Abstract
Polyethylene terephthalate (PET) films have been widely applied in the display industry. However, there is a problem in effectively protecting and extending the service life. To address this issue, a hyperbranched polyurethane acrylate (HPUA) has been synthesized with poly (hexylene glycol) neopentyl glycol ester (PNA), isophorone diisocyanate (IPDI), hyperbranched polyol Boltorn™H2004 and pentaerythritol triacrylate (PETA). Further, a series of high-performance UV curable coatings have been prepared with HPUA, hollow nano-SiO2 and silane coupling agents, along with other reagents. The structure of HPUA has been characterized by infrared spectroscopy (FT-IR), and the curing process has been monitored kinetically by real-time infrared spectroscopy to study its double-bond conversion ratio. The properties of the cured films have been investigated by thermogravimetric analysis (TGA). The water contact angle, aging and yellowing resistance, hardness, flexibility, abrasion resistance, light transmittance, and adhesion of the cured coatings on PET film have been tested. The results have shown that the addition of hollow nano-SiO2 and silane coupling agents has not only not affect the light transmittance of the coatings but also improved the surface properties and heat resistance. When the silane coupling agent has been γ-Methacryloxypropyltrimethoxysilane (KH-570), the water contact angle of the coating has been 101.8°, excellent resistance to aging and yellowing, and the hardness, flexibility, and abrasion resistance have reached 89.4 HD, 3 mm, and 500 g/350 times, respectively. It was worth mentioning that the cured coating has shown excellent adhesion to PET film with the best overall performance. This work has offered important guidance for the protection of PET films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, PET optical film has received widespread attention due to its good physical and chemical properties as well as transparency, which has been applied in the fields of packaging decoration, screen protection, optical-grade mirror surface protection, and so on [1,2,3,4]. However, the surface hardness and abrasion resistance of PET film were poor, which limits its service life [5,6,7,8]. Therefore, improving the surface hardness and abrasion resistance of PET film without damaging its excellent performance become particularly important. The simple and convenient method was to apply a protective coating to the surface of PET film [9, 10].
Comparing traditional solvent coatings and waterborne coatings, UV curable coatings have provided a faster processing speed for the PET film coating process without causing film damage due to the evaporation of organic solvents or heating and drying [11,12,13]. However, traditional low-function oligomer UV coatings have still found it difficult to meet the surface requirements of PET optical films, including higher hardness, better wear resistance, and light transmittance [14, 15]. In order to meet the requirements of the PET film, it has been necessary to develop fast curing UV curable coatings with high functional oligomers and diluents [16,17,18]. Hyperbranched polyurethane acrylates (HPUA), one of the most important oligomers for UV curable coatings, have attracted a lot of attention for their structural flexibility and excellent overall performance [19,20,21,22]. Because of its unique dendritic structure, it can achieve the purpose of high hardness; it also exhibits different properties from linear polymers [23,24,25], such as an acceptable viscosity even at high molecular weights. The soft section of the traditional HPUA resin synthesis process often used small-molecule diols or directly reacted hyperbranched polyol with isocyanate and was finally capped to generate HPUA resin. Fu et al. [26] have generated HPUA resins by reacting Trimethylolpropane diallyl ether (TMPDE), 4-Hydroxybutyl acrylate (HBA), and IPDI to first seal and then react with hyperbranched polyol. Although the resulting coating had excellent hardness and adhesion, the lack of soft segments to absorb a certain amount of stress leads to poor flexibility. Zhang et al. [16] synthesized long-chain Polyurethane acrylate (PUA) and HPUA and doped the two resins together to make light-curing coatings, which solved the problem of flexibility to a certain extent but also sacrificed part of the hardness of the coating and made the synthesis process more cumbersome. In contrast, the synergistic effect of HPUA and nano-SiO2 is more effective in preparing superhard coating on the PET optical film. Therefore, how to ensure the hardness of the coating while also having excellent flexibility and not affecting the light transmittance after the addition of nano-SiO2, has been the current urgent need to solve the problem.
In this work, hyperbranched polyurethane acrylate oligomers will be first prepared by polyester polyol (soft segment) and isocyanate (hard segment) so as to generate flexible long-chain intermediates with bisis-isocyanate groups, which will ensure a certain degree of flexibility and absorb most of the stress. Then, the hyperbranched polyol will be used as a chain extender to generate a hyperbranched structure to ensure excellent hardness. Finally, the end group will be terminated by a high-functional hydroxy acrylate so as to introduce a carbon-carbon double bond. The high-functionality HPUA will perfectly combines hardness and flexibility to form the basic skeleton of the UV curable superhard coating on the optical PET film. The UV curable superhard coatings will be mixed with a certain ratio of HPUA, multifunctional monomer, hollow nano-SiO2, silane coupling agent, photoinitiator, and co-initiator, and the comprehensive performance of the UV curable coating on the optical PET film will be discussed.
