Abstract
In order to solve the problem of plasticizers limited in alpine regions, in this paper, 1,6-hexanediol oleate (HD) was synthesized by a simple synthetic method using oleic acid and 1.6-hexanediol, and its epoxidation reaction was utilized to prepare a cold-resistant plasticizer (epoxidized 1,6-hexanediol oleate (EHD)) with excellent performances, which was structurally characterized by using Fourier transform infrared spectroscopy and 1HNMR. The properties of HD and EHD plasticized polyvinyl chloride (PVC) were tested and compared with dioctyl adipate (DOA). The results showed that the elongation at break, migration resistance and UV resistance of HD plasticized PVC films were slightly lower than those of DOA plasticized PVC films, but the temperature was 24.4 °C higher than that of DOA at 25% loss of mass, and the thermal stability was better; the epoxidized EHD plasticized PVC films showed 48.78% higher mechanical properties, 11.4 °C higher thermal stability at 25% loss of mass, higher migration resistance, compared with the DOA plasticized PVC films. The mechanical properties were 48.78% higher than those of DOA plasticized PVC films, the migration resistance was more stable in petroleum ether and anhydrous ethanol, and the UV resistance was better than that of DOA plasticized PVC films. Molecular dynamics simulations further verified the migration resistance of EHD/PVC in different solvents. In conclusion, EHD is suitable for low-temperature environments with excellent cold resistance, migration resistance and good stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyvinyl chloride (PVC) is a polymer produced by the free radical polymerization of vinyl chloride monomer under the action of an initiator. Its molecular weight is generally in the range of 50,000–110,000 [1,2,3], which has been widely used in agriculture, industry, construction, and other fields due to its low price and excellent thermal stability, abrasion resistance, electrical insulation, mechanical properties and other advantages [4,5,6]. In recent years, the worldwide demand for PVC has continued to increase, and only in 2020, the global demand for PVC had reached 49.5 million tons [7,8,9,10]. However, due to the PVC molecular chain contains a large number of C–Cl bonds, and each C–Cl bond in the PVC chain has a significant interaction force, the molecular chain of PVC is complicated to free movement, resulting in PVC material products have the disadvantage of hard and brittle [11,12,13]. As a result, PVC system varieties usually require the addition of a number of plastic additives to improve their properties, the largest percentage of which is consumed by plasticizers, whose main purpose is to reduce the plasticity of the PVC material and to give PVC better handling characteristics [14].
About 50% of the world's regions are in alpine areas; pure PVC resins are glassy at 25 ℃ and lack processability. Because of this, the use of PVC materials in alpine regions generally requires cold-resistant plasticizer, but the choice of plasticizers in the low-temperature environment is extremely limited, which seriously restricts the application of plasticized PVC in alpine regions [15,16,17,18].
Currently, straight-chain aliphatic diesters are industrialized cold plasticizers, of which dioctyl adipate (DOA) is the largest class of cold plasticizers, with a mature production process and superior plasticizing properties [19, 20]. Due to its small molecular weight and lack of polar groups, dioctyl adipate causes (DOA) poor migration resistance in PVC materials, which has a significant negative impact on PVC performance. At the same time, this plasticizer has the potential risk of carcinogenicity [21, 22]. DOA is more complicated during production, and the control conditions are more stringent. The efficiency of generating the product is lower, which results in higher costs and the phenomenon of waste of raw materials [23,24,25]. Based on this, there is an urgent need to find efficient cold-resistant plasticizers. Generally speaking, plasticizers with straight-chain alkyl groups have good cold resistance; the longer the alkyl chain, the better the cold resistance, while the more branched chains, the worse the cold resistance. In recent years, researchers have been committed to the development of new cold-resistant plasticizers in order to expand the applicable fields of PVC. Wang Shuqing et al. [26] synthesized the cold-resistant plasticizer n-octyl n-decyl adipate by using adipic acid and n-octanol, which has better low-temperature resistance, as well as low volatility and better heat and light resistance, but the synthesis process is complicated and the yield is low. Li Jinyu et al. [27] used neopentyl glycol, 1,2-propanediol, and adipic acid and synthesized polyester polyols such as 2-Propanediol neopentyl glycol adipate by high-temperature melt polycondensation, which have good oil resistance and migration resistance, and because of the presence of macromolecules, excellent thermogravimetric properties and better cold resistance, but poor mechanical properties and lack of plasticizing effect. Wang Fang et al. [28,29,30] synthesized a kind of epoxy oleic acid bis ester plasticizer by stepwise esterification and acid-catalyzed epoxidation using oleic acid, ethylene glycol and acetic anhydride as raw materials in order to solve the problem of easy migration of traditional epoxy fatty acid methyl ester plasticizers. The findings demonstrate that, although the epoxy oleic acid bis ester plasticizer has good plasticizing properties and has somewhat improved its migration resistance when compared to the traditional epoxy fatty acid methyl ester, its cold-resistant performance is subpar and its preparation is difficult. The ester plasticizers synthesized from acids and alcohols with longer carbon chains can confer excellent low-temperature properties to the products [31, 32]. Oleic acid is a straight-chain structure with a large molecular mass, and its structure contains a double bond, which can be subjected to epoxidation reaction to further increase the polarity and improve the compatibility with PVC, and it can be extracted from biomass; 1,6-hexanediol is mainly used to increase heat resistance and chemical resistance of the products [33, 34], belongs to straight chain diols, and is easy to esterify with acids. Therefore, the study was based on oleic acid with a straight chain length of 18 carbons and 1,6-hexanediol with a straight chain length of 6 carbons to synthesize new plasticizer EHD and HD. It was also compared with DOA to see how assess the effects of the prepared plasticizers affected on the mechanical properties, thermal stability, resistance to transference, and other aspects properties of PVC materials. The plasticizing mechanism of cold-resistant plasticizers was investigated, and the interaction relationship between PVC and plasticizers was simulated using molecular dynamics.
