Abstract
Poly(BA-MMA-PMPA-BPMA), a core-shell polyacrylate elastomer containing hindered amine light stabilizer (HALS) and benzophenone UV-absorber (UVA) in the shell phase, was prepared by two-stage seeded emulsion polymerization from n-butyl acrylate (BA), methyl methacrylate (MMA), 1,2,2,6,6-pentamethylpiperidin-4-ylacrylate (PMPA) and 2-hydroxy-4-(3-methacryloxy-2-hydroxyl-proroxy) benzophenone (BPMA). Poly(BA-MMA-PMPA-BPMA) was characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (1H-NMR), transmission electron microscopy(TEM), differential scanning calorimetry(DSC), thermogravimetric analysis (TG) and UV-visible (UV-vis) absorption spectroscopy. Furthermore, the obtained Poly(BA-MMA-PMPA-BPMA) was melt-blended with polyoxymethylene (POM). The effect of Poly(BA-MMA-PMPA-BPMA) on POM was compared with that of Poly (BA-MMA-PMPA) and Poly(BA-MMA-BPMA). Scanning electron microscope (SEM), metallographic microscopy, DSC and mechanical properties were employed to characterize POM blends before and after UV irradiation. The results showed that the size of surface cracks and changing degree of melting point of POM/poly(BA-MMA-PMPA-BPMA) were lower than that of POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-BPMA). The percentage retention of elongation at break and notched impact strength of POM/poly(BA-MMA-PMPA-BPMA) was 63 % and 80 % respectively after 800 h UV irradiation, which was higher than that of POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-BPMA). Therefore, it could conclude that the photostabilizing of Poly(BA-MMA-PMPA-PBMB) was greatly enhanced, attributing to the synergistic effect between HALS and UVA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyoxymethylene (POM), a kind of engineering plastics, is crystalline material and commonly used in industry. It is owing to excellent combination properties, such as high mechanical strength, low frictional coefficient, good processibility, dimensional stability and corrosion resistance [1–5]. However, its applications are restricted because of low-notched impact strength and poor UV aging characteristics. Generally, POM can be toughened with elastomers. In addition, adding UV-stabilizers into POM has been extensively employed, which is the primary way to improve POM photostability. HALS prevents the polymer from UV-damage. The mechanism is as follows: Under UV irradiation the POM products free radicals, leading to initiate chain degradation reaction to damage POM. At the same time, HALS scavenges free radicals to defend POM [6–11]. Different from HALS, UVA protects polymer from UV-aging by absorbing UV-light and transforming UV-light into heat [12]. Therefore, the photostabilizing of HALS carries out after the chain degradation reaction is taken, the photostabilizing of UVA carries out before the chain degradation reaction triggered by UV-light. So the two UV-stabilizers show a synergistic effect.
Very few studies are reported for the preparation of copolymers enriched with HALS and UVA. In our previous work, an elastomer containing HALS or UVA individually could protect POM from UV-damage [13, 14]. In this study, core-shell structure polyacrylate elastomer containing HALS and UVA was prepared by seeded emulsion polymerization and the synthetic was used as a modifier to improve the UV aging resistance to POM. The changes of structures and properties of the blends before and after UV irradiation were investigated. While, the effect of Poly(BA-MMA-PMPA) and Poly(BA-MMA-BPMA) on POM was compared with that of Poly(BA-MMA-PMPA-BPMA) on POM.
Experimental
Materials
2,4-Dihydroxybenzophenone (UV-0, purity above 99.5 %) and 1,2,2,6,6- pentamethyl-4-piperidinol (PMP, purity above 98.0 %) provided by Jinchun Meibang Chemical (Wuhan, China) was an industrial product with recrystallization renement. 2,3,5-trimethylhydroquinone (THQ, purity above 99.0 %) purchased from Tianjin Zhongxin Chemtech Co., Ltd (Tianjin, China) was used without further purification. Analytical reagent grade sodium hydroxide (NaOH), sodium dodecyl sulfate (SDS), potassium persulfate (KPS), tetrahydrofuran (THF), calcium chloride (CaCl2) and sodium bicarbonate (NaHCO3) were provided by Kelong Chemical Reagent Factory of China (Chengdu, China). Methyl methacrylate (MMA, analytical reagent) and n-Butyl acrylate (BA, analytical reagent), which were provided by Tianjin Bodi Chemical Co., Ltd of China (Tianjin, China), were distilled before application. Commercial POM copolymer, with weight-average molecular weight (Mw) about 1.16 × 105 g/mol and melting temperature (Tm) 165–167 °C, was procured from YunTianHua Corporation (China). BPMA was synthesized according to a patent [15]. Poly(BA-MMA-BPMA) were synthesized according to the literature [16]. PMPA and Poly(BA-MMA-PMPA) were prepared according to a previous study [14]. The components for the core and shell of Poly(BA-MMA-BPMA) and Poly(BA-MMA-PMPA) were shown in Table 1.
