of rosins with dienophile and phenolic resoles. These were then further esterified with polyfunctional hydroxyl compounds. The resulting compound was used as a vehicle for publication gravure printing inks. It was reported to exhibit excellent gloss property and fast curing rates. D.W. Kang et. al. [18] modified p-nonylphenol and p-formaldehyde based resole resin with rosin ester, prepared by esterification of rosin with diols such as ethylene glycol, propylene glycol, diethylene glycol, glycerol, and pentaerythritol, respectively. The physicochemical properties such as viscosity, softening point, acid value, glass transition temperature, and decomposition temperature of above-modified p-nonylphenol and p-formaldehyde based resole resin were also determined which supported its use as a self-structured ink vehicle for printing inks. Everett Crews [19] also prepared improved polyol esters from rosin modified phenol–formaldehyde resin and polyimide/polyamide derivatives which found their wide applications in lithographic inks.
The present research article reports the synthesis and comprehensive structural characterization of rosin modified o-cresol-formaldehyde resins, which is further, employed as the scaffold material for the synthesis of environment-friendly vinyl ester resin. The thermal, mechanical properties and chemical resistance of the synthesized vinyl ester resin were further evaluated for its application as coatings. Furthermore, these bio-derived VER containing non-volatile reactive diluents (MG & ME) can serve as an alternative to purely petroleum-based vinyl ester resins thereby reducing the environmental concerns.
Experimentation
Material
Gum rosin (WW grade), o-cresol (CDH) and formaldehyde (37–41% (wt/v) solution, Fisher scientific), and p-toluenesulfonic acid (pTSA) (Lobachemie, 99% pure) were used for the preparation of rosin modified o-cresol-formaldehyde novolac resin (AOCN). Epichlorohydrin (Avra, 99% pure), sodium hydroxide pellets (Avra, 85% pure) were used for the preparation of epoxy resin (AEOCN) from rosin modified o-cresol-formaldehyde novolac resin (AOCN). Toluene (Merck, 99% pure) was used for precipitating and separating salt (NaCl) from epoxy resin (AEOCN). Methacrylic acid (Lobachemie, 99% pure), triphenylphosphine (Lobachemie, 98% pure), and hydroquinone (Nice Chemicals Pvt. Ltd., Cochin) was used for the synthesis of vinyl ester resin (AVEOCN). Methacrylated eugenol (ME), methacrylated guaiacol (MG), and styrene (ACROS ORGANICS) were used as reactive monomers in the present study. All the reagents were used as received.
Procedures
Synthesis of vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin (AEOCN)
The synthetic route for vinyl ester based on rosin modified o-cresol-formaldehyde epoxy novolac resin (AVEOCN) is shown in Scheme 1. Initially, o-cresol-formaldehyde novolac resin (EOCN) was prepared by reacting the o-cresol and formaldehyde in 1:0.7. This novolac resin was subsequently condensed with rosin in a 1:2 molar ratio at temperature 150 ± 5 °C to give rosin modified o-cresol-formaldehyde novolac resin (AOCN). This AOCN (1 mol) was then epoxidized using epichlorohydrin (5 mol) and KOH (0.2 mol) to form epoxy resin (AEOCN). The detailed procedure for the synthesis of epoxy resin AEOCN and structural characterization of AOCN and AEOCN is reported in our previous study [20].
Vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin (AEOCN) was prepared using 1:0.9 molar ratios of rosin modified o-cresol-formaldehyde epoxy novolac resin (AEOCN) and methacrylic acid in addition to triphenylphosphine as a catalyst (1 phr by weight of epoxy resin) and hydroquinone as an inhibitor (200 ppm) at 85 °C [21]. The esterification reaction was done for 3.5 h to acquire a product with a yield of 96.2% and an acid value of ~ 7 mg of KOH/gm solids determined as per ASTM D 1636. Triphenylphosphine had been used as a catalyst (1 phr by weight of epoxy resin) in the esterification of epoxy resin because it acted effectively at moderately elevated temperatures [8, 22,23,24]. The reaction was carried out at 85 ± 1ºC in the presence of approximately 200 ppm of hydroquinone, as it prevented the premature gelation of vinyl ester resins. It has been reported that it is impracticable to esterify the epoxy resin on the whole as the gelation of the product was attained before the acid value approaches 10 mg KOH/g solids. Hence, esterification reactions are always carried out in excess epoxide resin [25]. The synthesized vinyl ester resin was then stored in a refrigerator at 10 °C.
Synthesis of bio-based reactive monomers
The synthetic route for the synthesis of lignin model compounds based reactive monomers methacrylated guaiacol (MG) and methacrylated eugenol (ME) is presented in Scheme 2. These reactive monomers were synthesized according to the procedure described in our previous study [26].
