Abstract
A novel pH-responsive Fe3O4/poly(2-succinyloxyethylmethacrylate-co-methylmethacrylate) (poly(SEMA-co-MMA)) nanocomposite was designed for anticancer drug delivery applications. For this propose, poly(2-hydroxyethyl methacrylate-co-methylmethacrylate) (poly(HEMA-co-MMA)) was synthesized via reversible addition-fragmentation transfer (RAFT) method. Then, poly(SEMA-co-MMA) was prepared by the esterification of poly(HEMA-co-MMA) copolymer through the reaction with an excess amount of succinic anhydride. The synthesized copolymers with acidic functional groups were adsorbed onto the surface of Fe3O4 nanoparticles and Fe3O4/poly(SEMA-co-MMA) nanocomposite was developed. The pH-sensitivity of Fe3O4/poly(SEMA-co-MMA) nanocomposite was confirmed via dynamic light scattering (DLS) measurements. The Doxorubicin (DOX) encapsulation efficiency was 92.6%. The release rates at pHs of 5.4 and 4 (37 °C) reached 55.7 and 62.4 wt%, respectively, and at pH = 7.4, it possessed a minimum amount around 38.3 wt% after 15 days. The synthesized nanocomposite may be find the drug delivery applications, in part thanks to their smart physicochemical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The inorganic nanocomposites have been prepared in developing new properties and superior performances. It is a research realm which covers the subjects ranging from chemistry, physics, and biology to material science [1,2,3]. The principal applications of magnetic nanoparticles were in the biotechnology and biomedicine such as magnetic resonance imaging (MRI) [4], site-specific drug delivery [5], magnetic hyperthermia in cancer therapy [6], magnetic field-assisted extraction of cells [7], enzyme catalysis [8] and radiotherapy [9]. Nanocomposites have been mainly applied as the drug delivery systems for their large specific capacity for drug loading, strong superparamagnetism, and efficient photoluminescence. Because these particles have an ability to target a specific site like a tumor, thereby decrease the systemic distribution of cytotoxic compounds and enhance uptake at the target site [10, 11]. On other hand the pH-responsive systems have been widely used for a variety of biomedical applications [12].
Doxorubicin (DOX) has been employed widely as effective anticancer drugs. However, DOX is inappropriate as a chemotherapeutic agent, due to its short- and long-term cardiac toxicity [13, 14]. Hence, it is a task to improve the anticancer activity and reduce the systemic toxicity of DOX with development of a drug delivery system. The methyl methacrylate (MMA) is a water insoluble monomer and it has been utilized as the optoelectronic biomaterials and optic fibers, because of its good optical subsuming the high transparency and clarity as well as mechanical properties [15]. In contrast, 2-hydroxyethyl methacrylate (HEMA) is a water soluble monomer and its solubility depends on the molecular weight of poly(HEMA), which has been widely used as hydrogel and biomedical fields such as drug delivery [16].
The polymers of magnetic nanocomposites were prepared using the reversible deactivation radical polymerization or living radical polymerization [17] including nitroxide-mediated polymerization (NMP) [18], atom transfer radical polymerization (ATRP) [19], and reversible addition-fragmentation chain transfer (RAFT) [20]. The ATRP polymerization is limited, because the toxic catalysts cannot be removed properly from the polymers. However, due to the variety of monomers that could be polymerized in water, the RAFT polymerization is suitable for the controlled delivery, biomedical, and pharmaceutical applications [21].
The purpose of this study was the synthesis of a pH-sensitive nanocomposite as the anticancer drug delivery system. Poly(2-hydroxyethylmethacrylate-co-methylmethacrylate) (poly (HEMA-co-MMA)) was synthesized by RAFT technique. The esterification of the hydroxyl groups on poly(HEMA) blocks with excess amount of succinic anhydride resulted in poly(SEMA-co-MMA). These copolymers with carboxylic functional groups were then adsorbed onto the surface of Fe3O4 nanoparticles through the interaction with hydroxyl groups on the nanoparticles surface. The doxorubicin hydrochloride (DOX) was interacted with nanocomposite via ionic interaction and hydrogen bonding. The strong interaction of DOX with the pH-responsive blocks and pH-sensitive drug release from polymer makes the system very useful as a controlled drug delivery system [22,23,24].