Synthesis of Poly(BA-MMA-PMPA-BPMA)
Poly(BA-MMA-PMPA-BPMA) was prepared through seeded emulsion polymerization. Table 2 was showed the components which was used to prepare Poly(BA-MMA-PMPA-BPMA).
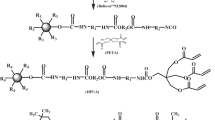
The molar ratio of BA, MMA, PMPA, and BPMA in the feed for the preparation of the core-shell particle Poly(BA-MMA-PMPA-BPMA), is 2.23 (1.929) : 1 (0.865) : 0.0954 (0.0825) : 0.0604 (0.0523), with a corresponding weight ratio of 2.857 (2.000) : 1 (0.700) : 0.214 (0.150) : 0.214 (0.150) as listed in Table 2. The procedure for the preparation of Poly(BA-MMA-PMPA-BPMA) was described as follows: The components for the core and the shell (except the initiator KPS) substances were vigorously stirred in a 250 ml four-necked flask for 0.5 h at room temperature to prepare core pre-emulsion and shell pre-emulsion respectively. Then, one-fourth of the core pre-emulsion and one-third of KPS were semi-continuous added in a 500 ml four-necked flask under moderate stirring in nitrogen atmosphere. The reaction temperature was raised to 80 °C. When the color of the emulsion was turned to blue, the remaining three-fourth of core pre-emulsion and one-third of KPS were dropped into the flask within 2 h. After completion of feeding, the system was kept at 80 °C for another 1 h to prepare core emulsion. Afterwards, shell pre-emulsion and the remaining one-third of KPS were added into the resulting core emulsion within 2 h. The reaction was carried out for additional 2 h at 85 °C to get the core-shell emulsion. The emulsion was coagulated with 5 % CaCl2 aqueous solution. Then the solid were filtered and washed with deionized water to demulsify. At last, the particles were dried in vacuum at 80 °C to constant weight. The synthesis routes of Poly(BA-MMA-PMPA-BPMA) were shown in Scheme 1.
Preparation of testing samples
POM/poly(BA-MMA-PMPA-BPMA) [10 wt % Poly(BA-MMA-PMPA-BPMA)], POM/poly(BA-MMA-PMPA) [10 wt % Poly(BA-MMA-PMPA)] and POM/poly(BA-MMA-BPMA) [10 wt % Poly(BA-MMA-BPMA)] were prepared via melt-blending process by a HT-30 twin-screw extruder (Nanjing Rubber and Plastics Machinery Plant, China) at 180 °C. Samples for mechanical properties determination and UV irradiation test were prepared through injection molding by a LS-26 injection molding machine (Nissei Plastic Industrial, Japan) at 190 °C.
UV irradiation
The specimens were placed in an exposure unit equipped with two 500-W Ga-In source lamps with a maximum intensity at 365 nm. The intensity of irradiation was 5.0 W/m2 measured by a UV irradiance meter model UV-A Tester (Photoelectric Instrument Factory of Beijing Normal University, Beijing, China). The irradiation tests were performed at 60 ± 2 °C in air circulation. Specimens for testing were taken at a series of intervals (0, 200, 400, 600, and 800 h).
Measurements and characterization
The FTIR spectroscopy was recorded with a Nicolet iS10 FTIR spectrometer (Nicolet Instruments Company, USA). The samples were dissolved in chloroform to prepare FTIR samples. The wavenumber was ranging from 4,000 to 400 cm−1.