Experimental section
Materials
Oleic acid (OA, acid value 195–200 mg/KOH/g, Tianjin Komeo Chemical Reagent Co., Ltd.) and tetrahydrofuran (THF, AR, Tianjin Obokai Chemical Co., Ltd.), 1,6-Hexanediol (98%, Shanghai McLean Biochemical Science and Technology Co), Hydrogen peroxide (H2O2, 30%, Tianjin Baishi Chemical Co), Formic acid (ACS, ≥ 98%, Sinopharm Chemical Reagent Co., Ltd.), petroleum ether (AR, Yantai Shuangshuang Chemical Co., Ltd.), anhydrous ethanol (AR, Tianjin Ou Bocai Chemical Co., Ltd.), dioctyl adipate (99%, Shanghai McLean Biochemistry & Technology Co., Ltd.), PVC (QingHai Salt Lake Industry Co., Ltd.).
Synthesis of 1,6-hexanediol oleate
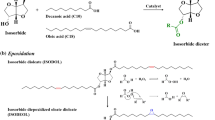
1,6-Hexanediol oleate (HD) was synthesized by esterification of oleic acid and 1,6-hexanediol as raw materials. Oleic acid (211.48 g) and 1,6-hexanediol (59.09 g) were mixed and poured into a three-necked flask equipped with a thermometer and a magnetic stirrer, and the reaction was carried out under nitrogen for 8 h. After the reaction was completed, the solution was washed with sodium bicarbonate to remove unreacted concentrated sulfuric acid and then washed with water for 3 to 5 times until the solution was neutral. Finally, the upper liquid 1,6-hexanediol oleate (HD) was obtained by standing for 3 days.
Synthesis of epoxy 1,6-hexanediol oleate
Hydrogen peroxide (42 g, 30%, V/V), formic acid (9 g), and concentrated sulfuric acid (9 g) were mixed homogeneously and dropped into a beaker containing a solution of HD (60 g), followed by continuous stirring for 8 h at 60 °C. At the end of the reaction, the mixture was washed with water to neutrality. The residual water was subsequently removed by spin evaporation to obtain epoxy 1,6-hexanediol oleate (EHD). The synthesis steps are shown in Fig. 1.
Preparation of plasticized PVC samples
First, 12 g of PVC resin powder and 150 mL of tetrahydrofuran were mixed in a beaker, which was sealed with plastic wrap to prevent volatilization of the tetrahydrofuran and placed on a magnetic stirrer for thorough stirring until the mixture became clear and transparent. Then, add a certain amount of plasticizer (DOA, HD, EHD) to the beaker and continue to stir until it is homogeneous and transparent. The homogenized solution is then poured into a 15-cm-diameter Petri dish. The Petri dish was allowed to stand at room temperature for 3 d. The Petri dish was placed in a thermostatic oven at 50 °C for 3 d. The PVC samples were then removed from the Petri dish. The composition of different PVC specimens is shown in Table 1.
Characterization of different plasticizers and PVC samples
Total reflection Fourier infrared spectroscopy (FT-IR)
The infrared spectrometer model is Nicolet 6700 and uses a DLaTGS detector. In addition, the FT-IR spectrometer has a scan count of 32 with a resolution of 4 cm−1 and a scan range of 500–4000 cm−1 for all samples.
Nuclear magnetic resonance hydrogen spectroscopy (1H NMR)
Depending on the environment of the protons in the molecular structure, it will show different chemical shifts, peak shapes, and splitting in the NMR hydrogen spectra, so it is a typical characterization method to determine the structure of compounds. The solvent used in this paper’s NMR hydrogen spectroscopy tests is deuterated chloroform (CDCl3), and the internal standard is tetramethylsilane (TMS).