Experimental
Materials
The RAFT agent (4-cyano-4-[(phenylcarbothioyl) sulfanyl] pentanoic acid) was synthesized in our laboratory [25]. The MMA and HEMA were distilled under reduced pressure, then were stored at −15 °C prior to use. Pyridine was dried over CaH2. The 2,2′-azobisisobutyronitrile (AIBN), dimethyl sulfoxide (DMSO) and succinic anhydride were purchased from Sigma-Aldrich. The ferrous chloride tetrahydrate (FeCl2. 4H2O, 98%), ferric chloride hexanhydrate (FeCl3. 6H2O, 99%), and NH4OH (25 wt% of ammonia) were purchased from Merck. The DOX was purchased from Zhejiang, China, and other reagents were purchased from Merck and purified according to the standard methods.
Synthesis of poly(HEMA-co-MMA) via RAFT reagent
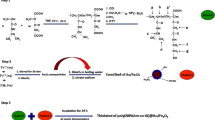
In a typical experiment, A 200 mL three-neck round-bottom flask was loaded with MMA monomer (0.7 mL, 6.54 mmol), HEMA(0.3 mL,2.4 mmol), AIBN (1 mg, 0.005 mmol), synthesized RAFT agent (2 mg, 0.007 mmol) and dried N,N-dimethyl formamide (DMF, 5 mL). The solution was degassed with three freeze-pump-thaw cycles, and then transferred in an oil bath at 75 ± 3 °C for about 22 h. At the end of reaction time, the ampoule was transferred in cooled ice/water bath for quenching the polymerization. The mixture was precipitated in cold diethyl ether (50 mL) and, subsequently, the product was dried under vacuum at 25 °C temperature (Scheme 1).
Synthesis of poly(SEMA-co-MMA)
Poly(SEMA-co-MMA) was prepared by the esterification of poly(HEMA-co-MMA) copolymer through the reaction between hydroxyl groups of the poly(HEMA) with an excess amount of succinic anhydride. In a 150 mL round-bottom flask, poly(HEMA-co-MMA) copolymer (0.5 g, 0.025 mmol) was dissolved in anhydrous pyridine (20 mL) at 25 °C under the argon flow. The succinic anhydride (0.5 g, 5 mmol) was added and the reaction was proceeded at 25 °C for about 50 h. The reaction was precipitated into 50 mL of methanol to remove the excess succinic anhydride. Simultaneously, during precipitation a part of succinic anhydride was converted into monomethyl succinate which was not removed. Thus, the precipitate was re-dissolved in 15 mL of pyridine and precipitated into 150 mL of diethyl ether (Scheme 2).
Synthesis of Fe3O4 nanoparticles
The iron oxide nanoparticles were synthesized by coprecipitation method [26, 27]. A three-neck round-bottom flask was loaded with 200 mL of double distilled water, and then degassed by pure argon gas for 40 min. Subsequently, 20 mL of ferrous chloride (0.1 mol L−1) and 40 mL of ferric chloride (0.1 mol L−1) were added to the reaction. The solution was then placed in an oil bath at 80 ± 5 °C, and 10 mL of NH4OH (25% of ammonia) was quickly added to mixture under stirring. The solution was maintained at 80 ± 5 °C for 1 h. Then, reaction was cooled and the produced black nanoparticles were washed four times with water and ethanol up to its pH became neutral. The black nanoparticles were dried under vacuum at 25 °C temperature for 24 h.