The 1H-NMR spectrum was recorded on a Varian INOVA-400 spectrometer (Varian Company, USA), the emission frequency was 400 MHz and the scanning range was 0–15 of 400 MHZ. Deuterated chloroform and tetramethylsilane were used as solvent and internal standard respectively.
TEM micrographs of Poly(BA-MMA-PMPA-BPMA) and POM/ poly(BA-MMA-PMPA-BPMA) was taken with HITACHI H-600 transmission electron microscope (Hitachi Company, Japan) at an accelerating voltage of 100 kV. Poly(BA-MMA-PMPA-BPMA) was diluted with deionized water and stained with 3 % phosphotungstic acid solution before measurement. POM/ poly(BA-MMA-PMPA-BPMA) samples were frozen microtome section before measurement.
For DSC analysis, a TA Q200 (TA Instruments Company) with nitrogen as the purge gas was utilized. Samples of Poly(BA-MMA-PMPA-BPMA) were heated from −90 °C to 200 °C at a rate of 20 °C/min. Powder samples scraped from the most up surface of POM/poly(BA-MMA-BPMA), POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-PMPA-BPMA) were heated from 50 to 200 °C at a heating rate of 10 °C/min.
TG was performed with a TG 209 F1 Iris (NETZSCH Company, Germany) under the nitrogen atmosphere at a heating rate of 10 °C/min from 50 to 800 °C.
The UV-vis absorption spectra of Poly(BA-MMA-PMPA-BPMA), Poly(BA-MMA-PMPA), Poly(BA-MMA-BPMA) and UV-0 were recorded on a T6 UV-vis spectrophotometer (PERSEE, China) in the range from 200 to 600 nm using THF as solvent.
The dispersion of Poly(BA-MMA-PMPA-BPMA) in POM was examined by a KYKY-2800 scanning electron microscope (SEM; KYKY Technology Development, China). The testing bar was broken at liquid nitrogen temperature (−196 °C) and etched by THF for 24 h at room temperature to eliminate Poly(BA-MMA-PMPA-BPMA). POM and POM/ poly(BA-MMA-PMPA-BPMA) samples were same broken at liquid nitrogen without THF etched.
Metallurgical microscopy observation of the surface of POM blends before and after UV aging was carried out using a UM200i metallurgical microscope (Chongqing UOP Photoelectric Technology Corp. Ltd, China).
Examination of mechanical properties of the blends
The tensile properties were determined by a RGM3010 tensile testing machine (Shenzhen Reger Instrument, China) with 10 kN load cell according to ISO 527–2:1993. The notched Izod impact strength was measured on a UJ-40 Tester (Chengdu Testing Machine Factory, China) according to ISO 180:2000.
Results and discussion
FTIR analysis
FTIR spectra of Poly(BA-MMA-PMPA-BPMA) was shown in Figs. 1 and 2. The main absorptions were as follows: The C-H stretching vibrations of saturated aliphatic hydrocarbons at 2,960 and 2,874 cm−1, a very strong absorption peak at 1,734 cm−1 associated with C=O ester carbonyl stretching vibration, two strong absorption bands at 1,246 and 1,165 cm−1 assigned to C-O-C stretching vibrations from ester groups and the -CH2- rocking vibrations centered at 753 cm−1. At the same time, it was notable that there was a doublet at 1,386 and 1,370 cm−1 attributed to the -C(CH3)2 symmetric bending vibrations from PMPA, which was the characteristic absorption peak for PMPA. So the spectra for Poly(BA-MMA-PMPA-BPMA) appeared a doublet at 1,378 and 1,360 cm−1. Meanwhile, the main absorption peaks of BPMA were as follows: a series of new absorption peaks at 3,444, 1,605, 1,343 and 706 cm−1 corresponding to the -OH stretching vibration, the C=O stretching vibration in benzophenone, the C-O stretching vibration in benzophenone and the single-substituted benzene ring respectively. So the spectra of Poly (BA-MMA-PMPA-BPMA) contained the main absorptions of BPMA. In addition, a strong absorption peak at 753 cm−1 assigned to the single-substituted benzene ring of BPMA overlaps with the -CH2- rocking vibrations absorbance. According to the analysis mentioned above, it could be concluded that PMPA and BPMA were successfully incorporated into the poly (BA-MMA-PMPA-BPMA) latex particles.