Thermogravimetric analysis (TGA)
Thermogravimetric analysis tests the change in sample mass with temperature or time by subjecting the sample to a certain temperature program (rise/fall/constant temperature). Thermogravimetric analysis can evaluate the sample's aging and high-temperature resistance. This paper’s test conditions for thermogravimetric analysis are as follows: nitrogen as carrier gas, nitrogen flow rate of 50 mL/min, test temperature range of 50–600 ℃, warming rate of 20 ℃/min, and sample mass of 8–10 mg.
Mechanical properties test
Tensile strength and elongation at break are essential parameters of PVC specimens and an essential basis for evaluating the plasticizing performance of plasticizers. In this paper, the mechanical properties of the PVC specimen test method refer to the standard ISO 527-5:2009 by using a specific mold from the PVC film cut out dumbbell-shaped test samples, each sample test length of 25 mm, width of 3.5 mm, thickness of 1 mm. The tensile rate of 50 mm/min, each PVC film samples to take the average of the results of the three independent samples as the final results.
Determination of aging resistance
The aging resistance of PVC film samples was tested in accordance with the standard GB/T 9349-2002 standards. Prepared PVC samples were cut into 20 mm (L) \(\times\) 20 mm (W) \(\times\) 1 mm (H) square test pieces; the different components of the PVC film samples were placed in the oven at 180 °C. Every certain period on the different components of the sample photo, record the color change.
Dynamic mechanical analysis (DMA)
The glass transition temperature is an important parameter of PVC samples according to the size of the glass transition temperature of plasticized PVC samples, which can be judged as plasticizer plasticizing effect. In this paper, the glass transition temperature of PVC samples is determined by a differential scanning calorimeter. Test method: PVC samples are cut into rectangular strips of 30 mm × 6 mm, and a film stretching fixture is used in the test process. Test conditions: the test temperature range was − 40–80 °C, the temperature increase rate was 3 °C/min, and the vibration frequency was 1 Hz.
Migration resistance test
Regarding the standard “ISO 175–2011 Plastics—Experimental methods for the determination of the immersion effect of liquid chemicals,” samples of PVC films were tested for their resistance to extraction. The solvents used in this paper are distilled water, polar solvent anhydrous ethanol, and non-polar solvent petroleum ether. The specific operation steps were as follows: The prepared PVC samples were cut into several square PVC sheets with specifications of 20 mm × 20 mm × 1 mm and then placed in a desiccator to dry for 6 h. Afterward, the mass of the PVC sheets was weighed using an analytical balance with an accuracy of 0.0001 g and recorded as W0. The PVC flakes were immersed in a 50-mL conical flask containing the same volume of solution, and the flask was placed in a thermostat at 30 °C. The PVC flakes were taken out at regular intervals, and the surface of the flakes was wiped clean with a piece of filter paper and then dried flat in an oven at 50 °C. After drying, the flakes were taken out, and the mass of the flakes was weighed and labeled as W. To minimize the experimental error, the same PVC samples were calculated for the mass loss rate of three independent samples, and finally, take the average value as the final result, the mass loss rate of the PVC sheet according to Eq. 1 for calculation
η2: migration loss rate, %; W0: mass of PVC sheet before immersion, g; W: mass of PVC sheet after immersion, g.
UV transmission test
The transmittance of the PVC specimens was tested by using a dual beam UV–Vis spectrophotometer scanning over the wavelength range of 200–800 nm.
Molecular dynamics simulation
Density functional theory (DFT) calculations were performed using the Dmol3 module of Material Studio 2020. The generalized gradient approximation (GGA) method of the Perdew–Burke–Ernzerhof (PBE) functional is used to describe the interactions between nuclei and electrons. The force and energy convergence criteria were set to 0.002 HaÅ−1 and 10–5 Ha, respectively. The binding energy (ΔE) is calculated as shown in Eq. 2
The binding energies between a molecule of EHD, DOA, and different substances with the same number of molecules (PVC, C2H5OH, H2O, and petroleum ether) are explored by forming a simple three-dimensional model.
Etotal: optimizing the energy of the system; E1: energy from EHD or DOA; E2: energy from PVC, C2H5OH, H2O, and petroleum ether.
