Abstract
Environmental concerns about coating material have led manufacturers towards considering bio-based alternatives to conventional petroleum–derived epoxy resins. Considering these concerns with rise in prices and high depletion of petroleum resources, a viable alternative polymer resin has been synthesized from characteristic cashew nut shell liquid (CNSL). In this study, cardanol, a major component of cashew nut shell liquid, was used to synthesize cardanol-based vinyl ester resole (CBVER) and cardanol-based vinyl ester novolac (CBVEN) resins. The vinyl ester resins synthesized were characterized using international standard methods. The molecular weight of the resins was determined to be 1069 and 1001 g/mol for CBVER and CBVEN, respectively, using gas chromatographic mass spectroscopy (GCMS). Fourier transform infrared (FTIR) and thermogravimetric (TGA) analysis were used to identify the functional groups and analyze the material weight changes with stability relative to temperature. FTIR results revealed functional groups typical of thermosetting resin. The results of the mechanical and thermal properties of the resins showed improved performance and material’s thermal stability (up to a temperature of 280 °C), respectively. The chemical resistance properties of the cardanol-based vinyl ester resins showed improved performance when subjected to solvent, alkalis, and acids, an indication of anti-corrosive properties. The better performances of cardanol-based vinyl ester resins obtained from this study is justifiable and suggest applicability in coatings, for both anticorrosion and interior/exterior decorations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthesis and development of polymeric products from sustainable origin have created great awareness among researchers who are in search of alternatives to petrochemical materials. This search is as a result of high cost of petroleum-based materials and increased diminution level associated with natural mineral deposits. These viable mineral deposits can function as substitute raw materials [1] for use in polymer industries. Currently, many concerns have been developed towards stabilization of chemical approaches with proficient predominant modifications to improve the level of effectiveness, at the same time, decreasing or maybe eluding utilization of poisonous substances, solvents, and costly catalysts [2]. Within this context is introduced a sustainable material such as cashew nut shell liquid (CNSL), which is targeted towards advanced and secured chemical approaches with waste reduction. This chemistry technique introduces opportunities of novel fabricating paths that are not only self-sustaining, but also eco-friendly [3]. Therefore, a salient factor in the modern-day research is to explore enhancement, in addition to appropriate designing of chemical synthesis, by means of alternative strategies, reduction of by-products, and the deployment of continuous [4] raw materials [5]. The need to utilize natural materials in coatings development is essential as it is cost-effective over importation of synthetic materials and at the same time serves as a source of revenue generation. Most researches on natural resins only centred on their production and curing characterization [6], without mechanical and end use properties. Raw materials such as vegetable oils from natural resources have been utilized to synthesize polymer products through the addition of functional groups like hydroxyl, epoxy, phenolic, and/or carboxyl groups. These functional groups are added to the fatty acid chains of the vegetable oil. Polymers manufactured from vegetable oils and their derivatives exhibit exceptional hydrolytic stability and thermo-mechanical properties owing to the hydrophobic nature of the side chain [7].
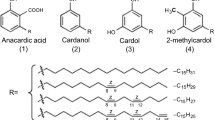
Cashew nut shell is one of the major agricultural waste products that are available in abundance, renewable, and cost-effective. Economically, protecting industrial and household equipment with these bio-based resins will be an avenue of converting waste to wealth. CNSL is a by-product of cashew nut industries which is a feasible raw material used for numerous industrial applications which include polymers, plastics, lubricants, resins, and antioxidants [8,9,10]. CNSL grasps significant prospective as a foundation for unsaturated hydrocarbon phenol which is an exceptional monomer for polymer manufacturing [11]. CNSL appears as a reddish brown viscous liquid within the soft honeycomb structure of the shell of cashew nuts, gotten from the cashew tree, Anacardium oxidentale. A number of research works have been reported on CNSL extraction [6], chemistry, and constitution [12,13,14,15]. Four major constituents of CNSL have been reported to be 3-pentadecylphenol (cardanol), 5-pentadecenyl resorcinol (cardol), 6-pentadecenyl salicylic acid (anacardic acid), and 2-methyl, 5-pentadecenyl resorcinol (2-methyl cardol) [11, 16,17,18]. The phenolic constituents of raw and technical CNSL are presented in Table 1.
An expansive range of chemical reactions can emanate from CNSL due to its chemical structure, and can be seen as presented in Fig. 1.
Reactions of CNSL [19]
CNSL have found applications in the production of exceptional phenolic resins for coatings applications, brake lining of automobiles, heat and waterproof paints, corrosion-resistant varnishes, and insulating enamels for the electrical industry, lamination, and as friction materials [17]. Generally, condensation reactions with electrophilic compounds like formaldehyde, chain polymerization by means of double bonds within the side chain with acid catalysts, or functionalization at the hydroxyl group are employed in the manufacture of polymeric products from CNSL and cardanol. These polymers subsequently undergo oligomerization to obtain functionalized pre-polymer [20, 21]. The literature on cardanol is loaded with numerous patents and reports, but its industrial utilization has been lagging behind. One of the most interesting aspects of the structure of cardanol is its ability to undergo polymerization by both step and chain reaction mechanisms. Added to this, is its amenability to manipulations through chemical modifications. Being phenolic in nature, cardanol undergoes the conventional aldehyde condensation reactions to give novolacs and resole resins owing to terms of polymerization. The procedure of cardanol isolation from decarboxylation residue gives rise to phenolic resins and polymer biocomposites. It is noteworthy that India and Nigeria are the second and third largest producers of cashew nut in 2010 with a production capacity of 613,000 and 594,000 metric tons (MT) respectively [22]. The kinetics of the alkali catalyzed cardanol through condensation of formaldehyde have been studied [23, 24]. The authors functionalized cardanol with orthophosphoric acid and oligomerized, which resulted in pre-polymers that performed as multi-functional additives. Saminathan [25] reported a study on the main-chain liquid crystalline polymer containing a phenyl azo group of cardanol. In his study, cardanol was oxidized with phase transfer catalyzed permanganate and the oxidized product 8-(3-hydroxyphenyl) octa-noic acid was copolymerized with p-hydroxy benzoic acid to produce a thermotropic liquid-crystalline copolymer [26]. Although, there are a few attempts on the synthesis, chemical modification and functionalization of cardanol and its polymers. Studies on detailed investigations on the structural properties and characterization of cardanol-based products are limited. Ranjana and Deepak [9] reported a study on the kinetics of acid catalyzed formaldehyde condensation of cardanol. Results revealed that phenolic resins obtained from condensation of cardanol and formaldehyde exhibited outstanding adhesive property and structural integrity [27]. However, they concluded that the results obtained could suffice for polymers with high temperature resistance [28]. Cardanol with diazotized p-anisidine/diazotized p-sulphanilic acid and pre-polymer resins was synthesized through condensation with formaldehyde and two organic compounds, o-hydroxy benzoic acid and α-naphthol, in the presence of acid catalyst. The spectral analyses on these resins were reported to be excellent [28].