Synthesis of poly(SEMA-co-MMA)/Fe3O4 nanocomposite
The synthesized poly(SEMA-co-MMA) (100 mg) and Fe3O4 nanoparticles (100 mg) were added into a flask containing 10 mL methanol and introduced into the ultrasonic reactor. The water was circulated and then ultrasonic generator was switched on. The ampoule was irradiated with ultrasonic vibrations for 30 min to obtain a homogenous suspension. The reaction condition was similar to the preparation of nanocomposite by ultrasonic irradiation. After 30 min, the ultrasonic generator was stopped at 25 °C and the reaction was then stirred for about 30 h. After elapsing the reaction time, the unmodified iron oxide nanoparticles were precipitated by centrifugation at 8000 rpm for about 15 min and dried under vacuum at 25 °C for 24 h. In addition, the Fe3O4/poly(SEMA-co-MMA) nanocomposite was purified through an external magnetic field (Fig. 1). Four microtubes in Fig. 1 demonstrate the intermediate purification step for the nanocomposite, in which the dark areas are composed of polymer and nanoparticles.
Preparation of doxorubicin hydrochloride (DOX)-loaded magnetic nanocomposite
In this study, DOX was selected as an anticancer drug model to evaluate the drug loading. Typically, 100 mg of poly(SEMA-co-MMA)/Fe3O4 nanocomposite was ultrasonically dissolved in deionized water (15 mL) under stirring at room temperature. Then, DOX solution (10 mg) was added to the mixture and stirred for about 48 h in the dark at room temperature to reach a maximum drug loading content. The DOX-loaded magnetic nanocomposite was collected by centrifugation at 13000 rpm for 15 min. The DOX encapsulation efficiency was determined by UV-Vis spectroscopy at 480 nm and calculated using Eq. (1) [28].
where CT is total DOX concentration taken for loading and CDOX is DOX concentration infiltrate solution. The DOX-encapsulation efficiency was calculated to be 94.6% and its loading efficiency was 9.3% (Fig. 2).
In vitro pH-dependent release of DOX
In the in vitro drug release experiment, DOX-loaded Fe3O4/poly(SEMA-co-MMA) magnetic nanocomposite (60 mg) was re-dispersed in buffer solution PBS (0.01 mol L−1,100 mL) at pHs of 7.4, 5.4, and 4, and was placed in a dialysis membrane bag with a molecular cutoff of 1 kDa. The release solution was stirred at 300 rpm at 37 °C, and then 2 mL of buffer solution was collected in different times to characterize with UV-Vis spectrophotometer at 480 nm. The percentage of the cumulative content of released DOX was deliberated from the standard calibration curve. The detected absorbance of DOX was compared with the corresponding calibration curve of DOX in the same buffer. The percent of drug released from nanocomposite was calculated based on Eq. (2) [29].
Characterization
Fourier transforms infrared (FT-IR) spectra of the polymer and Fe3O4/poly(SEMA-co-MMA) magnetic nanocomposite were taken in a Shimadzu 8101 M FT-IR (Shimadzu, Kyoto, Japan) at 400–4000 cm−1. The proton nuclear magnetic resonance (1HNMR) spectra were recorded in deuterated chloroform (CDCl3) at 25 °C by an FT-NMR (400 MHz) Bruker spectrometer (Bruker, Ettlingen, Germany). The size exclusion analyses were carried out using a Waters 1515 (USA) gel permeation chromatography instrument equipped with Breeze 1515 isocratic pump and 7725 manual injector. Dimethylformamide (DMF) was applied as eluent at a flow rate of 1 mL min−1 and column temperature of 25 °C. Magnetic properties of the Fe3O4/poly(SEMA-co-MMA) magnetic nanocomposite were evaluated by vibrating-sample magnetometer (VSM, AGFM, Iran) at 25 °C temperature. The ultraviolet-visible (UV-Vis) spectroscopic measurements were performed on a Shimadzu 1650 PC UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan). The field emission scanning electron microscope (FESEM) type 1430 VP (LEO Electron Microscopy Ltd., Cambridge, UK) was applied to determine the morphologies of the synthesized samples. The structural properties were investigated by means of a X-ray powder diffraction (XRD) with a X’pert-PRO advanced diffractometer and wavelength of 1.5406 Å, and operated at 40 kV and 40 mA at room temperature in the 2θ range of 20–70°. The average diameter was measured by a laser-scattering technique (Nanotrac Wave™ (Microtrac, San Diego, CA, USA) at 25 °C. The 0.5% (w/v) solutions were prepared in deionized water, stirred for 2 h and stored for 24 h. The samples were then filtered using 0.20 μm nylon membranes (Micron Separations, Westboro, MA, USA).