Results and discussion
FT-IR spectra of plasticizers
The infrared spectra of the different types of plasticizers synthesized are shown in Fig. 2, where Fig. 2a shows the line graph of the infrared spectra of oleic acid, Fig. 2b shows the infrared spectra of 1,6-hexanediol oleate, and Fig. 2c shows the infrared spectra of epoxy 1,6-hexanediol oleate. The telescopic vibration peak of a hydroxyl group (–OH) on the carboxyl group in the molecular structure of oleic acid at 937 cm−1 in Fig. 2a, the characteristic peak disappeared after the first step of the esterification reaction; in addition, the telescopic vibration peak of the carbonyl group (C = O) in the carboxyl group at 1709 cm−1 move to 1735 cm−1, which indicated the successful esterification reaction [35]. In the second step of the epoxidation reaction, the stretching vibration peak of the epoxy bond (C–O–C) at 829 cm−1 in Fig. 2c appears concurrently with the disappearance of the unsaturated C–H bond’s stretching vibration peak at 3007 cm−1 in Fig. 2b, which is a good indication of the unsaturated double bond's successful conversion to the epoxy bond in the HD structure [36].
1H NMR spectra of plasticizers
Nuclear magnetic resonance hydrogen spectroscopy was utilized to verify the molecular structure of the products after each step of the reaction, and the results are shown in Fig. 3. In Fig. 3a is the 1H NMR curve of oleic acid, b is the 1H NMR curve of HD, c is the 1H NMR curve of EHD. Comparing the 1H NMR curves of oleic acid and 1,6-hexanediol oleate, the peak at 3.9–4.1 ppm of HD is attributed to the proton signals on the carbon atoms in the ester molecule, indicating that the esterification reaction was successfully carried out [37]. In the 1H NMR curves of esters and epoxides, the peak at 5.3–5.4 ppm for HD was shifted, while the peak at 2.8 ppm for EHD appeared, which indicated that the unsaturated double bond of the ester was basically converted to epoxide group [38]. It also demonstrates the accomplishment of the esterification and epoxidation processes.
TGA analysis
Figure 4 shows the TGA and DTG curves of PVC films after blending with different plasticizers. The thermal stability of EHD is higher than that of DOA, as shown in Fig. 4a. In the DTG curves of EHD, the weight loss between 200–300 ℃ is due to the fact that a small amount of saturated 1,6-hexanediol oleate is still present in EHD. As shown in Fig. 4b, all the films showed no mass loss until 150 ℃, indicating that THF was removed from the PVC films. After that, the thermal decomposition of each specimen was mainly applicable in the range between 150 and 550 ℃. It can be seen from Fig. 4b that the first stage of thermal decomposition is mainly concentrated between 150 and 350 ℃, and the weight loss is mainly due to the volatilization of the plasticizer and the dehydrochlorination of the PVC skeleton. Thermal degradation data, including weight loss temperatures (T25 and T50) for 25% and 50% of the degraded mass, are summarized in Table 2. The corresponding temperatures for the PVC samples (DOA-50, HD-50, and EHD-50) when 25% of the weight loss of each sample was reached were 305.2 ℃, 314.5 ℃, 329.6 ℃, and 316.6 ℃, respectively. Subsequently, when the mass loss reached 50%, the corresponding temperatures for the different plasticizer specimens were 319.5 ℃, 364.5 ℃, and 352.6 ℃. Regardless of the mass loss, the temperature of EHD in the first stage is higher than that of the commercially available samples. This could be due to two different factors. One is that EHD is more heat resistant and has a higher boiling point due to its larger molecular weight than both DOA. Another explanation is that oleate plasticizers with straight-chain type structures have significantly longer chains than DOA after mixing with PVC; hence, their long alkyl chains are more densely entangled with PVC chains. Along with the more significant contact force between the structure’s uniformly distributed polar groups, PVC molecules also prevent the plasticizer from precipitating in the PVC matrix, giving PVC greater thermal stability. The second stage of decomposition is mainly concentrated between 350 and 450 °C, in which the molecular structure of PVC is altered at high temperatures, the carbon skeleton is destroyed [39], and the conjugated polyenes form aromatic compounds by cyclization; in the third stage at 450–550 °C, the polyene structure is decomposed into olefins [40, 41]. In conclusion, the compatibility and interfacial interaction between EHD and PVC are better, and the thermal stability of plasticizer EHD in PVC is better than that of DOA.
Cold resistance
The standard application of PVC products in low-temperature environments depends on whether the plasticizer can have an excellent plasticizing effect in this environment. The glass transition temperature (Tg) can evaluate the cold resistance of PVC samples. In general, plasticized PVC specimens with lower Tg have greater flexibility at low temperatures—that is, greater resistance to cold at lower temperatures. By comparing the mechanical properties of DOA and EHD, migration resistance, etc., it can be seen that EHD has superior performance. Finally, the superiority of cold resistance is determined by comparing the Tg of the two best performers, DOA-50 and EHD-50. The Tg of different specimens can be accurately tested by DMA.