Characterization
Structural characterization
The structural characterization of AVEOCN was done using FT-IR, 1H-NMR, and 13C-NMR spectroscopy. For recording the FTIR spectra, the samples were dissolved in chloroform and subsequently smearing the solution on the KBr disc followed by evaporation of the solvent. Perkin Elmer FT-IR Spectrometer was used for this purpose. The 1H-NMR, 13C-NMR, and DEPT-135° spectra of these samples were recorded with a Bruker Avance II 400 MHz NMR spectrometer in DMSO, and tetramethylsilane was used as an internal standard.
Gel permeation chromatography (GPC)
The weight average molecular weights \(\overline{{(M_{w} )}}\) and number average molecular weights \(\overline{{(M_{n} )}}\) of all the products synthesized during the research work were recorded with Perkin Elmer Turbo matrix-40 (U.S.) Gel Permeation Chromatograph. Crosslinked polystyrene column was used for GPC calibration and Tetrahydrofuran (THF) was used as a mobile and stationary phase.
Physical properties of VERs
The refractive indices (RIs) of the vinyl ester resin (AVEOCN) samples were measured via an A.KRUSS Optronic Abbe’s refractometer AR2008 (Germany). Viscosities of the vinyl ester resin (AVEOCN) samples containing MG, ME, and styrene (40% w/w), were determined via LVDV II + Pro Brookfield viscometer (USA) with spindle no L62 at 120 rpm. The density of the above samples was determined using a Pycnometer at 20 °C.
Dynamic curing and thermal stability of VERs
Different samples for curing and thermal stability analysis were prepared by mixing 10/4/0.2 (w/w) of vinyl ester resin (AVEOCN), styrene/methacrylated guaiacol (MG)/methacrylated eugenol (ME) as reactive monomers, and benzoyl peroxide (free radical initiator), respectively, in a beaker and stirred briskly at 30 °C to give a homogeneous mixture. DSC scans of the VER samples were recorded using Perkin Elmer Diamond Differential Scanning Calorimeter (U.S.) under a nitrogen atmosphere with ≈ 2 mg of the sample and the scans were performed at a program heating rate of 10 °Cmin−1 ranging from 30 °C to the temperature where the completion of exothermic reactions occur.
The thermal stability of the samples cured isothermally at 90 ± 2 °C in a hot air oven was evaluated by thermogravimetry. TG/DTG/DTA analysis was carried out on EXSTAR TG/DTG SII by taking 11 ± 5 mg of the sample under nitrogen atmosphere having a flow rate of 200 ml min−1 and heating rate of 10 °Cmin−1. The quantitative estimation of relative thermal stability of the vinyl ester resin samples was done by comparing the temperatures for a particular degree of weight loss.
Mechanical properties
The samples for analyzing the mechanical performance were prepared by mixing 10/4/0.2 (wt/wt) of the vinyl ester resin, styrene, MG, and ME as reactive monomers, respectively, and free radical initiator-benzoyl peroxide in a beaker. The mixture was then poured into specially designed molds of dimension (150 × 150 × 5.0 mm3) and cured at a temperature determined from the DSC scan for a particular sample under a load of 100 kg/cm2 for 12 h in a compression-molding machine. The samples were cut apart with the help of a metallurgical saw into different sizes required for the type of test to be carried out according to the test specifications.
For the analysis of the mechanical performance of all the samples such as tensile, compressive, and flexural strength, a Hounsfield-25KN H50KS (U.K.) universal testing machine at a strain rate of 2 mm/min was used. For determining the tensile strength (ASTM D3039), the span length was fixed as 50 mm. In the case of compressive strength (ASTM D3410) the test specimens were held between two platforms of the machine and the compressive load was applied over 25 mm span length. The three-point flexural strength (ASTM D790) was determined by fixing the span length to 50 mm. The impact tests (ASTM D256) were also carried out for these samples using a plastic impact tester Tinius Olsen IT 406 (U.S.A) that determines the notch impact strength having 2 mm depth under the notch. Three replicates of each sample were used for the measurement of each mechanical property. The mean value and standard deviation of each mechanical property were determined.
Chemical resistance and corrosion resistance of Cured AVEOCN resin samples
Mild steel panels (15 cm × 10 cm), prepared according to British standard specification 1449, were used to evaluate the chemical and corrosion resistance of AVEOCN samples containing styrene, MG, and ME as reactive monomers. One side of the panel was coated with coal tar epoxy primer to protect against the chemical environment used to evaluate the chemical and corrosion resistance. While the tested side of the panel was coated with AVEOCN resin containing styrene, MG, and ME as reactive monomers and cured at 90 ± 5 °C for 24 h. Chemical resistance behavior as a function of % weight loss of the cured VER samples coated on mild steel panels was determined by dipping the layered panels of known weights in 1 M HCl, 1 M NaOH, and 1 M NaCl aqueous solutions at room temperature for 90 days. % weight loss was then calculated as follows:
where, Wi = Dry weight of the sample before immersion
Wf = Dry weight of the sample after immersion
Scanning electron microscopy
The morphological changes that appeared on the surface of coated AVEOCN resin samples, cured with MG, ME, and styrene, caused by chemical exposure were studied via the Scanning electron microscopic technique (SEM) for corrosion resistance of the cured AVEOCN resin samples. For SEM analysis, a JSM-6610 scanning electron microscope was used, and to increase the conductivity a thin film of gold was mounted on the samples before taking the SEM images.