Vinyl ester resins (VERs) are thermosetting polymers from esterification reactions of epoxidized resins and carboxylic acids with unsaturation at the chain end. Several other epoxy resins like diglycidyl ether of bisphenol A or higher homologues, epoxidized phenol–formaldehyde novolac resin, and polypropylene oxide diepoxide are also employed as thermosetting matrix for the manufacture of a range of reinforced structures such as pipes, tanks, scrubber, and ducts. Besides the above applications, vinyl esters find applications in coating materials, adhesives, molding compounds, structural laminates, electrical insulation products, etc. Vinyl esters of epoxy novolac/resole resins are developed for the production of chemical storage tanks, pipes and ducting, fume extraction systems, and gas cleaning units due to their superior toughness and chemical resistance properties at elevated temperatures [29,30,31,32].
However, the use of cinnamic acid for the production of vinyl ester resins has not been reported thus far, to the best of our knowledge. In this study, the effectiveness of utilizing cardanol for vinyl ester resin synthesis through condensation of phenolic resin and cinnamic acid (an unsaturated monocarboxylic acid) was employed. This study was also aimed at synthesizing resins from cost-effective and sustainable materials with excellent mechanical and thermal properties which can be utilized in coatings systems and other polymer nanocomposites for automotive applications. Consequently, in spite of purity of products, functioning clarity, duration, cost, VOCs, waste management, and in the design of an enhanced approach for the manufacture of products that uses cardanol as starting material, this study also compared two different types of vinyl ester resins, evaluated their performances and confronted results obtained for future prospects on the development and production of coatings and nanocomposites for automotive applications.
2 Experimental
2.1 Materials
The cashew nuts (CN) used in this study was obtained from fresh cashew-nuts assembled from cashew plants (A. occidentale) of Abia State University, Uturu, Abia State, Nigeria. The chemicals NaOH, KMnO4, NaClO2, HNO3, NaCl, NaHCO3, and H2SO4 were sourced from a chemical shop in Onitsha Head Bridge and used as received. Anhydrous sodium sulfate (Na2SO4 — 99%), hexane (C6H14 — 98.5%), methanol (CH3OH — 99.8%), ammonium hydroxide (NH4OH — 28–30%), hydrochloric acid (HCl — 37%), formaldehyde (CH2O — 36.5–38%), diethylenetriamine (DETA, C4H13N3 — 99% — Sigma-Aldrich), toluene (C7H8 — 99.5%), epichlorohydrin (CH3H5ClO — 92.5%), 2- napthol (C10H8O — 99%), cinnamic acid (C9H8O2 — 50%), and triethylamine (C6H15N — 99.5%) were used without any preceding modification.
2.2 Synthesis of cardanol-based vinyl ester resins
CNSL extraction, cardanol isolation, and vinyl ester resin synthesis were carried out utilizing similar methods reported in the literature [33, 34] with slight modifications. Cardanol-based vinyl ester novolac (CBVEN) and resole (CBVER) resins with mole ratios 1:0.8 and 1:1.2 of cardanol (C) to formaldehyde (F) were prepared respectively, using sodium bicarbonate (NaHCO3), an acid catalyst and sodium hydroxide (NaOH) catalyst at a temperature of 40 °C to form dimethylol resin. The dimethylol resin formed was further reacted with 2-napthol to condense it to phenolic resin. The reaction product was cooled and dried under vacuum at 60 °C while maintaining the pH between 7 and 8 by adding 10 wt% of sulphuric acid. The cardanol-based phenolic resins (CPR) thus formed was further epoxidized by treatment with molar excess of epichlorohydrin at a temperature of 60 °C with the addition of 50% solution of sodium hydroxide. The temperature was further raised to 120 °C, after addition of NaOH with continuous stirring to produce triglycidyl ether of cardanol resin which was further vacuum-distilled to remove excess epichlorohydrin. The product obtained was subjected to additional reaction through esterification to obtain cardanol-based epoxydized vinyl ester resin using cinnamic acid. The reaction was performed using triethylamine catalyst and hydroquinone (200 ppm as inhibitor) at 90 °C in nitrogen atmosphere for about 5 h to attain a product that contained less than 10 acid value [35]. Possible reactions for the synthesis of the cardanol-based vinyl ester resin are shown in Fig. 2.
Mild steel panels with dimensions 120 × 100 mm were produced using mechanical press, and a small hole was produced on the coupons to support the thread. The prepared metal coupons were degreased in xylene, washed with distilled water, cleaned, dried, weighed, and stored for subsequent use. Calculated amounts of the resin samples were weighed into 250-mL capacity beakers, and for each sample, methyl ethyl ketone peroxide (MEKP) and co-octoate (catalysts) were mixed in a concentration of 4 and 2 wt% respectively, and were poured into the beakers. The prepared coupons were dipped totally in 250-mL volume glass beakers having 200 mL of the resin samples with the help of a glass rod and thread. After immersion, the test samples were carefully removed and allowed to cure with the help of laboratory oven at different temperatures, 40, 60, 80, and 120 °C for 6 h.