The results are shown in Fig. 5a, and it can be seen that the Tan δ curves of the two PVC specimens have only one peak, which indicates that these two plasticizers are more compatible with PVC, and the Tg of DOA-50 is 16.89 °C, and the Tg of EHD-50 is 23.05 °C. It can be seen from the results that DOA has relatively low Tg value. However, the loss factor Tan δ of EHD is lower than that of DOA at the same temperature, and the loss factor is used to describe the viscoelastic characteristics of the material, and the lower the value of Tan δ, the better the elasticity of the material, which indicates that the plasticizing effect of EHD is better than that of DOA at low temperatures. Figure 5b shows different PVC specimens’ energy storage modulus curves. The degree to which these PVC specimens can absorb deformation is indicated by the magnitude of their energy storage modulus, and this can be interpreted as a measure of their stiffness. The figure shows that the stored energy modulus of EHD-50 is larger than DOA, which indicates that EHD-50 requires more energy for deformation and has more substantial stiffness. The conclusion shows that EHD has better compatibility with PVC at low temperatures and has better plasticizing effect.
Optical performance analysis
The XRD curves of PVC films after plasticizing with different plasticizers are shown in Fig. 6. The crystallinity is shown in Table 3, from which it can be seen that the crystallinity of EHD-50 is reduced by 4.5 compared to pure PVC, which indicates that plasticizing improves the transparency of the material due to the scattering and refraction of light caused by the crystalline regions of the polyvinyl chloride, which results in the opaque and semi-transparent appearance of the film. At the same time, the decrease in crystallinity makes the polyvinyl chloride material have better elasticity because the material with high crystallinity has more ordered grain structure inside, which can effectively hinder the dislocation movement, which leads to the material having higher strength. This is consistent with the findings of the mechanical properties mentioned in the text.
Transparency is a key feature of PVC film products, especially in applications such as window medical, device food and packaging. Superior transparency enhances product visibility and attractiveness and draws the attention of consumers. The physical drawings of PVC films with different plasticizers added are shown in Table 4 which shows that both HD and EHD plasticized PVC films have excellent light transmission and can be used in many applications, giving more applicability to PVC films.
Optical properties of PVC different PVC samples
The UV transmittance of different PVC specimens is shown in Fig. 7, which shows that the transmittance of PVC specimens decreases with the increase in plasticizer content, and the obstruction of UV light increases. In the UV region of wavelength 200–300 nm, the UV transmittance of commercial DOA specimens is around 30% with the same addition of 50 parts of plasticizer. On the other hand, the UV transmittance of HD and EHD was only 5%; in comparison, HD and EHD enhanced the UV-resistant property to a certain extent.
Mechanical properties test
The mechanical properties of these plasticized PVC were evaluated by tensile tests. The tensile stress–strain curves of different PVC specimens are shown in Fig. 8, and the related mechanical property data are shown in Table 5. From Fig. 8, it is evident that as the plasticizer concentration grew, the PVC specimens’ tensile strength gradually dropped and their elongation at break gradually increased. The elongation at break of PVC samples following plasticizer HD plasticization is only roughly 510%, even after adding the same amount of plasticizer (30 parts, 40 parts, and 50 parts). Its tensile strength is much smaller than the other PVC samples, which indicates that HD basically does not play a plasticizing effect, because the plasticizer structure of the carbon–carbon double bond cannot enter the rotation, so that the molecular flexibility is significantly reduced. Because plasticizer and PVC are compatible, precipitating from the PVC substrate is simple and does not result in the plasticizing effect. For this reason, epoxidized vegetable oils can be used as secondary plasticizer applications, while unprocessed vegetable oils cannot be used directly for plasticizing. Comparing DOA and EHD the tensile strength is 28.76% greater and the elongation at break is 7.13% higher than that of DOA at the same content. This is because the addition of EHD increases the free volume of the PVC chain between the PVC molecules to give the free movement of the space between the PVC molecules to reduce the interaction between the PVC molecules and can also act as a lubricant in the process of PVC moving to reduce the sliding resistance, so that PVC molecules are more likely to be accessible to move, which has played the role of the effect of plasticizing.
Molecular dynamics simulation
The binding energies between EHD and DOA with PVC, C2H5OH, H2O, and petroleum ether, respectively, are shown in Table 6. It can be seen that in PVC the binding energy of EHD is 0.86, DOA binding energy is 0.79, indicating that the binding ability between EHD and PVC is strong, in PVC compared to DOA is more stable; in anhydrous ethanol the binding energy of EHD is 0.69, DOA binding energy is 0.74, this indicates that DOA has a stronger binding force in polar solutions and is easier to migrate to polar solutions, indicating that EHD is more stable in polar solutions; in distilled water, the binding energy of EHD is 0.68, the binding energy of DOA is 0.73, indicating that DOA has a stronger binding force in distilled water solution and is easier to migrate to it, indicating that EHD is more stable in distilled water solution; in petroleum ether, the binding energy of EHD is 0.32, the binding energy of DOA is 0.49, indicating that DOA has a stronger binding force with polar solutions in non-polar solutions and is easier to migrate to non-polar solutions, indicating that EHD is more stable in non-polar solutions. In summary, EHD has better stability in PVC and is more stable in water, petroleum ether, and anhydrous ethanol.