Results and discussion
Synthesis and characterization of AVEOCN resin
To investigate the effect of rosin modification of the o-cresol-formaldehyde novolac resins being used as a scaffold material for the synthesis of respective vinyl ester resins and to investigate their subsequent application in coatings, linear rosin modified o-cresol-formaldehyde novolac resin has been synthesized via electrophilic aromatic substitution [27].
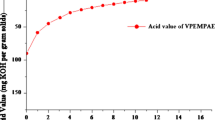
Rosin modified o-cresol-formaldehyde epoxy novolac resin (AEOCN) based vinyl ester resin (AVEOCN) was prepared by reacting rosin modified o-cresol- formaldehyde epoxy novolac resin (AEOCN) with methacrylic acid in 1:0.9 molar ratio, in the presence of triphenylphosphine as catalyst and hydroquinone as an inhibitor. The reaction was monitored for the acid value and the product with acid value ~ 7 mg KOH/g solid, was obtained. Figure 1 illustrates the change in the acid value with reaction time. It can be observed from the figure that the acid value decreased with the reaction time and the behavior is similar to the typical catalyzed polyesterification reactions. The non-linear behavior of acid value with reaction time at the initial stage was attributed to the greater availability of the reactive sites and the larger possibility of association of acid and epoxide groups.
The FT-IR spectrum of synthesized vinyl ester resin (AVEOCN) in Fig. 2 presented the distinguishable broad absorption band at 3431 cm−1 owing to secondary hydroxyl groups. The widening of the absorption peak at 1715 cm−1 attributable to the merging of the stretching vibrations of carbonyl C = O of the ester group attached to the rosin skeleton and carbonyl C = O of the methacrylate end group. The intensity of the stretching bands due to the conjugated alkene bonds at 1636 and 1613 cm−1 present in the phenanthrene ring of rosin also increased as a result of the introduction of more C = C bond of methacrylate groups. The 1H-NMR spectrum of AVEOCN has been shown in Fig. 3. A broad proton resonance signal due to secondary –OH group (proton 5) was observed at 2.7–3.0 ppm The methacrylated epoxy groups showed distinct resonance signals in 1H-NMR spectra of AVEOCN, where the methylene protons of methacrylate group appeared at 6.2 ppm and 5.5 ppm (protons 6 and 7) and the signal at 1.9 ppm was due to methyl protons (proton 8) of methacrylate end group. Figure 4 showed the 13C-NMR spectrum of AVEOCN in which resonance signals for –CH3 (carbon 6), = CH2 (carbon 4), and –C = CH2 (carbon 3) of methacrylate group appeared at 17.8, 123, and 136.8 ppm, respectively. The resonance signals at 167.42 ppm and 179 ppm region were due to the carbonyl group of an ester of methacrylate group (carbon 2) and the carbonyl group of ester (carbon 5) present on the rosin skeleton present in AVEOCN, respectively.
The resin (AVEOCN) thus formed, was further used to study the thermal and mechanical properties using styrene (Sty), methacrylated guaiacol (MG), and methacrylated eugenol (ME) as reactive monomers. The physio-chemical properties of the vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin samples have also been summarized in Table 1.
Molecular weight determination
The molecular weight of vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin has also been determined by calculating the number of methacrylate groups present on the o-cresol-formaldehyde epoxy novolac based vinyl ester resin component and rosin component of the AVEOCN [28]. The number of methacrylate groups (A) was evaluated using the 1H-NMR spectroscopic technique.
The number of methacrylate groups (A1) present on the o-cresol-formaldehyde epoxy novolac based vinyl ester resin component of AVEOCN can be calculated by taking the ratio of the integration of aromatic ring protons observed between 7.5 and 6.5 ppm to the –CH2 proton of the methacrylate group (proton 6,7) which appeared at 5.6 and 6.1 ppm (Fig. 3).
Similarly, the number of methacrylate groups (A2) present on the rosin component of AVEOCN can also be determined by taking the ratio of the integration of methyl (-CH3) proton present on rosin which appeared between 1.2 and 0.8 ppm to the –CH2 proton of the methacrylate group (proton 6,7) appeared at 5.6 and 6.1 ppm (Fig. 3)
The addition of the number of methacrylate groups present on the o-cresol-formaldehyde epoxy novolac based vinyl ester resin component (A1) and vinyl ester resin based on rosin moiety (A2) in the AVEOCN molecule gave the total number of methacrylate groups (A) which was found to be 4.3. The value of A can be employed to calculate the molecular weight of AVEOCN using the formula (1368 + 86A), where 1368 g/mol is the molecular weight of AEOCN, 86 is the molecular weight of one methacrylate group and A is the number of total methacrylate groups per molecule present in AVEOCN. Using 1H-NMR spectroscopy, the molecular weight of AVEOCN is computed to be 1738 g/mol, which is almost comparable to the value obtained from the GPC graph of the AVEOCN shown in Fig. 5. The value of the molecular weight of AVEOCN determined from the GPC technique has been tabulated in Table 2.