Results and discussion
Magnetic nanoparticles have highly biocompatible properties; because their uptake, transport, and storage features in the liver are largely regulated by proteins such as ferritin and transferrin [30]. The aim of this study was synthesis of pH-responsive poly(SEMA-co-MMA)/Fe3O4 nanocomposite and study of its application as a nanocarrier for the anticancer drug delivery.
Characterization of poly(SEMA-co-MMA) copolymer
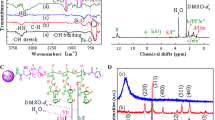
The FT-IR spectra of poly(HEMA-co-MMA) and poly(SEMA-co-MMA) are illustrated in Fig. 3. The FT-IR peaks of poly(HEMA-co-MMA) as followed: aliphatic C–H stretching vibrations at 2925 cm−1, strong absorption band at stretching vibration of the carbonyl group at 1720 cm−1, C–H bending vibration at 1458 cm−1, stretching vibration of C–O group at 1375 cm−1 and C–O–C stretching vibration at 1255 cm−1. Furthermore, the stretching vibration of hydroxyl group as a strong broad band centered at 3456 cm−1 (Fig. 3). After esterification of diblock copolymers with succinic anhydride, no conspicuous alteration was detected in FT-IR spectra.
The FT-IR spectrum of Fe3O4 nanoparticles demonstrated the absorption bands of 567 cm−1 for oxygen-metal stretching vibration. The bands around 1592 and 3367 cm−1 were also ascribed to the bending and stretching vibrations of the hydroxyl groups on the surface of Fe3O4 nanoparticles, respectively. In FT-IR spectrum of the Fe3O4/poly(SEMA-co-MMA) nanocomposite, after adsorbing of polymer onto Fe3O4 nanoparticles, the absorption bands of C–H at 2831 cm−1, carbonyl groups at 1766 cm−1, C–H bending vibration at 1500 cm−1, C–O–C stretching vibration at 1180 cm−1, the stretching vibration of hydroxyl group as a strong broad band centered at 3393 cm−1 and oxygen-metal stretching vibration band at 567 cm−1 were detected (Fig. 4).
The synthesis of poly(HEMA-co-MMA) and poly(SEMA-co-MMA) samples were confirmed by means of 1HNMR spectroscopy, as shown in Fig. 5a and b. The chemical shifts around 1.02–1.27 ppm (a) were attributed to the protons of methyl groups and the chemical shifts at 1.42–1.44 ppm (b) were associated with the methylene groups in the backbone of poly(HEMA-co-MMA) chain. The chemical shifts at 1.81–1.93 ppm (c) were also related to the methylene protons of RAFT backbone. The chemical shifts at 3.60 ppm were attributed to the methyl of PMMA and methylene protons of poly(HEMA) backbones (d and f), respectively. The protons of –CH2OH were observed at 3.84 ppm (e) and the chemical shift at 4.12 ppm was assigned to the hydroxyl groups of poly(HEMA). The chemical shift at 8.01 ppm (e) was also related to the aromatic protons of the RAFT agent (Fig. 5a). In addition, 13CNMR spectrum of poly(HEMA-co-MMA) copolymers (Fig. 5b) showed the chemical shifts at 16.1–18.2 ppm (CH3 − C), 30.7 ppm (C − CH3), 58.48 ppm (OCH3) and 66.30 ppm (O − CH2 − CH2 − O), 122.47 ppm (C=C aromatic) and 162.3 ppm (CO). The successful esterification of poly(HEMA) segments into poly(SEMA) blocks was verified by the appearance of new chemical shifts at 2.22 and 2.43 ppm, relating to –CH2 groups of the succinyloxy moiety. The peak of the hydroxyl group of poly(HEMA) at 4.12 ppm was disappeared, demonstrating that all of the hydroxyl groups were converted to the succinyloxy groups through the esterification (Fig. 5c). The degree of esterification (= 94.34%) was achieved by 1HNMR using succinic anhydride as a judgment. The GPC chromatograms of poly(HEMA-co-MMA) sample is represented in Fig. 6. The polydispersity index of poly(HEMA-co-MMA) (PDI = 1.23) synthesized via RAFT polymerization was relatively low. The low dispersity suggested an excellent control over the RAFT polymerization (Fig. 6).