Migration loss by leaching tests of PVC films
Migration resistance is one of the essential properties of flexible PVC products, which determines the application areas of PVC products. The mass loss of different PVC specimens in the extraction resistance and volatility resistance tests is shown in Fig. 9. Due to HD's poor plasticizing action and poor compatibility with PVC due to double-kin in its molecular structure, a significant mass loss occurs in petroleum ether and anhydrous ethanol in a relatively short period of time. For the mass loss of other PVC specimens, it can also be seen from Fig. 9 that the mass loss rate of all PVC specimens in the distilled water environment is less than 2% in the 7-day test time. No migration occurs so that these PVC specimens can maintain excellent stability in the distilled water. In the nonpolar solution petroleum ether, only in 1 day, as shown in Fig. 9a, the mass loss rate of DOA was very high and gradually stabilized after that, which indicated that DOA were poorly stabilized in the nonpolar environment. They all migrated out of the PVC matrix in less than one day. HD and EHD had better extraction resistance in non-polar environments compared to HD and EHD, which had a loss rate of 15% in 1 day, but then the loss was slower and stabilized. As shown in Fig. 9d, EHD had a loss rate of less than 10% after 7 days in petroleum ether and no further loss. In the polar environment of anhydrous ethanol, the loss rate of the same plasticizer increased with the increase of the amount of plasticizer within 7 days of testing. In comparing equal amounts of plasticizers, the mass loss rate of EHD-30, EHD-40, and EHD-50 was less than 12%, while the loss rate of DOA-50 was 15%, indicating that DOA-50 was less stable in the polar environment. The stability of EHD was relatively better. This is because the molecular chain of EHD is longer than that of DOA, so it is more tightly intertwined with PVC molecules. Furthermore, EHD has superior migration resistance because its ester and epoxy groups exhibit particular subvalent bonding interactions with PVC molecules that prevent EHD from escaping the polymer.
Plasticizing mechanisms for migration resistance of plasticizers in polyvinyl chloride
Since different plasticizers have varied plasticizing effects due to differences in their molecular structures, combining them with the plasticizing mechanisms that are now known (lubrication theory, gel theory, and free-volume theory) [42, 43]. Figure 10 depicts the potential plasticizing mechanisms of EHD. Without the use of plasticizers, PVC molecules can freely move within the extremely small volume. The PVC molecular structure also exists in a large number of polar parts. Hence, the PVC molecules are between the existence of a significant interaction force, so pure PVC shows hard and brittle characteristics. When EHD is mixed with PVC, they become embedded in the PVC molecules, increasing the free volume between neighboring PVC chains and allowing more PVC molecules to move around. PVC molecules move when the plasticized PVC film is subjected to external forces. The contact force between the PVC molecules is the most important factor at this point. To reduce the sliding resistance of PVC, the EHD plasticizers within the material can function as a lubricant. The addition of plasticizer increases the distance between PVC molecules, thus reducing the force between PVC molecules, making it easier for PVC molecules to move, thus playing a plasticizing effect. Since the molecular chain of EHD is longer than that of DOA, it is more tightly entwined with the PVC chain. Additionally, because of the degree of subvalent bonding force between the epoxy and ester groups in EHD's molecular structure and the PVC molecules, EHD has a better migration performance than DOA.
Conclusions
The cold-resistant plasticizer HD was successfully synthesized from oleic acid and 1,6-hexanediol, and then, EHD was produced by epoxidation, with the addition of epoxy functional groups to improve its polarity. Thermogravimetric analysis showed that the thermal stability of PVC films plasticized with EHD was 11.4 °C higher than that of films plasticized with DOA at 25% mass loss. In terms of mechanical properties, EHD can improve the flexibility of PVC very well, and the plasticizing effect is better than that of DOA; moreover, the migration resistance of PVC films containing EHD in polar and nonpolar solutions is significantly improved compared with that of DOA, and this result has been verified by molecular simulation. UV transmittance tests show that EHD plasticized PVC films have higher UV resistance than DOA, and DMA results show that EHD plasticized PVC films with a Tg of 23.05 °C are also suitable and more stable in cold regions. In conclusion, EHD can improve the thermal stability, mechanical properties, optical properties and migration resistance of PVC film, and it is a good cold-resistant plasticizer.