1H-NMR analysis
The chemical structure of Poly (BA-MMA-PMPA-BPMA) was analyzed by 1H-NMR the spectrum demonstrated in Fig. 3. In Fig. 3, the chemical shifts of -O-CH3 protons in MMA and -O-CH2 protons in BA were observed at 3.60 and 4.03 ppm [17–19] respectively. In view of the number of proton and proton coupling, the intensity ratio of -O-CH3 protons in MMA and -O-CH2 protons in BA was 1:1.56 (13.3:20.8), so the molar ratio of MMA and BA introduced in the poly(MA-MMA-PMPA-PBMA) was calculated to be 1:2.34 (1/3 : 1.56/2), which was close to the molar ratio of comonomers of MMA and BA at 1:2.23 (0.28:0.625) in the initial feed as shown in Table 2. A weak peak around 5.04 ppm assigned to the chemical shift of -O-CH proton in PMPA was observed, indicating that PMPA had been successfully incorporated into the polyacrylate latex particles. A peak at d = 12.66 ppm was attributed to the proton of R-OH of BPMA, which was conformable with the data of previous study [20]. The 1H-NMR resonance peaks at 7.5, 7.0 and 6.5 ppm were due to the aromatic protons of benzophenone. Moreover, the intensity of –O-CH in PMPA was 0.19 and the intensity of R-OH in BPMA was 0.12, so the protons intensity ratio (1.58) was similar to the molar ratio of PMPA and BPMA (0.0267 mol:0.0169 mol). Therefore, 1H-NMR analysis provided evidence that PMPA, BPMA, BA and MMA monomers effectively participated in the seeded emulsion polymerization.
Morphology of Poly (BA-MMA-PMPA-BPMA) latex particles
The microstructure of Poly (BA-MMA-PMPA-BPMA) particles was presented in Fig. 4(a). A clear core-shell structure of the particles was observed, which was due to the difference of electron penetrability to the core and shell phases. The white and gray regions in the particles represent PBA core and Poly (MMA-PMPA-BPMA) shell respectively, which confirmed the microstructure of the core-shell particles. Furthermore, the average diameter of the particles was about 100 nm. Hence, it proved that the core-shell particles were obtained as desired through two-stage seeded emulsion polymerization. Moreover, the microstructure of POM/poly(BA-MMA-PMPA-BPMA) was showed in Fig. 4(b), it can be seen that Poly(BA-MMA-PMPA-BPMA) latex particles was relatively homogeneous dispersed in POM matrix.
Thermal stability
The thermal stability of Poly (BA-MMA-PMPA-BMPA) was investigated by TG. Figure 5 provided the TGA curve of Poly (BA-MMA-PMPA-BMPA). The Tonset was 323 °C (based on 5 % mass loss) as shown in Fig. 5. So Poly (BA-MMA-PMPA-BMPA) was of excellent thermal stability, which was due to thermal decomposition of Poly (BA-MMA-PMPA-BMPA) started from the shell and the shell was constituted by copolymerizing between PMPA and MMA. PMPA could quench the radicals which were generated by PMMA chains thermal decomposition to make the synthetics steady [21]. According to the analysis above, Poly(BA-MMA-PMPA-BMPA) was stable when it was mixed with POM at 180 °C.
UV-vis absorption spectroscopy analysis
The UV-absorbing performance of Poly(BA-MMA-PMPA-BPMA), Poly(BA-MMA-PMPA), Poly(BA-MMA-BMPA) and UV-0 were studied. The UV-vis absorption spectra of Poly(BA-MMA-PMPA-BMPA), Poly(BA-MMA-PMPA), Poly(BA-MMA-BMPA) and UV-0 were provided in Fig. 6.
Absorption bands of Poly(BA-MMA-BPMA) were similar to the absorption peaks of UV-0. The only difference was that those of Poly(BA-MMA-BPMA) were just slightly broader. Absorption bands of Poly(BA-MMA-PMPA-BPMA) at 280–380 nm were carried out. However, the absorption intensity was about a half of that of Poly(BA-MMA-BPMA). This was because the former contained a half of UVA than that of the latter. It also could be seen that there was an extremely weak absorption peak near 300 nm for Poly(BA-MMA-PMPA), indicating weak UV absorption ability and it was impossible for Poly(BA-MMA-PMPA) to achieve light stability by absorbing ultraviolet.