The X-ray diffraction study of Fe3O4/poly(SEMA-co-MMA) nanocomposite
The X-ray diffraction patterns of Fe3O4 nanoparticles and Fe3O4/poly(SEMA-co-MMA) nanocomposite are shown in Fig. 7. The XRD pattern for the Fe3O4 nanoparticles indicated distinct peaks at 2θ = 29.6, 35.5, 43.2, 53.1, 57.1, 62.2, and 74.2°, corresponding to the (220), (312), (400), (422), (512), (440), and (533) of Fe3O4 crystalline structure, respectively. The Fe3O4/poly(SEMA-co-MMA) nanocomposite also indicated similar peaks corresponding to the Fe3O4 nanoparticles.
Morphology and elemental composition of Fe3O4/poly(SEMA-co-MMA) magnetic nanocomposite
The FESEM images of poly(SEMA-co-MMA) copolymers, Fe3O4 nanoparticles and Fe3O4/poly(SEMA-co-MMA) nanocomposite are exhibited in Fig. 8a–c, respectively. FESEM micrograph of poly(SEMA-co-MMA) (Fig. 8a) showed the formation of a non-uniform surface. The Fe3O4 nanoparticles had spherical shapes with an average diameter of 30 ± 10 nm. The Fe3O4 nanoparticles had homogeneous spherical morphologies. The FESEM images demonstrated the uniformity in shape and size of prepared nanoparticles. The FESEM image of the nanocomposite (Fig. 8c) depicted that the Fe3O4 nanoparticles were homogeneously coordinated into the copolymer matrix through the interactions between the carboxyl and hydroxyl groups. The nanocomposite had spherical morphology with an average diameter of 40 ± 10 nm. On the basis of FESEM micrographs of Fe3O4/poly(SEMA-co-MMA) nanocomposite, there was a homogeneous dispersion of Fe3O4 nanoparticles in poly(SEMA-co-MMA) and no aggregations were detected after Fe3O4 nanoparticles and polymer coating.
Magnetic properties of Fe3O4/poly(SEMA-co-MMA) nanocomposite
The VSM was a principle analysis for evaluation of nanomagnetic property. The magnetic properties of Fe3O4/poly(SEMA-co-MMA) nanocomposite were described using VSM at room temperature. As reported in Fig. 9, the saturation magnetizations (δs) of the Fe3O4 and Fe3O4/poly(SEMA-co-MMA) nanocomposite were around 22.23 and 13.25 emug−1, indicating that the synthesized nanocomposite was capable of being employed in the drug delivery applications [31, 32].