2.3 Microstructural analysis
The morphological architectures of the synthesized resins were analyzed using JEOL-SEM (JSM-6390LV) within the acceleration range of 5–20 kV. The fractured surfaces of the cured resins were characterized using SEM micrographs. Samples of substrates were smeared with Pt prior to observation.
2.4 Fourier transform infrared spectroscopy
The functional groups present in the cardanol based vinyl ester novolac (CBVEN) and cardanol based vinyl ester resole (CBVER) resins were recorded utilizing FTIR with attenuated total reflectance (ATR). FTIR spectra within the range of 500–4000 cm−1 at a resolution of 4 cm−1 were used in the analysis.
2.5 Gas chromatography-mass spectrometry
Fractions were analyzed via Shimadzu GC-17A/MS QP5050A (GC–MS system): DB-5HT capillary-column (30 m × 0.251 mm, 0.1 µm film thickness); carrier-gas: helium 1.7 mL/min; column-inlet pressure 107.8 kPa; column flow = 1.7 mL/min; linear velocity = 47.3 cm/s; total flow 24 mL/min; carrier flow 24 mL/min; injector temperature 280 °C; detector temperature 300 °C; column temperature 100 (1 min) — 310 °C at 10 °C/min (15 min). Operational conditions of the mass spectrometer were 70 eV of ionization energy. Recording of mass spectra occurred within 40 – 450 m/z.
2.6 Thermogravimetric analysis
The vinyl ester resins TGA was studied through a thermal-analyzer using nitrogen at a flow-rate of 30 mL/min and a steaming speed of 10 °C/ min between 25 and 600 °C.
2.7 Mechanical behaviour
The mechanical behaviour of the resins such as Young’s modulus, tensile strength, and elongation at break were conducted using Zwick/Roell (Z005) universal testing machine according to ASTM Standard D638-14 at a tensile velocity of 50 mm/min under room temperature. Dumbbell-shaped films of dimension 30 mm long, 15 mm width, and 1 mm thickness were made for each sample.
2.8 Chemical resistance
A blank acid solution of 0.6 M HCl (aggressive medium) was prepared using distilled water. The dried resin film samples were dipped entirely in 250-mL glass beakers consisting of distilled water, 0.6 M sodium hydroxide (NaOH), sodium chloride (NaCl), sodium carbonate (Na2CO3), acetic acid (CH3COOH), hydrochloric acid (HCl), and sulfuric acid (H2SO4). The solutions were kept under room temperature and monitored every 24 h. At the expiration of each time duration, the test samples were removed from the solution and the resistance of the resin films to acid, alkalis and solvent were determined. The film samples were also subjected to ultraviolet light (UV) to determine their resistance to environmental conditions.
3 Results and discussions
3.1 Physical–chemical characteristics of cashew nut shell liquid
The percentage yield of CNSL was 45.85 ± 0.2% indicative of high oil yield. The percentage yield of 30.61 ± 0.200% and within the range of 15–30% for CNSL have been reported [36, 37]. The results of the physicochemical characterization of the CNSL are presented in Table 1. From the result, it was observed that the oils contained high molecular weight fatty acids, which was considered to be non-edible oil, indicating points of unsaturation, a very important material property for coating systems. It is worthy of note that unsaturated fatty acids contain active sites which can be functionalized for polymerization reactions on coating materials with suitable approaches. However, with unsaturation, polymers can be modified by polymerization through auto-oxidation and chemical reactions using their internal active sites. Built into unsaturation (double bonds) allows functionalization of polymer products through various reactions such as epoxidation, hydrogenation, halogenations, hydroxylation, acrylation, maleinization, ozonolysis, and dimerization [38,39,40,41,42,43,44,45] (Table 2).
A decreased saponification value was observed for cardanol which was found to be lower than the raw CNSL. Raw CNSL has a high relative density due to the anarcadic acid present in the oil. There was an observed intermolecular attraction between the electronegative oxygen atom and the partially positive hydrogen atom of the phenol core, which resulted in the closely-packed molecules. The specific gravity of the cardanol was decreased due to removal of hydrogen bonds in the course of decarboxylation and isolation; however, the viscosity (at 100 °C) of the resins were determined to be 50 and 43 for CBVER and CBVN respectively, typical of vinyl ester resin [46]. The cardanol exhibited an increased iodine value which is a measure of the amount of unsaturation in a given oil. The unsaturation of the samples owing to the side chain was slightly different for raw CNSL and cardanol (Table 1). The iodine values of more than 140 in the fractions indicate drying oils [47]. The iodine value CNSL obtained from this study is an indicative of non-drying oil. A process of trans-esterification was employed during the synthesis to aid drying. There was a variation in the viscosity values. Raw CNSL contained anarcardic acid as the major constituent with the –COOH group in the ortho position of the phenol core. It, therefore, displayed strong dipole–dipole interaction between the partially positively charged hydrogen atom and the strong electronegative oxygen atom. However, the molecules were held together by the presence of intramolecular hydrogen bonding.
3.2 Gas chromatographic mass spectroscopic analysis of CNSL
Gas chromatographic analysis of cardanol reveals attainment of morphine, ethyl ether, 7-octyl-2-ol, 2-methyl-6-methylene, 6-oxabicyclo-3-hexane, 3, 4-epoxycyclopentanone, 11(2-cyclopentene-1-yl) undecanoic acid, O-cresol; 2-methylphenol; 95–48-7; 2-hydroxytoluene; 2-Cresol; O-methylphenol, benzenepropanenitrile, 2-methyl-1-nonene-3-yne [2-methylnon-1-en-3-yne IUPAC], and 1, 4-cyclohexadiene-1-carboxylic acid. The GCMS of these chemicals are shown in Fig. 2, while Table 3 reveals the peaks obtained.
From Fig. 3, the compounds were detected in the positive chemical ionization-mode (PCI) via their quasi-molecular ions [M + H] + (m/z 55.1), [M − H]+ (m/z 59.0). All mass spectra in Fig. 3 exhibit characteristic alkyl-amine ions: [C4H9NH2]+ (m/z 55.1), [CH3OH+CH2]+ (m/z 69), [C6H13NH2]+(m/z 85), and [C7H15NH2]+(m/z 86). The molecular weight of the resins was determined to be 1069 and 1001 g/mol for CBVER and CBVEN respectively.