Dispersion of particles in POM
The dispersion of Poly(BA-MMA-PMPA-BPMA) in POM was studied by SEM. The SEM micrographs were magnified 3,000 times in Fig. 7. The fracture surface of POM and POM/poly(BA-MMA-PMPA-BPMA) blend was illustrated in Fig. 7(a) and (b). The SEM micrograph demonstrated two phase morphology clearly in Fig. 7(c), because of POM/poly(BA-MMA-PMPA-BPMA) blend etched by THF, and the holes on the surface represented the core-shell polyacrylate elastomers. Some of the larger diameter hole, which is composed of some gel and elastomer shell structure imperfections caused by particle agglomeration. As shown, Poly(BA-MMA-PMPA-BPMA) was homogeneous dispersed in POM matrix.
Surface morphology analysis
After 200, 400, 600, and 800 h UV irradiation, the surface of POM/poly(BA-MMA-PMPA-BPMA), POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-BPMA) were observed by using metallurgical microscopy. The micrographs were shown in Figs. 8, 9, 10 and 11 respectively. As shown, the surface crack width of POM/poly(BA-MMA-BPMA), POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-PMPA-BPMA) reached 8 um, 6 um and 5 um respectively after 200 h UV irradiation. After 400 h UV irradiation, the surface crack width for POM/poly(BA-MMA-BMPA), POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-PMPA-BPMA) were still 8 um, 6 um and 5 um, but all the crack edges became smooth and glossy decreased. After 600 h UV irradiation, the surface crack length for all blends was significantly increased. After 800 h UV irradiation, the crack morphology for all blends become blurred,and the surface crack width of POM/poly(BA-MMA-PMPA-BPMA) was still the smallest among that of all the blends. It could be concluded that Poly(BA-MMA-PMPA-BPMA) was the most suitable material among Poly (BA-MMA-PMPA-BPMA), Poly(BA-MMA-PMPA) and Poly(BA-MMA-BPMA) to protect POM from UV irradiation.
Change of melting point
Figures 12, 13 and 14 illustrated the DSC curves of the POM/poly(BA-MMA-BPMA), POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-PMPA-BPMA) before and after UV irradiation. As Table 3 showed, all the melting points of blends were decreased with the irradiation time increasing, which was consistent with the previous study [22]. The decrease of melting point was attributed to the reduction of the molecular weight for the blends and the formation of imperfect crystalline in the amorphous regions by UV irradiation [22]. As Table 4 showed, The shift of melting point for POM/poly(BA-MMA-PMPA-BPMA) was the smallest among POM/poly(BA-MMA-PMPA), POM/poly(BA-MMA-BPMA) and POM/poly(BA-MMA-PMPA-BPMA) with the irradiation time increasing, indicating that POM/poly(BA-MMA-PMPA-BPMA) could bear UV irradiation best.
Mechanical properties
The changes in mechanical properties of POM/poly(BA-MMA-PMPA-BPMA), POM/poly(BA-MMA-PMPA) and POM/poly(BA-MMA-BPMA) before and after UV irradiation were shown in Figs. 15 and 16.
According to Fig. 15, it could be observed that the elongation at break for all the samples declined at different degrees with the aging time increasing. Wherein, the elongation at break of POM/poly (BA-MMA-BPMA) decreased more than that of the POM/poly(BA-MMA-PMPA-BPMA) and POM/poly(BA-MMA-PMPA), indicating the degree of aging of the former was severer than that of the other two. After 800 h UV irradiation, the elongation at break of POM/poly(BA-MMA-PMPA), POM /poly(BA-MMA-BPMA), and POM/poly(BA-MMA-PMPA-BPMA) reduced 42 %, 50 %, and 37 % respectively. It was obvious that elongation at break of POM/ poly(BA-MMA-PMPA-BPMA) was the highest retention rates, up to 63 %.
As Fig. 16 shown, the notched impact strength of POM/poly(BA-MMA-BPMA) decreased faster than that of the other two with the aging time increasing. After 800 h UV-aging, the impact strength retention rates of POM/poly(BA-MMA-BPMA), POM/poly(BA-MMA-PMPA) and POM/poly (BA-MMA-PMPA-BPMA) were 65 %,74 %, and 80 %, of which poly(BA-MMA-BPMA) was of the lowest retention rate and the retention rate of Poly(BA-MMA-PMPA-BPMA) was the highest.