The number average molecular weight of AOCN, AEOCN, and AVEOCN calculated from the 1H-NMR have been presented in Table 2 and were found to be comparable to the number average molecular weight obtained from the GPC technique.
Dynamic curing studies
The curing behavior of the samples containing vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin, styrene/methacrylated eugenol (ME)/methacrylated guaiacol (MG) as reactive monomers, and benzoyl peroxide as free radical initiator has been studied using Differential Scanning Calorimeter (DSC). The curing reaction between vinyl ester resin (AVEOCN) and styrene, MG, and ME as reactive monomers follow a similar reaction scheme as reported in previous research articles [29]. The DSC exotherms shown in Figs. 6a-c represent the curing of vinyl ester resin (AVEOCN) with reactive monomers MG, ME, and Styrene, respectively, at a programmed heating rate of 10˚C/min. A single exothermic peak in the range of 85–150˚C due to the heat flow for the curing of vinyl ester resin (AVEOCN) with all the reactive monomers was observed. The DSC curing exothermic temperatures and their corresponding enthalpies (ΔH) for all the cured vinyl ester resin systems have been summarized in Table 3. The decomposition of the benzoyl peroxide under the influence of temperature generated free radicals which open the double bond of vinyl groups of resins and reactive monomers which subsequently rapidly undergoes an exothermic reaction and formed a thermosetting network [30]. On comparing the onset (Tonset) temperatures 85.2, 86.7, 94.4˚C and peak (Tpeak) temperatures 107.3, 112.3, 114.1˚C for vinyl ester resin (AVEOCN) cured with MG, ME, and styrene, respectively, it was examined that the AVEOCN samples containing MG and ME have lower onset and peak temperatures as compared to the sample containing styrene. The proposed crosslinked network structure formed by curing of synthesized vinyl ester resin with different reactive monomers has been shown in Scheme 3. The lesser onset and peak temperatures for the samples containing MG and ME demonstrated their higher reactivity towards vinyl ester resin based on rosin modified o-cresol-formaldehyde epoxy novolac resin (AVEOCN) than that of styrene which must be due to the more polar methacrylate radicals formed by MG and ME as compared to styrene. It can also be observed that the reactivity of methacrylated eugenol (ME) was less than that of methacrylated guaiacol (MG). The lower reactivity of ME was due to the formation of resonance stabilized allylic free radical present at the para position to methacrylate group in ME. This free radical competed with the normal propagation reaction step during the curing reaction between ME and AVEOCN resin system by degradative chain transfer (DCT) reaction as reported in our previous research articles. The results of this study were compared to the o-cresol epoxy novolac based vinyl ester resin (VEOCN), based on petroleum products, synthesized in our previous study [31] that showed onset temperatures as 94.1, 97.7, and 105.2˚C and peak temperatures as 110.2, 115.6 and 118.2˚C on curing with MG, ME and styrene, respectively. The VEOCN resin samples exhibited high temperature curing as compared to AVEOCN samples which may be attributed to more functionality in AVEOCN that enhances the possibility of crosslinking and hence increasing the reactivity of rosin modified vinyl ester resin. The results were also compared to cardanol-based VER cured with styrene as a reactive monomer that reported curing temperatures from 96–128˚C which is higher than that of rosin modified vinyl ester resin (AVEOCN) [32]. Furthermore, AEOCN epoxy resin which is a predecessor of AVEOCN vinyl ester resin was cured with imidoamine curing agents in our previous study [20] showed high-temperature curing (117–115˚C) as compared to vinyl ester resin AVEOCN that ensures the higher reactivity of vinyl ester resins as compared to epoxy resins. The enthalpy of reaction (∆H) for AVEOCN containing styrene, MG, and ME has also been mentioned in Table 3. It can be observed from the table that the AVEOCN samples containing MG and ME have a lower enthalpy of reaction (∆H) thus indicating the higher degree of curing or crosslinking in AVEOCN samples cured with styrene [33]. The DTA runs were carried out for cured VERs to analyze the residual curing and the absence of exotherms authenticate the complete curing of samples.
Thermal stability of vinyl ester resins
The thermogravimetric (TG/DTG/DTA) analysis of cured vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin containing styrene/methacrylated eugenol (ME)/methacrylated guaiacol (MG) as a reactive monomer and benzoyl peroxide as free radical initiator was conducted under dynamic conditions with 10 °C/min heating rate ranging from 30 °C to the temperature where the exothermic reactions were completed. The comparative thermogravimetric (TGA) scans for isothermally cured samples of AVEOCN containing styrene, methacrylated eugenol (ME), methacrylated guaiacol (MG) as reactive monomers have been illustrated in Fig. 7 that displays the % weight loss with temperature for all the vinyl ester resin samples. The cured samples of vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin containing styrene, methacrylated eugenol (ME), methacrylated guaiacol (MG) showed the single-step mass loss in TG trace which depicted the single-step decomposition behavior of the samples.