Mass spectra for a morphine; b ethyl ether; c 7-octen-2-ol, 2-methyl-6-methylene; d 6-oxacyclobi-3-hexane; e 3, 4-epoxycyclopentanone; f 11(2-cyclopentene-1-yl) undecanoic acid, O-cresol; g O-methylphenol, benzenepropanenitrile h 2-methyl-1-nonene-3-yne [2-methylnon-1-en-3-yne IUPAC]; and i 1, 4-cyclohexadiene-1-carboxylic acid
3.3 Microstructural analysis
Figure 4 shows the SEM micrograph of the resins (Fig. 4a) CBVER and (Fig. 4b) CBVEN. It was observed that the micrographs of both resins exhibited layered structure with rough surface which may be attributed to deformation [48] and/or degradation during the curing process. However, the SEM micrograph in Fig. 4b exhibited continuous crack-propagations on a straight path towards the direction of the thickness. The observed rough surface appearances on CBVER and CBVER are ideal and implied typical brittle fracture surface of thermosetting resins [49, 50]. The surface roughness was seen to be more pronounced with CBVEN. However, some clusters were seen on the SEM image (Fig. 4a) which revealed bond-cleavage interaction attributed to agglomeration. A SEM micrograph of commercial epoxy resin with uninterrupted crack propagated along the thickness direction path was reported by Tiimob et al. [51]. Several researches have reported similar trend of rough SEM micrographs of commercial epoxy resins with continuous crack propagation along the thickness direction [52,53,54]
3.4 Functional group analysis
The FTIR spectral knowledge of the samples revealed availability of varying linkages, such as carboxylic groups, unsaturated aliphatic hydrocarbons (alkenes), and other distinctive heights. FTIR analysis presented in Fig. 5 revealed peaks at wave number within 500 and 4000 cm−1 range as a result of C-H external bending vibration, C-H in-plane bending, and out-of-plane C–H wagging [55]. An observed peak depicted around 3410–3380 cm−1 was ascribed to O–H stretching of H-bonding which consisted of alcohols and phenols on the surface of cardanol, and the vinyl ester resins. Detected peak around 3000 cm−1 was ascribed to the O–H stretch of carboxyl functional groups. The height situated at 2900 cm−1 was ascribed to C-H symmetric stretch vibration of cardanol, while that situated at 2850 cm−1 was ascribed to stretching frequency of C-H for vinyl ester resins. The absorption peak at 1560 and 1370 cm−1 depicts the C–C and C-H stretching vibration of cardanol, while the band at 1120 cm−1 was ascribed to C-O bonds stretching of alcohols, carboxylic, esters, and ethers for the vinyl ester resins, which confirmed a characteristic cardanol resin. The peaks at 550 and 900 cm−1 were ascribed to C–Br stretch and C-Hoop (epoxy group) present in the vinyl ester resins, respectively. The absence of a peak at 1000 cm−1 confirmed the completion of esterification reaction and formation of vinyl ester resins as seen in CBVER and CBVEN FTIR graphs. Furthermore, the C–H out-of-plane bending absorption peaks of the terminal vinyl group for cardanol and the resins were observed between 890 and 910 cm−1. Similar results for C-H bending of vinyl ester linkages for cardanol at 991 and 910 cm−1 were reported [56]. Furthermore, the FTIR of the sample resin films during cure for various temperatures (at 3-h interval) is presented in Fig. 5b. Characteristic peaks at 2880 and 2900 cm−1 were ascribed to C–H stretch vibrations of alkane for CBVER (Fig. 5a) and CBVEN (Fig. 5b) at 40 °C respectively. Notable peaks at 2920, 2940, 2980, and 2960 cm−1 ascribed to C–H stretch vibrations were observed for CBVER and CBVEN for 60 and 80 °C respectively. However, the CBVER and CBVEN resins exhibited O–H stretch of alcohols at 3350 cm−1 peak (Fig. 5c–f). Other notable peaks observed were 1200 – 1390 cm−1 and 940–900; 740–650 cm−1 ascribed to C–F stretch of alkyl halides and = C–H bend of alkenes for the resins at 40–80 °C. The FTIR spectra of specified samples are represented in Fig. 5.
3.5 Mechanical properties of vinyl ester resin films
The results of the optimum tensile strength and tensile stress with reference to strain are represented in Fig. 6. The ultimate tensile strength of the CBVER and CBVEN at 120 °C curing temperature were determined to be 20.8 and 18.5 MPa (Fig. 6a, b) respectively. Similarly, the Young’s modulus of the resins cured at 120 °C was determined to be 2.60 and 2.47 MPa for CBVER and CBVEN respectively. It was observed that the tensile strength of the vinyl ester resins relied on the degree of curing. The high values observed for the mechanical properties of the resins may be attributed to the process of epoxidation which may have increased the crosslink density of the resins [34]. With increased crosslink density, more polymer chains were consolidated together and became tougher to be broken.
Subsequently, in this study, the samples with higher degree of curing exhibited improved tensile strength. However, vinyl ester resins cured at 120 °C exhibited higher tensile strength and elongation than those cured at 40 °C. Interestingly, the values obtained for the failure strain for CBVER and CBVEN showed a gradual increase. The increased tensile strength and elongation at break observed may be ascribed to shear yield instigated by the deformed structure of the resins cured at 120 °C. Furthermore, increased failure strain exhibited by the resins may also be due to the presence of C15 long chains existing in cardanol [57]. As polymers are hardened and cross-link density is increased, elongation at break generally drops.
On the other hand, the microgels formed at high temperature may not be as good as those formed at low temperature. These structures may exhibit rubberlike particles in vinyl ester resins, which can bring about the shear yield of the matrix [58]. The values of the mechanical properties of CBVER were higher than those of CBVEN. This may be due to the fact that CBVER had less terminal cardanol units in the system than CBVEN.