The analysis above showed that ultraviolet aging could make the elongation at break and notched impact strength decreased. The retention rate of breaking elongation and notched impact strength of POM/poly(BA-MMA-PMPA-BPMA) was the highest, and indicating that Poly (BA-MMA-PMPA-BPMA) improved the performance of UV aging resistance of POM best.
Conclusions
The Poly(BA-MMA-PMPA-BPMA), a core-shell polyacrylate elastomer bound with HALS and UVA, were successfully prepared by emulsion polymerization and was of a good compatibility with POM. For POM/poly(BA-MMA-PMPA-BPMA), the percentage retention of elongation at break was 63 % and notched impact strength was 80 % after 800 h UV-irradiation, both higher than that of POM/Poly(BA-MMA-PMPA) and POM/Poly(BA-MMA-BPMA). The size of surface cracks, the shift of melting point were lower than that of POM/Poly(BA-MMA-PMPA) and POM/Poly(BA-MMA-BPMA). Therefore, it could be concluded that the photo-stabilizing of Poly(BA-MMA-PMPA-PBMB) was greatly enhanced, owing to the synergistic effect between hindered amine light stabilizer and benzophenone UV-absorber.
References
Hu YL, Ye L, Zhao XW (2006) Polym 47:2649–2659
Shi J, Jing B, Zou X, Luo H, Dai W (2009) J Mater Sci 44:1251–1257
Pielichowski K, Leszczynska A (2004) J Therm Anal Calorim 78:631–637
Zhang Q, Chen YH (2011) J Polym Res 18:293–303
Jiang Z, Chen YH, Liu Z (2014) J Polym Res 21:451
Sun GJ, Jang HJ, Kaang S, Chae KH (2002) Polym 43:5855–5863
Mosnacek J, Chmela S, Theumer G, Habicher WD, Hrdlovic P (2003) Polym Degrad Stab 80:113–126
Malik J, Ligner G, Avirr L (1998) Polym Degrad Stab 60:205–213
Kikkawa K (1995) Polym Degrad Stab 49:135–143
Binet ML, Commereuc S, Lajoie P, Lacoste J (2000) J Photochem Photobiol A Chem 137:71–77
Liu NC, Pan JQ, Lau WWY (1998) Polym Degrad Stab 62:307–314
Catalan J, Fabero F, Soledad Guijarro M, Claramunt RM, Santa Maria MD, Foces-Foces MDLC, Cano FH, Elguero J, Sastre R (1990) J Am Chem Soc 112:747–759
Zhou DJ, You B, Wu GB, Ren XC (2012) Polym Int 61:971–981
You B, Zhou DJ, Yang F, Ren XC (2011) Colloid Surf A: Physicochem Eng Asp 392:365–370
Goldberg AI, Joseph F, Martin S. Ethylenically unsaturated derivatives of 2,4-dihydroxybenzophenone. United State Patent NO.3162676 A
Yang F, Wu GB, Zhang SL, Ren XC (2011) Adv Mater Res 239:2431–2434
Xiao XY, Wang Y (2009) Colloid Surf A: Physicochem Eng Asp 348:151–156
Montaudo MS, Montaudo G (1999) Macromolecules 32:7015–7022
Kanmuri S, Moholkar VS (2010) Polym 51:3249–3261
Zhao Y, Dan Y (2007) Eur Polym J 43:4541–4551
Kashiwagi T, Inaba A, Brown JE, Hatada K, Kitayama T, Masuda E (1986) Macromolecules 19:2160–2168
Ren XC, Chen L, Zhao HJ, Dan Y, Cai XF (2007) J Macromol Sci B 46:411–421
Acknowledgments
We would like to thank the generous financial support by the following grant: National Natural Sciences Foundation of China, Grant NO. 51273130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Zy., Zeng, MQ., Liu, L. et al. Synthesis and characterization of HALS/UV-absorbers bifunctionalized core-shell elastomer and Its application in polyoxymethylene. J Polym Res 21, 589 (2014). https://doi.org/10.1007/s10965-014-0589-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0589-y