The relative thermal stability of the AVEOCN samples containing styrene, MG, and ME as reactive monomers was compared by noting the initial decomposition temperature (IDT), final decomposition temperature (FDT), the temperature of maximum weight loss (Tmax), and also by determining percent char yield at 700 °C. From Fig. 7, the above temperatures i.e. IDT, FDT, Tmax, and char yield have been summarized in Table 4. The cured AVEOCN samples containing MG, ME, and styrene showed IDT at 350, 365, and 340 °C and Tmax at 431, 436, and 405 °C, respectively. These findings revealed that the AVEOCN samples containing ME and MG possessed elevated IDT, FDT, and char yield values than that of styrene as a reactive monomer. This confirmed the enhanced thermal stability of AVEOCN samples cured by using MG and ME as reactive monomers than samples containing styrene as reactive monomers.
The activation parameter i.e. activation energy (Ea) for the cured AVEOCN samples containing styrene, MG, and ME as reactive monomers has also been worked out by using the well-known non-isothermal integral method by Dharwadkar and Kharkhanawala [34] as reported in the research article [26]. The calculated activation energies of decomposition (Ea) and the decomposition temperatures for every 5, 10, and 20% weight loss of the cured AVEOCN samples containing styrene, MG, and ME as reactive monomers, calculated from the data obtained from the TGA analysis, have also been reported in Table 4. According to the observations drawn from Table 4, it is evident that cured AVEOCN samples containing MG and ME showed higher values of activation energies of decomposition (Ea) and elevated decomposition temperatures for every 5, 10, and 20% weight loss as compared to the AVEOCN samples containing styrene as reactive monomers. This observation revealed the enhanced thermal performance of the AVEOCN samples containing ME and MG than that of AVEOCN samples containing styrene. Increased thermal strength in AVEOCN samples containing MG and ME could be attributed to the increased degree of crosslinking in cured vinyl ester resin-MG/ME resin system which might be due to the presence of more reactive and polar methacrylate moiety in MG and ME monomers. Furthermore, the thermal data shows the preeminent results for the VER cured with ME which may be due to the presence of both allylic and vinylic free radical reactive sites in ME that undergo copolymerization with C = C of AVEOCN resulting in increased cross-linked density subsequently increasing the thermal stability of the cured VPEMPAE sample containing ME. These results were compared to a previously reported study [35] wherein cardanol-based vinyl ester resins were cured with styrene and glycidyl methacrylate as reactive monomers that showed initial thermal decomposition at 210ºC and maximum decomposition up to 460ºC which is lesser than that of rosin-modified vinyl ester resin (AVEOCN). Furthermore, the rosin-modified o-cresol based vinyl ester resin (AVEOCN) showed higher thermal performance than o-cresol based vinyl ester resin cured (VEOCN) with MG, ME, and styrene in our previously reported study [31]. The cured VEOCN samples exhibited lower IDT and Tmax values as compared to AVEOCN samples and the reason may be the higher cross-linked structure of cured resins formed by more functional AVEOCN resin. The thermal properties of rosin modified o-cresol based vinyl ester resin was also compared to rosin modified o-cresol based epoxy resins (AEOCN) cured with different curing agents in our previous study [20] which showed thermal stability of cured epoxy resins to a temperature range of 310-420ºC whereas the cured vinyl ester resin exhibited thermal stability to 340-455ºC temperature range. The activation energies and the char residue were also found to be higher in the case of rosin-modified VER samples cured with different reactive diluents. These results specify the superior performance of rosin-modified vinyl ester resin thermosets than epoxy resin thermosets thus possibly will be a preferred choice for polymer industries.