Dispersibility investigation
Based on the image of Fig. 10 for Fe3O4/poly(SEMA-co-MMA) nanocomposite in deionized water, the nanocomposite was a homogeneous solution. Hence, the Fe3O4/poly(SEMA-co-MMA) nanocomposite was stabilized in deionized water as a solvent.
pH-responsivity of Fe3O4/poly(SEMA-co-MMA) nanocomposite
The pH is an important signal, which can be addressed through pH-responsive materials. The weak acids and bases such as carboxylic acids are capable of either accepting or releasing protons in response to the environmental pH changes [33]. The DLS results at pHs of 4 and 9 are illustrated in Fig. 11. The average size increased at pH = 4 and decreased at pH = 9. This is consistent with a deprotonation of poly(SEMA) at basic pH, leading to the low diameters. However, at a lower pH having a stronger repulsive interaction resulted in the larger sizes.
In vitro DOX release behavior
The use of nanoparticles as therapeutic carriers is important for their unique features like large specific quality for drug loading as well as strong magnetism. A system of magnetically targeted drug-delivery includes binding a drug to small magnetic particles, injecting these into the bloodstream, and using a high gradient magnetic field to pull them out of suspension in the target region [34, 35]. In this study, a pH-sensitive drug-delivery system consisting of DOX-loaded Fe3O4/poly(SEMA-co-MMA) nanocomposite have been successfully prepared as a drug carrier. The conjugated DOX with the magnetic nanocomposite was released in the acidic pHs. The in vitro DOX release behaviors of DOX-loaded Fe3O4/poly(SEMA-co-MMA) nanocomposite in different buffers, i.e., pH = 4, 5.4, and 7.4 were examined at 37 °C. As represented in Fig. 12, a quick release behavior was detected at pHs of 4 and 5.4 after 15 days. Partly at pH = 5.4 and completely at pH = 4, the protonation of carboxyl groups of nanocomposite, due to ionic interaction between DOX and nanocomposite, occurred and the release rates reached to the maximal amounts of 55.7 and 62.4 wt%, respectively (Fig. 12). The release rate in a buffer solution at pH = 7.4 reached to a minimum amount of 38.3 wt% after 15 days; because at pH of 7.4, the strong ionic interaction between DOX and nanocomposite, thanks to the deprotonation of carboxylic acids of SEMA section and protonation of DOX prohibited the drug release. At buffer with pH of 7.4, the slowest release of DOX was observed. As depicted in the inset panel of Fig. 12, within somehow 5 h, the drug release was less than 10%.
Conclusion
A pH-responsive magnetic nanocomposite was successfully prepared by RAFT polymerization, and its structure and delivery application were studied. FESEM images for the Fe3O4/poly(SEMA-co-MMA) nanocomposite demonstrated the spherical shapes and average diameter of 30 ± 10 nm. The VSM analysis demonstrated a saturation magnetization of 13.25 emug−1 for the Fe3O4/poly(SEMA-co-MMA) nanocomposite. The dispersibility tests also exhibited that the synthesized Fe3O4/poly(SEMA-co-MMA) nanocomposite was stable more than five months at ambient condition in deionized water solvent. The pH sensitivity of nanocomposite was also represented by DLS. The DOX release behaviors of DOX-loaded Fe3O4/P(SEMA-co-MMA) nanocomposite were accelerated at pHs of 4 and 5.4 compared to 7.4 at 37 °C. The results exhibited that the DOX-loaded Fe3O4/poly(SEMA-co-MMA) nanocomposite could be considered as an superlative candidate for drug delivery.