References
Jia P, Ma Y, Zhang M et al (2019) Designing rosin-based plasticizers: effect of differently branched chains on plasticization performance and solvent resistance of flexible poly (vinyl chloride) films. ACS Omega 4(2):3178–3187
Zheng T, Wu Z, Xie Q et al (2018) Structural modification of waste cooking oil methyl esters as cleaner plasticizer to substitute toxic dioctyl phthalate. J Clean Prod 186:1021–1030
Liu D, Jiang P, Nie Z et al (2020) Synthesis of an efficient bio-based plasticizer derived from waste cooking oil and its performance testing in PVC. Polym Testing 90:106625
Navarro R, Pérez Perrino M, Gómez Tardajos M et al (2010) Phthalate plasticizers covalently bound to pvc: plasticization with suppressed migration. Macromolecules 43(5):2377–81
Xie Y, Yu B, Zhang Y et al (2021) Antibacterial plasticizers based on bio-based engineering elastomers for medical PVC: synthesis, characterization and properties. Polym Chem 12(8):1114–1124
Qiu G, Guo Y (2022) Current situation and development trend of titanium metal industry in China. Int J Miner Metall Mater 29(4):599–610
He W, Zhu G, Gao Y et al (2020) Green plasticizers derived from epoxidized soybean oil for poly (vinyl chloride): Continuous synthesis and evaluation in PVC films. Chem Eng J 380:122532
Montero de Espinosa L, Gevers A, Woldt B et al (2014) Sulfur-containing fatty acid-based plasticizers via thiol–ene addition and oxidation: synthesis and evaluation in PVC formulations. Green Chem 16(4):1883–1896
Zhang Q, Guo Y, Marek AA et al (2019) Design, fabrication and anti-aging behavior of a multifunctional inorganic–organic hybrid stabilizer derived from co-intercalated layered double hydroxides for polypropylene. Inorg Chem Front 6(9):2539–2549
Jia P, Hu L, Shang Q et al (2017) Self-plasticization of pvc materials via chemical modification of mannich base of cardanol butyl ether. ACS Sustainable Chem Eng 5(8):6665–6673
Tong H, Hai J (2018) Synthesis of a novel environmental friendly plasticizer based on tung oil fatty acid for poly (vinyl chloride) blends. Polish J Chem Technol 20(2):92–7
Feng Y, Chu Z, Man L et al (2019) Fishbone-like polymer from green cationic polymerization of methyl eleostearate as biobased nontoxic PVC plasticizer. ACS Sustainable Chem Eng 7(23):18976–18984
Boran H, Terzi S (2017) Stress-induced transcriptional changes and dna damage associated with Bis (2-ethylhexyl) adipate exposure in zebrafish (Danio rerio) larvae. Bull Environ Contam Toxicol 99(3):308–314
Fukuwatari T, Suzuki Y, Sugimoto E et al (2002) Elucidation of the toxic mechanism of the plasticizers, phthalic acid esters, putative endocrine disrupters: effects of dietary di (2-ethylhexyl) phthalate on the metabolism of tryptophan to…. Biosci Biotechnol Biochem 66(4):705–710
McCombie G, Biedermann S, Suter G et al (2017) Survey on plasticizers currently found in PVC toys on the Swiss market: banned phthalates are only a minor concern. J Environ Sci Health Part A Toxic/Hazard Subst Environ Eng 52(5):491–496
Biedermann-Brem S, Biedermann M, Pfenninger S et al (2008) Plasticizers in PVC toys and childcare products: What Succeeds the Phthalates? market survey 2007. Chromatographia 68(3):227–234
Xu Y, Xiong Y, Guo S (2020) Effect of liquid plasticizers on crystallization of PCL in soft PVC/PCL/plasticizer blends. J Appl Polym Sci 137(24):48803
Schutyser W, Koelewijn S-F, Dusselier M et al (2014) Regioselective synthesis of renewable bisphenols from 2,3-pentanedione and their application as plasticizers. Green Chem 16(4):1999–2007
Yang Y, Huang J, Zhang R et al (2017) Designing bio-based plasticizers: Effect of alkyl chain length on plasticization properties of isosorbide diesters in PVC blends. Mater Des 126:29–36
Goulas AE, Riganakos KA, Ehlermann DAE et al (1998) Effect of high-dose electron beam irradiation on the migration of doa and atbc plasticizers from food-grade pvc and pvdc/pvc films, respectively, into olive oil. J Food Prot 61(6):720–724
Bazilio FS, Bomfim MVJ, R. J. d. Almeida, et al (2014) Intralaboratory validation of an analytical method for determining the migration of bis (2-ethylhexyl) adipate from packaging to fat foods. Accreditation Qual Assur 19(3):195–204
Yang Y, Miao C, Wang R et al. (2024) Advances in morphology-controlled alumina and its supported Pd catalysts: synthesis and applications. Chem Society Reviews
Chaibakhsh N, Rahman MBA, Vahabzadeh F et al (2010) Optimization of operational conditions for adipate ester synthesis in a stirred tank reactor. Biotechnol Bioprocess Eng 15(5):846–853
Yimeng L, Jiangong S, Yi Z et al (2015) Market demanding analysis of PVC cold-resistant plasticizer-DOA. Ind Technol Innovation 2(2):155–159
Carbonell-Verdu A, Garcia-Sanoguera D, Jorda-Vilaplana A, et al. (2017) Corrigendum: A new biobased plasticizer for poly(vinyl chloride) based on epoxidized cottonseed oil. J Appl Polym Sci 134(17).