Mechanical performance of AVEOCN samples
The mechanical performance i.e. compression, flexural, tensile, and impact strength, young’s modulus of the rosin modified o-cresol-formaldehyde epoxy novolac resin-based vinyl ester resin (AVEOCN) prepared using styrene, methacrylated eugenol (ME), and methacrylated guaiacol (MG) have been reported in Table 5. The compression, flexural and tensile strength for all the VER samples have been compared and represented in a bar graph as shown in Fig. 8. The data from Table 5 and Fig. 8 clearly shows the better strength for samples cured with bio-based reactive diluents MG and ME as compared to petroleum-based styrene. The mechanical behavior of the vinyl ester resins depends on the physical structure and chemical composition of the vinyl ester resin and the reactive monomers. Secondary interactions between the polymeric chains and the degree of crosslinking of the cured vinyl ester resin system also affect the mechanical properties of the vinyl ester resin samples. Moreover, the increased polymeric chain interactions between the secondary hydroxyl group of AVEOCN and carbonyl group of MG and ME due to intermolecular hydrogen bonding and polar attraction also tended to decrease the chain mobility and resisted the deformation and AVEOCN matrix breakup. The mechanical performance of the AVEOCN samples containing ME was found to be lesser as compared to that of MG which might be attributed to the presence of two functionalities i.e. allylic group as well as methacrylic group in ME which could undergo copolymerization with the vinyl C = C group of AVEOCN and gave a highly crosslinked network. This in turn led to the brittleness in the samples and hence, deterioration of the mechanical performance of these samples as compared to the AVEOCN samples containing MG. The mechanical properties of cured AVEOCN samples containing ME were still observed to be higher than the AVEOCN samples containing styrene as reactive monomers due to the presence of more reactive methacrylate free radical present in ME, which resulted in the formation of a better-crosslinked network. The results were compared to VER based on bisphenol-A, a petroleum product, cured with styrene and α-methyl styrene as reported by Siva et. al. [36]. The paper reports the tensile and flexural strength as 30–35 MPa and 68–78 MPa, respectively, which was much lesser than the synthesized resin systems. Moreover, the rosin-modified VER samples exhibited better mechanical performance than the o-cresol based vinyl ester resin system (VEOCN) when cured with MG/ME/styrene which may be attributed to the increased functionality and incorporation of rosin phenanthrene rings that provide rigidity to the crosslinked network structure of resin system. When compared with rosin-modified o-cresol novolac epoxy resin (AEOCN) cured with imidoamine curing agents, cured AVEOCN system showed superior tensile, flexural, and compression strength that reveals vinyl ester resins lead to strong and tough thermosetting materials than epoxy resins.
Evaluation of chemical resistance
Chemical resistance studies of the AVEOCN samples cured using styrene, methacrylated eugenol (ME), methacrylated guaiacol (MG) as reactive monomers had been performed as a function of % weight loss upon plunging the vinyl ester resin-coated mild steel panels to the chemical environment i.e. 1 M HCl, 1 M NaOH and 1 M NaCl solutions for 90 days. The data obtained from the chemical resistance studies of VER samples containing styrene, MG, and ME as reactive monomers has been tabulated in Table 6. The results are quite encouraging in terms of coating applications. The degree of curing or crosslinking between the vinyl ester resin and the reactive monomers i.e. styrene, MG, and ME (determined using DSC studies) play a decisive role in determining the chemical resistance of the cured AVEOCN samples. It had been observed from Table 6 that the cured AVEOCN samples containing styrene exhibited higher percent weight loss as compared to the AVEOCN samples containing MG and ME as reactive monomers. This substantiated the enhanced acid, base, and salt resistance of AVEOCN samples containing MG and ME as compared to that of AVEOCN samples containing styrene, which could be attributed to increased crosslinking in the polymer network structure and more polar functionalities present in the vinyl ester sample network containing ME and MG. The chemical resistance of the AVEOCN samples containing ME was found to be even greater than that of AVEOCN samples containing MG due to its potential ability to undergo copolymerization using two cross-linkable functionalities i.e. allylic group as well as methacrylic group hereby enhancing crosslinking in the resin system. These results were compared to o-cresol based vinyl ester (VEOCN) cured with MG/ME/Styrene and were found to be less stable in the chemical environment as compared to rosin-modified VER samples due to increased functionality of AVEOCN and more rigid structure formed by the introduction of rosin moieties. Also, when compared with the chemical resistance shown by AEOCN epoxy cured with imidoamine curatives, the VER samples displayed much higher resistance to chemical solutions that authenticate the use of vinyl ester resins for coating applications.
Evaluation of surface morphology by SEM
The mild steel panels coated with cured AVEOCN samples (containing styrene, MG, and ME as reactive monomers) taken for the chemical resistance studies, were also evaluated for topographical or morphological changes that occurred due to exposure to the chemical environment via scanning electron microscopy (SEM). Figure 9 shows the SEM images for all the samples before and after immersing to 1 M HCl, 1 M NaOH, and 1 M NaCl chemical solutions and it was observed that maximum surface deterioration or cracks had been observed on the surface of cured rosin modified o-cresol-formaldehyde epoxy novolac resin (AVEOCN) samples cured with styrene whereas only coarseness on the surface of VER samples cured with MG and ME as reactive diluents had been observed which indicated better chemical resistance of vinyl ester resin samples containing bio-based reactive monomers MG and ME than petroleum-based styrene. This study also holds a good agreement with the % weight loss data of the cured AVEOCN samples during the study of chemical resistance behavior.