References
Bhowmick A, Saha A, Pramanik N, Banerjee S, Das M, Kundu PP (2015) Novel magnetic antimicrobial nanocomposites forbone tissue engineering applications. RSC Adv 5:25437–25445
Massoumi B, Mousavi-Hamamlu SV, Ghamkhari A, Jaymand M (2017) A novel strategy for synthesis of polystyrene/Fe3O4 nanocomposite: RAFT polymerization, functionalization, and coordination techniques. Polym-Plast Technol Eng 56:873–882
Zhu L, Wang D, Wei X, Zhu X, Li J, Tu C, Su Y, Wu J, Zhu B, Yan D (2013) Multifunctional pH-sensitive superparamagnetic iron-oxide nanocomposites for targeted drug delivery and MR imaging. J Control Release 169:228–238
Wang W, Zhang Z (2007) Hydrothermal synthesis and characterization of carbohydrate microspheres coated with magnetic nanoparticles. J Dispers Sci Technol 28:557–561
Cheng R, Meng F, Deng C, Klok H-A, Zhong Z (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmedsite-specific drug delivery. Biomaterials 34:3647–3657
Hergt R, Dutz S, Muller R, Zeisberger M (2006) Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancertherapy. J Phys Condens Matter 18:S2919–S2934
Karumanchi RS, Doddamane SN, Sampangi C, Todd PW (2002) Field-assisted extraction of cells, particles and macromolecules. Trends Biotechnol 20:72–78
Jiang Y, Guo C, Xia H, Mahmood I, Liu C, Liu H (2009) Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. J Mol Catal B Enzym 58:103–109
Caoa J, Wanga Y, Yu J, Xiaa J, Zhanga C, Yina D, Hafeli UO (2004) Preparation and radiolabeling of surface-modifiedmagnetic nanoparticles with rhenium-188 formagnetic targeted radiotherapy. J Magn Magn Mater 277:165–174
Zhang X, Yang P, Dai Y, Ma P, Li X, Cheng Z, Hou Z, Kang X, Li C, Lin J (2013) Multifunctional up-converting nanocomposites with smart polymer brushes gated mesopores for cell imaging and thermo/pH dual-responsive drug controlled release. Adv Funct Mater 23:4067–4078
Oha JK, Park JM (2011) Iron oxide-based superparamagnetic polymeric nanomaterials: design, preparation, and biomedical application. Prog Polym Sci 36:168–189
Sivudu KS, Rhee KY (2009) Preparation and characterization of pH-responsive hydrogel magnetite nanocomposite. Colloids Surf A Physicochem Eng Asp 349:29–34
Lee JE, Lee DJ, Lee N, Kim B, Choi SH, Hyeon T (2011) Multifunctional mesoporous silica nanocomposite nanoparticles for pH controlled drug release and dual modal imaging. J Mater Chem 21:16869–16872
Poorgholy N, Massoumi B, Jaymand M (2017) A novel starch-based stimuli-responsive nanosystem for theranostic applications. Int J Biol Macromol 97:654–661
Pietsch C, Hoogenboom R, Schubert US (2010) PMMA based soluble polymeric temperature sensors based on UCST transition and solvatochromic dyes. Polym Chem 1:1005–1008
Chi W, Liu S, Yang J, Wang R, Ren H, Zhou H, Chenb J, Guo T (2014) Evaluation of the effects of amphiphilic oligomers in PEI based ternary complexes on the improvement of pDNA delivery. J Mater Chem B 2:5387–5396
Fran J, Lutz C (2006) Solution self-assembly of tailor-made macromolecular building blocks prepared by controlled radical polymerization techniques. Polym Int 55:979–993
Delaittre G, Save M, Gaborieau M, Castignolles P, Rieger J, Charleux B (2012) Synthesis by nitroxide-mediated aqueous dispersion polymerization, characterization, and physical core-crosslinking of pH- and thermoresponsive dynamic diblock copolymer micelles. Polym Chem 3:1526–1538
Beers KL, Boo S, Gaynor SG, Matyjaszewski K (1999) Atom transfer radical polymerization of 2-hydroxyethyl methacrylate. Macromolecules 32:5772–5776
Ahmed M, Narain R (2013) Progress of RAFT based polymers in gene delivery. Prog Polym Sci 38:767–790