Chen J, Liu Z, Li X et al (2016) Thermal behavior of epoxidized cardanol diethyl phosphate as novel renewable plasticizer for poly(vinyl chloride). Polym Degrad Stab 126:58–64
Kumar S (2019) Recent developments of biobased plasticizers and their effect on mechanical and thermal properties of Poly (vinyl chloride): a review. Ind Eng Chem Res 58(27):11659–11672
Jia P, Zhang M, Hu L et al (2015) Synthesis and application of environmental castor oil based polyol ester plasticizers for poly(vinyl chloride). ACS Sustainable Chem Eng 3(9):2187–2193
Pokharel P, Choi S, Lee DS (2015) The effect of hard segment length on the thermal and mechanical properties of polyurethane/graphene oxide nanocomposites. Compos A Appl Sci Manuf 69:168–177
Lee B-M, Jung J, Gwon H-J et al (2023) Synthesis and properties of isosorbide-based eco-friendly plasticizers for poly (Vinyl Chloride). J Polym Environ 31(4):1351–1358
Jia P, Zhang M, Hu L et al (2018) A strategy for nonmigrating plasticized PVC modified with mannich base of waste cooking oil methyl ester. Sci Rep 8(1):1589
Carbonell-Verdu A, Garcia-Sanoguera D, Jordá-Vilaplana A et al. (2016) A new biobased plasticizer for poly(vinyl chloride) based on epoxidized cottonseed oil. J Appl Polym Sci 133(27).
Kardar P (2015) Preparation of polyurethane microcapsules with different polyols component for encapsulation of isophorone diisocyanate healing agent. Prog Org Coat 89:271–276
Li H, Wang X (2022) Preparation of microcapsules with IPDI monomer and isocyanate prepolymer as self-healing agent and their application in self-healing materials. Polymer 262:125478
Gui X, Ding Y, Yun Z (2016) Preparation of cottonseed-based epoxy fatty acid methyl esters by an integrated approach %J The Canadian journal of chemical engineering %J. The Canadian J Chem Eng 94(3):424–429
Kurańska M, Beneš H, Prociak A et al (2019) Investigation of epoxidation of used cooking oils with homogeneous and heterogeneous catalysts. J Clean Prod 236:117615
Scotti N, Ravasio N, Evangelisti C et al (2019) Epoxidation of karanja (Millettia pinnata) oil methyl esters in the presence of hydrogen peroxide over a simple niobium-containing catalyst. Catalysts. https://doi.org/10.3390/catal9040344
Kurańska M, Benes H, Polaczek K et al (2019) Effect of homogeneous catalysts on ring opening reactions of epoxidized cooking oils. J Clean Prod 230:162–169
Jia P, Hu L, Zhang M et al (2017) Phosphorus containing castor oil based derivatives: potential non-migratory flame retardant plasticizer. Eur Polymer J 87:209–220
Chen J, Liu Z, Nie X et al (2018) Synthesis and application of a novel environmental C26 diglycidyl ester plasticizer based on castor oil for poly(vinyl chloride). J Mater Sci 53(12):8909–8920
Jia P, Feng G, Bo C et al (2018) A composition of phosphaphenanthrene groups-containing castor-oil-based phosphate plasticizer for PVC: synthesis, characterization and property. J Ind Eng Chem 60:192–205
Bocqué M, Voirin C, Lapinte V et al (2016) Petro-based and bio-based plasticizers: chemical structures to plasticizing properties. J Polym Sci, Part A: Polym Chem 54(1):11–33
Lee Y-M, Lee J-E, Choe W et al (2019) Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ Int 126:635–643
Acknowledgements
This project is supported by the Science and Technology Funding Program of Xining City, Qinghai Province (2024-Y-03). Qinghai University Innovation and Entrepreneurship Training Program (2023-QX-44). The innovation project of Kunlun elitist of Qinghai Province, and training program for famous teachers of Qinghai University. Thousand Talents Program of Qinghai Province.
Author information
Authors and Affiliations
Contributions
Kuiyun Dong wrote the text of the manuscript, which was reviewed by others.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, K., Xue, C., Nan, H. et al. Synthesized the cold-resistant plasticizer epoxidized 1,6-hexanediol oleate and effects on the properties of polyvinyl chloride. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05420-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05420-9