3.6 Thermogravimetric analysis
The TGA and derivative thermogravimetric (DTG) curves are a characteristic property which determines the thermal behaviour and identifies the decomposition of materials at a certain temperature. The degradation of vinyl ester resins were investigated and shown in Fig. 7. The resins were stable up to a temperature of about 280 °C for CBVER and CBVEN respectively. Initial degradation of both resins occurred at 280 °C with an initial weight loss of 10 and 21% for CBVER and CBVEN respectively. The initial degradation of these resins may be attributed to elimination of moisture contained in the resin. However, the TG curves maintained a similar pattern/behaviour which is in agreement with TG curves for conventional resins [59,60,61]. Degradation occurred in four stages, at 280, 370, 480, and 550 °C for CBVEN with corresponding weight loss percentages at 89, 62, 53, and 18%. For CBVER, the degradation occurred at 280, 320, 400, and 420 °C with percentage weight loss at 78, 62, 51, and 28% respectively. The vinyl ester resins exhibited maximum weight loss at 420 and 550 °C with total volatilization at 620 and 680 °C for CBVEN and CBVER respectively. Additionally, the TGA analysis conducted under nitrogen atmosphere may have enhanced the rate of the resin’s decomposition, which consequently reduced the activation energies of decomposition resulting in improved thermal stability exhibited by the resins [62]. Moreso cross-links introduced during curing may have also contributed to the improved thermal stability exhibited by the resins. Improved thermal stability exhibited by the CBVEN over CBVER may be attributed to aromatic content of CBVEN resin. It is worthy of note that higher aromatic contents in vinyl ester resins enhance its thermal stability. However, the TGA curves of both resins followed a similar route. A slightly lower value of maximum degradation temperature of 500 °C for vinyl ester resin was reported by [63]. Similar TGA with initial degradation of 265 °C for cardanol based vinyl ester resin was reported [64]. The improved thermal stability of this study is promising and can compare favourably with the commercial-based epoxy resins [65].
3.7 Dynamic mechanical analysis
The storage modulus (E′), loss modulus (E″), and damping (tan δ) of the vinyl ester resins have been studied using DMA (Fig. 8). The glass transition temperature (Tg) by E′ was determined as the temperature at which the E′ begins to decline (onset temperature), while that of E″ and tan δ showed peaks at the glass transition. Figure 8 shows the storage modulus, loss modulus (E″), and damping (tan δ) as function of temperature for the vinyl ester resins cured at 120 °C. From Fig. 8a, the Tg by E′ onset was determined to be 115 °C and 105 °C, while the Tg of E″ by peak was determined to be 120 and 118 °C for CBVER and CBVEN respectively. However, the Tg by tan δ was determined to be 150 and 140 °C (Fig. 8b) for CBVER and CBVEN respectively. The high Tg exhibited by the vinyl ester resins studied is desirable for thermosetting polymer resins [65]. Changes in Tg are very important for the performance of thermosetting polymers. Tg can provide insight into fundamental changes in molecular chain dynamics and can have a critical impact on polymer applications. It can be seen from Fig. 8 that the temperature has a great influence on the storage modulus and loss factor of the resins. The storage modulus of the resins decreased sharply with increased temperature. The reason is that at low temperature, the material is in a glass state, but as the temperature increases, the material changes from a glass state to a rubber state and the materials become rubber-like. However, the figure revealed that at lower temperature, below Tg, the storage modulus of the vinyl ester resins increased. This was attributed to the fact that at lower temperatures, the resins take longer time to relax (relaxation time). At this time, the vibrational energy is rapidly distributed over the entire volume of the polymer resins. In this case, little mechanical loss was encountered [66]. Similar results reported that vinyl ester resin’s Tg by E′ onset and Tg by E″ peak increased from 106 to 108 and 131 to 133 °C [67]. Figure 8 b reveals one single peak of tan δ which implied complete cure of the resins. However, this result indicated complete elimination of residual reactions that can adversely affect the mechanical properties of the resins.
3.8 Chemical resistance properties
The chemical resistant properties of the resins with their corresponding weight loss are presented in Fig. 9 and Table 4. The chemical resistance and solvent compatibility are the major decisive parameters for adhesion in coatings. Table 4 shows the comparative study on the alkalis and acid resistance of the cured resin films. From Table 4, it was clearly revealed that the cured films prepared exhibited maximum resistance towards distilled water (Fig. 9a, d). The cured films immersed in distilled water exhibited no effect, and maintained homogeneity throughout the period of immersion. This could most probably be ascribed to the hydrophobic nature of the resin’s backbone, which repelled any probable interaction between the cross-linked resins backbone and water. More so, the cured films immersed in 0.6 M NaOH and Na2CO3 also exhibited good resistance to alkalis (Fig. 9b–c, e–f). This trend could further be interrelated to the highly cross-linked structure of the vinyl ester resins synthesized, which could also be confirmed by their excellent thermal stability exhibited. The ether functional groups coupled with the unsaturated hydrocarbon side chains, such as C = C double bonds present in the synthesized resins, led to improved resistance to solvent and alkalis. Mukesh and Anagha [44], reported excellent chemical resistance on a study of epoxy resin from cardanol as partial replacement of bisphenol A-based epoxy for coating. However, weak acid resistance (0.6 M acetic, hydrochloric, and sulphuric acids) was observed for the cured films (Fig. 9g–h, j–k). The cured films immersed in 0.6 M H2SO4 were affected to a large extent. These coated films exhibited either dissolution, loss in gloss, scorching, blushing, or shrinkage in the course of immersion period (Fig. 9i, l), presumably, as a result of saponification of ester groups [68]. The above result was evident of highly cross-linked structure exhibited by the resin, which could have brought about increased polarity of the system. The CBVEN was adversely affected (Fig. 9i). Accordingly, the prepared CBVEN system possessed poor strong acid resistance, irrespective of exceptional alkali and solvent resistance. Additionally, the cured films of CBVER were found to exhibit better chemical resistance properties compared to CBVEN (Fig. 9d–f, j–l). However, in the case of weight loss, it was evident from Table 4 that the resin film coupons showed more dissolution in hydrochloric, sulphuric, and acetic acids. From Table 4, the film’s behaviour was certain that the resins regulated the dissolution of mild steel coupons exposed to NaOH, Na2O3, and H2O. It can be concluded that sulphide ion was more aggressive to mild steel than chloride ion. This may be attributed to the stability of mild steel in hydrochloric acid compared to sulphuric acid as a result of variation in the electronegative potentials of sulphide and chloride ions [69]. These results could be inferred from the lower values of material loss exhibited by the materials upon immersion in the media. The acid solutions initially impeded the dissolution process, but thereafter, gradual weakening effect sets in with time on the resin film layers which resulted in deformation; Fig. 9g, h, i, l). On the other hand, it was observed that the material loss varied linearly with time in UV exposure and showed some discolouration from deep brown to light brown (Fig. 9m, n) for CBVER and CBVEN respectively. However, the linear correlation could be ascribed to the fact that the resin films exhibited some level of resistance to UV within the time of exposure. Conversely, the CBVEN film exhibited discolouration upon salt water immersion, while the CBVER film showed evidence of rust and blistering upon salt water immersion (Fig. 9o, p).