SEM images of cured AVEOCN samples containing Styrene, MG and ME as reactive monomers. (a), (b) and (c) Styrene, MG and ME (unexposed); (d), (e) and (f) Styrene, MG and ME (exposed to 1 M HCl); (g), (h)and (i) Styrene, MG and ME (exposed to 1 M NaOH) and (j), (k) and (l) Styrene, MG and ME (exposed to 1 M NaCl)
Conclusion
The research deals with the modification of petroleum-based o-cresol-formaldehyde novolac resin with gum rosin, which originated from renewable resources. This rosin modified o-cresol-formaldehyde novolac resin (AOCN) has been utilized to synthesize the respective epoxy resin (AEOCN) and finally for the synthesis of the vinyl ester resin (AVEOCN). The chemical structures of vinyl ester resin (AVEOCN) based on rosin modified o-cresol-formaldehyde epoxy novolac resin was authenticated by FT-IR, 1H-NMR, and 13C-NMR spectroscopic techniques. The article also elucidated the results of a detailed study on the curing behavior, thermal stability, mechanical and chemical resistance of rosin modified o-cresol-formaldehyde epoxy novolac resin-based vinyl ester resin (AVEOCN) with bio-derived lignin-based reactive monomers, methacrylated guaiacol (MG), and methacrylated eugenol (ME), and petroleum-derived styrene. The AVEOCN samples containing MG and ME showed fast curing rates and elevated thermal stability as compared to that of AVEOCN samples containing styrene. Also, the mechanical performance and the chemical resistance in terms of % weight loss of AVEOCN samples containing MG and ME were found to be superior to those of the samples cured with styrene. The morphological studies via SEM for these chemically exposed samples also validate the results obtained from the chemical and corrosion resistance studies. The results showed that the bio-derived reactive monomers MG and ME are optimal substitutes to styrene and the synthesized VER samples with ME as reactive diluent displayed foremost outcomes among all. The synthesized rosin-modified VER exhibited better performance than petroleum-derived o-cresol based vinyl ester resin that elucidates the role of rosin moiety and enhanced functionality in the VER systems. Furthermore, the performance of VERs has been compared to that of epoxy resin system wherein the vinyl ester resin showed low temperature curing and enhanced thermal, mechanical, and chemical resistance as compared to epoxy resin system indicating profitable exploitation of vinyl ester resins over epoxy resins for thermosetting and coating industries.
Availability of data and material
Data will be available on request.
References
Jaswal S, Gaur B (2014) New trends in vinyl ester resins. Rev Chem Eng 30:567–581. https://doi.org/10.1515/revce-2014-0012
Gaur B, Rai JSP (1992) Curing and decomposition behaviour of vinyl ester resins. Polymer (Guildf) 33:4210–4214. https://doi.org/10.1016/0032-3861(92)90631-6
Jin L, Agag T, Ishida H (2013) Use of allyl-functional benzoxazine monomers as replacement for styrene in vinyl ester resins. Polym Int 62:71–78. https://doi.org/10.1002/pi.4279
Ragauskas AJ, Williams CK, Davison BH et al (2006) The path forward for biofuels and biomaterials. Science 311:484–489. https://doi.org/10.1126/science.1114736
Bu Z, Hu J, Li B (2014) Thermochimica Acta Novel silicon-modified phenolic novolac resins : Non-isothermal curing kinetics, and mechanical and thermal properties of their biofiber-reinforced composites. Thermochim Acta 575:244–253. https://doi.org/10.1016/j.tca.2013.11.003
Pan G, Du Z, Zhang C et al (2007) Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety. Polymer 48:3686–3693. https://doi.org/10.1016/j.polymer.2007.04.032
Hirano K, Asami M (2013) Phenolic resins-100 years of progress and their future. React Funct Polym 73:256–269. https://doi.org/10.1016/j.reactfunctpolym.2012.07.003
Chen GF (1992) Developments in the field of rosin chemistry and its implications in coatings. Prog Org Coatings 20:139–167. https://doi.org/10.1016/0033-0655(92)80002-E
Scala JJ, Logan MS, Sands JM, Palmese GR (2008) Composites based on bimodal vinyl ester resins with low hazardous air pollutant contents. Compos Sci Technol 68:1869–1876. https://doi.org/10.1016/j.compscitech.2008.01.003
Zhang J (2012) Rosin-based chemicals and polymers. Smithers Rapra Technology Ltd, United Kingdom
Thakur T, Jaswal S, Parihar S, Singha AS (2020) Bio-based Epoxy Thermosets with Rosin Derived Imidoamine Curing Agents and their Structure-Property Relationships. Express Polym Lett 14:512–529. https://doi.org/10.3144/expresspolymlett.2020.42
Jaswal S, Gaur B (2015) Structure-property correlation study of bio-based multifunctional vinyl ester resin in presence of methacrylated lignin model compounds. Polym Sci - Ser B 57:417–433. https://doi.org/10.1134/S1560090415050048