Davaran S, Ghamkhari A, Alizadeh E, Massoumi B, Jaymand M (2017) Novel dual stimuli-responsive ABC triblock copolymer: RAFT synthesis, Schizophrenic” micellization and its performance as an anticancer drug delivery nanosystem J Colloid Interface Sci 488:282–293
Yang C, Guo W, Cui L, An N, Zhang T, Lin H, Qu F (2014) pH-Responsive magnetic core-shell nanocomposites for drug delivery. Langmuir 30:9819–9827
Bastakoti BP, Guragain S, Nakashima K, Yamauchi Y (2015) Stimuli-induced core–corona inversion of micelle of poly(acrylic acid)-block-poly(N-isopropylacrylamide) and its application in drug delivery. Macromol Chem Phys 216:287–291
Huang G, Zhang K-L, Chen S, Li S-H, Wang L-L, Wang L-P, Liu R, Gao J, Yang H-H (2017) Manganese-iron layered double hydroxide: a theranosticnanoplatform with pH-responsive MRI contrast enhancement and drug release. J Mater Chem B 5:3629–3633
Ghamkhari A, Massoumi B, Jaymand M (2017) Novel ‘schizophrenic’ diblock copolymer synthesized via RAFT polymerization: Poly(2-succinyloxyethyl methacrylate)-b-poly[(N-4-vinylbenzyl),N,N-diethylamine]. Des Monomers Polym 20:190–200
Ma WF, Wu KY, Tang J, Li D, Wei C, Guo J, Wang SL, Wang CC (2012) Magnetic drug carrier with a smart pH-responsive polymer network shell for controlled delivery of doxorubicin. J Mater Chem 22:15206–15214
Li PY, Lai PS, Hung WC, Syu WJ (2010) Poly(L-lactide)-vitamin E TPGS nanoparticles enhanced the cytotoxicity of doxorubicin in drug-resistant MCF-7 breast cancer cells. Biomacromolecules 11:2576–2582
Massoumi B, Ghamkhari A, Agbolaghi S (2017) Dual stimuli-responsive poly(succinyloxyethylmethacrylate-b-N-Isopropylacrylamide) block copolymers as nano-carriers and respective application in Doxorubicin delivery. Int J Polym Mater. https://doi.org/10.1080/00914037.2017.1300901
Salehi R, Rasouli S, Hamishehkar H (2015) Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int J Pharm 487:274–284
Bol’bukha YN, Tertykha VA, Yurkovb GY, Ovchenkov EA (2011) Synthesis and properties of nanocomposites based on magnetite and biocompatible polymers. Russ J Appl Chem 84:847–853
Rahimi M, Shojaei S, Safa KD, Ghasemi Z, Salehi R, Yousefi B, Shafiei-Irannejad V (2017) Biocompatible magnetic tris(2-aminoethyl)amine functionalized nanocrystalline cellulose as a novel nanocarrier for anticancer drug delivery of methotrexate. New J Chem 41:2160–2168
Massoumi B, Poorgholy N, Jaymand M (2017) Multi-stimuli responsive polymeric nanosystems for theranostic applications. Int J Polym Mater 66:38–47
Shivakumar HG, Fathima SJ, Radha V, Khanum F (2016) pH and thermosensitive 5-fluorouracil loaded poly (NIPAM-co-AAc) nanogels for cancer therapy. RSC Adv 6:105495–105507
Bawa P, Pillay V, Choonara YE, du Toit LC (2009) Stimuli-responsive polymers and theirapplications in drug delivery. Biomed Mater 4:022001
Cheng R, Wang X, Chen W, Meng F, Deng C, Liuband H, Zhong Z (2012) Biodegradable poly(3-caprolactone)-g-poly(2-hydroxyethyl methacrylate)graft copolymer micelles as superior nano-carriers for “smart” doxorubicinrelease. J Mater Chem 22:11730–11738
Acknowledgements
We express our gratitude to the Payame Noor University for supporting this project.
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ghamkhari, A., Agbolaghi, S., Poorgholy, N. et al. pH-responsive magnetic nanocomposites based on poly(2-succinyloxyethyl methacrylate-co-methylmethacrylate) for anticancer doxorubicin delivery applications. J Polym Res 25, 37 (2018). https://doi.org/10.1007/s10965-017-1431-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1431-0