4 Conclusion
Novel low-viscosity cardanol-based vinyl ester resins were synthesized through reaction with cinnamic acid. The curing investigation of the two different vinyl ester resins at varying temperatures showed a similar process. However, low cured properties of the resins showed that these cardanol-based vinyl ester resins would be useful for moderate temperature applications. The resins molecule exhibited significant improvement in TGA, tensile, and chemical resistance properties. These improvements may be due to complete reaction of the functional groups which resulted in a highly cross-linked network thereby significantly improved TGA, tensile, and chemical resistance properties of the resins. The presence of C15 aliphatic chain, two anhydride functionalities, and aromatic ring could be the factors accountable for exceptional mechanical, in addition to chemical resistance properties observed. Similar results are expected to be achievable by the use of diverse methods to fully or partially methacrylate the cardanol molecule. Overall, it can be concluded that the synthesized cardanol-based vinyl ester resins can be utilized in composites and coating applications, for both industrial and architectural purposes and anticorrosion.
References
N.V. Sadavarte, Difunctional monomers starting from cashew nut shell liquid (CNSL) and high-performance polymers therefrom. Doctoral dissertation, University of Pune, India (2012)
M.B. Gawande, V.D.B. Bonifácio, R. Luque, P.S. Branco, R.S. Varma, Solvent free and catalysts-free chemistry: a benign pathway to sustainability. Chem. Sus. Chem. 7, 24–44 (2014)
P. Anastas, N. Eghbali, Green chemistry: principles and practice. Chem. Soc. Rev. 39, 301–312 (2010)
K.P. Unnikrishnan, E.T. Thachil, Synthesis and characterization of cardanol-based epoxy systems. Des. Monomers Polym. 11, 593–607 (2008)
R.C. Cioc, E. Ruijter, R.V.A. Orru, Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem. 16, 2958–2975 (2014)
C.C. Ugoamadi, Comparison of cashew nut shell liquid (CNS) resin with polyester resin in composite development. Niger. J. Technol. Dev. 10(2), 17–21 (2013)
A. Zlatanic, Z.S. Petrovic, K. Dusek, Structure and properties of triolein-based polyurethane networks. Biomacromol 3, 1048–1056 (2003)
S. Gopalakrishnan, N.T. Nevaditha, C.V. Mythili, Synthesis and characterization of bifunctional monomers for high performance polymers from renewable resource. Int. J. Chem. Technol. Res. 4(1), 48–54 (2012)
R. Yadav, D. Srivastava, Blends of cardanol based epoxidised novolac resins and CTBN for applications in surface coatings. J. Coat. Technol. Res. 7(5), 557–568 (2010)
X. Li, L.G. Tabil, S. Panigrahi, Chemical treatments of natural fibre for use in natural fibre reinforced composites; A Review. J. Polym. Environ. 15, 25–33 (2007)
D. D’Amico, L. Longo, C. Stifani, A. Tarzia, Cardanol-based novolac resins as curing agents of epoxy resins Pietro Campaner. J. Appl. Polym. Sci. 114(1), 3585–3591 (2009)
S.K. Kyei, O. Akaranta, G. Darko, U.J. Chukwu, Extraction, characterization and application of cashew but shell liquid from cashew but shells. Chem. Sci. Int. J. 28(3), 1–10 (2019)
S. Mohapatra, G.B. Nando, Cardanol: a green substitute for aromatic oil as a plasticizer in natural rubber. Royal Soc. Chem. Adv. 4, 15406–15418 (2014)
M. Yuliana, B.T. Nguyen-Thi, S. Faika, L.H. Huynh, F.E. Soetaredjo, Ju. Yi-Hsu, Separation and purification of cardol, cardanol and anacardic acid from cashew (Anacardium occidentale L.) nut-shell liquid using a simple two-step column chromatography. J. Taiwan Inst. Chem. Eng. 45, 2187–2193 (2014)
R. Ikeda, H. Tanaka, H. Uyama, S. Kobayashi. Synthesis and curing behaviors of a crosslinkable polymer from cashew nut shell liquid. Polymer. 43(12), 3845-3481 (2002)
S. Kanehashi, K. Yokoyama, R. Masuda, T. Kidesaki, K. Nagai, T. Miyakoshi, Preparation and characterization of cardanol-based epoxy resin for coating at room temperature curing. J. Appl. Polym. Sci. 2468–2478 (2013). https://doi.org/10.1002/app.39382
J.-H. Lee, J. Jeon, S. Kim, Green adhesives using tannin and cashew nut shell liquid for environment-friendly furniture materials. J. Korea Furnit. Soc. 22(3), 219–229 (2011)
V. Kathir, F. Emerson, A process for selective extraction of cardanol from cashew nut shell liquid (CNSL) and its useful applications Solomon. Int. J. Sci. Eng. Res. 4(3), 1–4 (2013)
C. Voirin, S. Caillol, N.V. Sadavarte, B.V. Tawade, B. Boutevinab, P.P. Wadgaonkar, Functionalization of cardanol: towards biobased polymers and additives. Polym. Chem. 5, 3142 (2014)
R. Paramashiyappa, P. Phani Kumar, J. Vithayathil, A. Srinivasa Rao, Novel method for isolation of major phenolic constituents from Cashew (Anacardium occidentale L) nut shell Liquid. J. Agric. Food Chem. 49, 2548–2551 (2001)