Wang Z, Gao P, Chen P (2000) Synthetic reaction of rosin-modified phenolic resin for offset inks. Pigment Resin Technol 29:88–92. https://doi.org/10.1108/03699420010317834
Tuck N, Coyard H, Deligny P (2000) Resins for surface coatings. Wiley, New York
Panda H (2005) Handbook on Speciality Gums, Adhesives, Oils, Rosin & Derivatives, Resins, Oleoresins, Katha. Asia Pacific Business Press Inc, New Delhi, Chemicals with Other Natural Products
Okoshi N, Kudo K, Shimoyama S (1981) Process for producing oil-modified and rosin-modified phenolic resin for printing inks. US patent 4(391):640A
Deblasi DS, Walsh JP (1989) Phenolic-modified rosin ester printing inks. US patent 4(857):624
Kang DW, Yoon DK, Ji SH (2000) Synthesis and characterization of rosinester modified with p-nonylphenolic resole. J Ind Eng Chem 6:256–261
Sathe, Sharad S (1986) Process for the preparation of N-acetyl-P-aminophenol. US Patent 4,565,890
Thakur T, Jaswal S, Gaur B, Singha AS (2020) Thermo-mechanical properties of rosin-modified o-cresol novolac epoxy thermosets comprising rosin-based imidoamine curing agents. Polym Eng Sci 1–21. https://doi.org/10.1002/pen.25562
Pal N, Srivastava A, Rai JSP (2003) Synthesis of vinyl ester resins in the presence of monoepoxies: A kinetic study. Polym - Plast Technol Eng 42:105–122. https://doi.org/10.1081/PPT-120016338
(1954) Division of Paint, Plastics and Printing Ink Chemistry. Chem Eng News Arch 32:3845–3846. https://doi.org/10.1021/cen-v032n039.p3845
Jaswal S, Gaur B (2013) Curing and decomposition behaviour of cresol novolac based vinyl ester resin. Chem Eng Trans 32:1591–1596. https://doi.org/10.3303/CET1332266
Mohan P (2013) A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym - Plast Technol Eng 52:107–125. https://doi.org/10.1080/03602559.2012.727057
Agrawal S, Singhal R, Rai JSP (1999) Curing and rheological behavior of vinyl ester resins prepared in the presence of tertiary amines. J Macromol Sci Pure Appl Chem 36A:741–757. https://doi.org/10.1081/ma-100101561
Jaswal S, Gaur B (2015) Green methacrylated lignin model compounds as reactive monomers with low VOC emission for thermosetting resins. Green Process Synth 4:191–202. https://doi.org/10.1515/gps-2015-0005
Atta AM, El-Saeed SM, Farag RK (2006) New vinyl ester resins based on rosin for coating applications. React Funct Polym 66:1596–1608. https://doi.org/10.1016/j.reactfunctpolym.2006.06.002
Jaillet F, Nouailhas H, Boutevin B, Caillol S (2015) Synthesis of novel vinylester from dicyclopentadiene prepolymer. Eur Polym J 71:248–258. https://doi.org/10.1016/j.eurpolymj.2015.08.002
Jaswal S, Thakur T, Gaur B, Singha AS (2021) High-performance gum rosin-modified hyperbranched vinyl ester resin derived from multifunctional pentaerythritol. Polym Bull. https://doi.org/10.1007/s00289-020-03511-x
McCool DJ (2000) Van Nostrand’s scientific encyclopedia, revised 8th edition. J Chem Educ 77:36. https://doi.org/10.1021/ed077p36.1
Jaswal S, Gaur B (2015) Morphological, Mechanical and Physio-chemical Performance of ortho-Cresol Epoxy Novolac Based Vinyl Ester Resin. Polish J Chem Technol 17:1–7. https://doi.org/10.1515/pjct-2015-0041
Sultania M, Rai JSP, Srivastava D (2010) Studies on the synthesis and curing of epoxidized novolac vinyl ester resin from renewable resource material. Eur Polym J 46:2019–2032. https://doi.org/10.1016/j.eurpolymj.2010.07.014
Smith CA (1987) Characterisation of polymers used in printed circuit board manufacture. Polym Test 7:79–84. https://doi.org/10.1016/0142-9418(87)90002-X
Dharwadkar SR, Kharkhanawala MD (1969) In Thermal Analysis in Organic Materials and Physical Chemistry. Academic Press, New York, Vol, p 2
Garg MS, Srivastava D (2014) Effect of glycidyl methacrylate (GMA) content on thermal and mechanical properties of ternary blend systems based on cardanol-based vinyl ester resin, styrene and glycidyl methacrylate. Prog Org Coatings 77:1208–1220. https://doi.org/10.1016/j.porgcoat.2014.03.029
Siva P, Varma IK, Patel DM, Sinha TJM (1994) Effect of structure on properties of vinyl ester resins. Bull Mater Sci 17:1095–1101. https://doi.org/10.1007/BF02757587
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
No conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaswal, S., Thakur, T. & Gaur, B. Rosin-modified o-cresol novolac based vinyl ester thermosets containing methacrylated lignin model compounds: synthesis, curing and thermo-mechanical analysis. J Polym Res 28, 111 (2021). https://doi.org/10.1007/s10965-021-02475-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02475-4