A. Devi, D. Srivatsava, Studies on the blends of cardanol- based epoxidised nonolac resin and CTPB. Eur. Polymer J. 43(6), 2422–2432 (2007)
FAOSTAT, Food and Agriculture Organization of the United Nations Data. Statistical Databases. (2012). http://faostat.fao.org/default.aspx?lang=en. Accessed 11/01/2022
C.K.S. Pillai, V.S. Prasad, J.D. Sudha, S.C. Bera, A.R.R. Menon, J. Appl. Polym. Sci. 41, 2487 (1990)
A.R.R. Menon, C.K.S. Pillai, G.B. Nando, J. Adhes. Sci. Technol. 9, 443 (1995)
M. Saminathan, C.K.S. Pillai, C. Krishn, C. Pavithran, Macromolecules 26, 7103 (1993)
C.K.S. Pillai, D.C. Sherrington, A. Sneddon, Polym. Commun. 33(18), 3968–3970 (1992)
R. Yadav, D. Srivastava. Material chemistry and physics. 1(15), 74-81 (2007)
L.K. Aggarwal, P.C. Thapliyal, S.R. Karade, Anticorrosive properties of the epoxy cardanol, based paints. Prog. Org. Coat. 59(1), 76–80 (2007)
K. Yamada, H. Yamane, K. Kumada, S. Tanabe, T. Kajiyama, Plasma-graft polymerization of a monomer with double bonds onto the surface of carbon fiber and its adhesion to a vinyl ester resin. J. Appl. Polym. Sci. 90, 2415–2419 (2003)
B.F, Howell, De. Realf. Pigmented coatings cured with visible light, Polym. Mater. Sci. Eng. 74, 387–38 (1996)
S. Masujiro, J. Kazuyoshi, Thermosetting printing ink. JP 74(67), 709 (1974)
M. Henne, C. Breyer, M. Niedermeir, P.A. Ermanni, New kinetics and viscosity model for liquid composite molding simulations in an industrial environment. Polym. Compos. 25, 255–269 (2004)
P. Rahmawati, A.H. Ramelan, S.D. Marliyana, N.S. Suharty, S. Wahyuningsih, Synthesis of cardanol-based novolac resin from cashew nut shell liquid. J. Eng. Sci. 15, 23–33 (2019)
S.K. Shukla, K. Srivastava, D. Srivastava, Studies on the thermal, mechanical and chemical resistance properties of natural resource derived polymers. Mater. Res. 18(6), 1217–1223 (2015). https://doi.org/10.1590/1516-1439.007715
M. Sultania, J.S.P. Rai, D. Srivastava, Studies on the synthesis and curing of epoxidized novolac vinyl ester resin from renewable resource material. Eur Polym J 46, 2019–2032 (2010)
S. Rwahwire, B. Tomkova, A.P. Periyasamy and B.M. Kale (2019). Green thermoset reinforced biocomposites. Green composites for automotive applications. Chapter 3, 61–80 (2019). https://doi.org/10.1016/B978-0-08-102177-4.00003-3
B. Lochab, S. Shukla, I.K. Varma, Naturally occurring phenolic sources: Monomers and polymers. RSC Adv. 4(42), 21712–21752 (2014). https://doi.org/10.1039/c4ra00181h
N. Zora, T. Rigaux, J.C. Buvat, D. Lefebvre, S. Leveneur, Influence assessment of inlet parameters on thermal risk and productivity: application to the epoxidation of vegetable oils. J. Loss Prev. Process Ind. 72, 104551 (2021)
U.P. Laverdura, L. Rossi, F. Ferella, C. Courson, A. Zarli, R. Alhajyoussef, K. Gallucci, Selective catalytic hydrogenation of vegetable oils on lindlar catalyst. Asc Omega 5, 22901–22913 (2020)
S. Soares, F.R.P. Rocha, Multi-energy calibration to circumvent matrix effects in the determination of biodiesel quality parameters by UV-Vis spectrophotometry. Talanta 209, 120584 (2020)
V.B. Borugadda, V.V. Goud, Hydroxylation and hexanoylation of epoxidized waste cooking oil and epoxidized waste cooking oil methyl esters: Process optimization and physico-chemical characterization. Ind. Crop. Prod. 133, 151–159 (2019)
Y. Su, S. Zhang, Y. Chen, T. Yuan, Z. Yang, One-step synthesis of novel renewable multi-functional linseed oil-based acrylate prepolymers and its application in UV-curable coatings. Prog. Org. Coat. 148, 105820 (2020)
F. Dominici, M.D. Samper, A. Carbonell-Verdu, F. Luzi, J. López-Martínez, L. Torre, D. Puglia, Improved toughness in lignin/natural fiber composites plasticized with epoxidized and maleinized linseed oils. Materials 13, 600 (2020)
Z. Zhou, J.P.D. Abbatt, Formation of gas-phase hydrogen peroxide via multiphase ozonolysisi of unsaturated lipids. Environ. Sci. Technol. Lett. 8, 114–120 (2021)
P.M. Paraskar, R.D. Kulkarni, Synthesis of isostearic acid/dimer fatty acid-based polyesteramide polyol for the development of green polyurethane coatings. J. Polym. Environ. 29, 54–70 (2021)
W.R. Slama. Polyester and vinyl ester coatings. Journal of Protective Coatings & Linings. 88–109 (1996)
F.W. Njuku, P.M. Nwangi, G.T. Thiongo, Evaluation of cashew nut liquid based products as a reactive diluents for alkyd coatings. Int. J. Adv. Res. 2(3), 5–10 (2014)
J. Zhu, S. Wei, J. Ryu, M. Budhathoki, G. Liangd, Z. Guo, In situ stabilized carbon nanofiber (CNF) reinforced epoxy nanocomposites. J. Mater. Chem. 20, 4937–494 (2010). https://doi.org/10.1039/c0jm00063a
L. Preeti, N. Anil, Thermal and mechanical properties of environment-friendly ‘green’ plastics from stearic acid modified-soy protein isolate. Ind. Crop. Prod. 21(1), 49–64 (2005). https://doi.org/10.1016/j.indcrop.2003.12.006
M.M. Rahman, A.N. Netravali, B.J. Tiimob, V.K. Rangari, Bioderived green composite from soy protein and eggshell nanopowder. ACS Sustain. Chem. Eng. 2, 2329–2337 (2014)
B.J. Tiimob, S. Jeelani, V.K. Rangari, Eggshell reinforced biocomposite – an advanced “green” alternative structural material. J. Appl. Polym. Sci. 133, 1–10 (2016). https://doi.org/10.1002/app.43124
C. Wang, Q. Sun, K. Lei, C. Chen, L. Yao, Z. Peng, Effect of toughening with different liquid rubber on dielectric relaxation properties of epoxy resin. Polymers 12, 433 (2020). https://doi.org/10.3390/polym12020433
K.S.M. Barczewski, R. Gorny, A. Klozinski, Evaluation of highly filled epoxy composites modified with walnut shell waste filler. Polymer Bullerin 75, 2511–2528 (2018). https://doi.org/10.1007/s00289-017-2163-3
S. Ma, W. Liu, N. Gao, Z. Yan, Y. Zhao, Synthesis and properties of LED-packaging epoxy resin toughened by a novel polysiloxane from hydrolysis and condensation. Macromol. Res. 19(9), 972–979 (2011). https://doi.org/10.1007/s13233-0110911-z
X. Jing, H. Mi, B. Napiwocki, X. Peng, L. Turng, Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 125, 557–570 (2017). https://doi.org/10.1016/j.carbon.2017.09.071
M. Shibata, Y. Itakura, H. Watanabe, Bio-based thermosetting resins composed of cardanol novolac and bismaleimide. Polym. J. 45, 758–765 (2013)
E. Kinaci, E. Can, J.J. LaScala, G.R. Palmese, Influence of epoxidized cardanol functionality and reactivity on network formation and properties. Polymers 12, 1956 (2020). https://doi.org/10.3390/polym12091956
Xi. Zhang, V. Bitaraf, S. Wei, Z. Guo, Vinyl ester resin: rheological behaviors, curing kinetics, thermomechanical, and tensile properties. Am. Inst. Chem. Eng. (2013). https://doi.org/10.1002/aic.14277266-274
F. Yeasmin, A.K. Mallik, A.H. Chisty, F.N. Robel, Md. Shahruzzaman, P. Haque, M. Mizanur Rahman, N. Hano, M. Takafuji, H. Ihara. Remarkable enhancement of thermal stability of epoxy resin through the incorporation of mesoporous silica micro-filler Heliyon, 7(1), e05959 (2021). https://doi.org/10.1016/j.heliyon.2021.e05959
S.A. Kolawole, S.A. Danladi, U.S. Ishiaku, B.M. Dauda, Thermogravimetry analysis of epoxy and unsaturated polyester filled with some agricultural waste of dates palm (Phoenix dactylifera) and African Elemi (Canarium shweinfurthii) particulate composites. J. Mater. Sci. Res. Rev. 3(4), 1–9 (2019)
K. Strzelec, Studies on the properties of epoxy resins cured with polythiourethanes. Int. J. Adhes. Adhes. 27(2), 9–101 (2007)
A.J. Farhan, Characterization the thermal degradation E kinetic of unsaturated polyester and polyester/silica nanoparticles composites by TGA and DSC analysis. J Adv. Res. Fluid Mech. Thermal Sci. 71(1), 10–20 (2020)
F. Jaillet, H. Nouailhas, R. Auvergne, A. Ratsimihety, B. Boutevin, S. Caillol. Synthesis and characterization of novel vinylester prepolymers from cardanol. Eur. J. Lipid Sci. Technol. 116, 928–939 (2014). https://doi.org/10.1002/ejlt.201300487
M.S Garga, K. Srivastavab, D. Srivastava. Physical and chemical toughening of cardanol-based vinyl ester resin using CTBN: A study on spectral, thermal and morphological characteristics. Prog. Org. Coat. 78, 307–317 (2014). https://doi.org/10.1016/j.porgcoat.2014.08.004
J.S. Terry, A.C. Taylor, The properties and suitability of commercial bio-based epoxies for use in fiber-reinforced composites. J. Appl. Polym. Sci. 138, e50417 (2021). https://doi.org/10.1002/app.50417
F. Wang, J. Liao, C. Huang, H. Yu, J. Yan, H. Li, Study on the damping dynamics characteristics of a viscoelastic damping material. Processes 10, 635 (2022). https://doi.org/10.3390/pr10040635
B. Herzog, D.J. Gardner, R. Lopez-Anido, B. Goodell, Glass transition temperature based on dynamic mechanical thermal analysis techniques as an indicator of the adhesive performance of vinyl ester resin. J. Appl. Polym. Sci. 97, 2221–2229 (2005)
M. Kathalewar, A. Sabnis, Epoxy resin from cardanol as partial replacement of bisphenol A-based epoxy for coating application J. Coat. Technol. Res. 11, 601–618 (2014). https://doi.org/10.1007/s11998-014-9570-2
M. Pourbaix, Atlas of electrochemical equilibria in aqueous solution, 2nd edn. (NACE, Houston, 1974)
Acknowledgements
The authors acknowledge Project-200 of Prof. Charles Esimone, Vice Chancellor, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Nwuzor, I.C., Okolie, P.C., Ezeani, O.E. et al. Production of epoxidized cardanol–based vinyl ester resins with cinnamic acid for eco-friendly coating materials. emergent mater. 5, 2061–2074 (2022). https://doi.org/10.1007/s42247-022-00396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-022-00396-6