Experimental
Materials
Poly(neopentyl glycol adipate) (PNA, Mn = 2000.00 g/mol) was provided by Jiangsu Xuchuan Chemical Co. Isophorone diisocyanate (IPDI, Mn = 222.32g/mol) was provided by Bayer, Germany. Pentaerythritol acrylate (PETA, Mn = 298.00 g/mol) was provided by Jiangsu Sanmu Group Co. Photoinitiator (1-hydroxycyclohexyl phenyl ketone (184), Mn = 204.30 g/mol; Benzophenone (BP), Mn = 182.22 g/mol) was provided by Tianjin Jiuri Chemical Co. Co-initiator (P115, Mn = 500.00 g/mol) was provided by Jiangsu Asahikawa Chemical Co. Dipentaerythritol hexaacrylate (DPHA, Mn = 578.56 g/mol) was provided by Jinan Rongzheng Chemical Co. Hollow nano-SiO2 (100nm) was provided by Nanjing Nanorainbow Biotechnology Co. γ-Aminopropyltriethoxysilane (KH−550, Mn = 221.37 g/mol), γ-Glycidyl etheroxypropyltrimethoxysilane (KH−560, Mn = 236.34 g/mol), γ-Methacryloxypropyltrimethoxysilane (KH−570, Mn = 248.35 g/mol), and hyperbranched polyol (Boltorn™H2004, Mn = 3200.00g/mol) were commercially available analytically pure reagents.
Preparation of HPUA oligomers
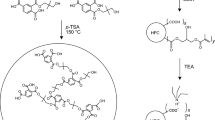
Poly(neopentyl glycol adipate) (PNA, 200.00 g) has been placed into a 500 mL four-necked bottle equipped with a thermometer, an evacuation device, and a mechanical stirrer and evacuated for 1 h at 100 °C, then cooled down to 70 °C to add isophorone diisocyanate (IPDI, 88.80 g), added 0.02 g of the catalyst dibutyltin dilaurate (DBTDL), and then heated up to 90 °C freely. After heating to 90 °C, the content of −NCO in the reaction system was determined by the di-n-butylamine method. When the mass fraction of −NCO was 8.73%, 160.00 g of Boltorn™H2004 was added, and when the −NCO mass fraction was 2.87%, pentaerythritol acrylate (PETA, 89.40 g) was added, and the end point of the reaction has been reached when there was no characteristic peak of the −NCO group at 2270 cm−1 in the FT-IR spectrum. A hyperbranched polyurethane acrylate oligomer, denoted as HPUA, has been obtained, and the synthesis process is shown in Fig. 1.
Preparation of UV curable coatings with HPUA oligomers
With 30.00 wt% HPUA oligomer, 59.00 wt% Dipentaerythritol hexaacrylate (DPHA), 3.00 wt% 1-hydroxycyclohexyl phenyl ketone (184), 3.00 wt% Benzophenone (BP), and 5.00 wt% co-initiator (P115) as raw materials, the UV curable coatings was prepared by mixing and stirring under the condition of light avoiding for 30 minutes and was recorded as UV-1. Then, 1 wt% hollow nano-SiO2 particles were added to UV-1, and 5 wt% KH-550, KH-560, and KH-570 were added to the coatings with hollow nano particles and mixed and stirred for 30 minutes under the condition of avoiding light. The obtained UV curable coatings were recorded as UV-2, UV-3, and UV-4, respectively. After the air bubbles in the coatings had disappeared, the coatings was applied to PET film with a coatings thickness of 9.1 μm, after leveling for 3 minutes, the sample was cured under a UV lamp, 10 cm away from the lamp, and irradiated for 30 seconds to obtain the UV curable superhard coating. Under the same conditions, according to different tests, the UV curable coatings were poured into the corresponding size of the PTFE mold to prepare the required sample, and then tested and characterized.
Instruments and characterization
ZMUV200-2 UV mercury lamp curing machine was used to cure the coating with power density of 100W/cm, wavelength range of 254~450 nm, and main wavelength of 365nm. The structure of HPUA oligomers was characterized using an Avatar-type Fourier infrared spectrometer, Nicolet360, and the samples were coated on the KBr pellet. The number of scans was 32, and the scanning interval was 400–4000 cm−1 with a resolution of 2 cm−1. The molecular weight of the HPUA oligomers was determined using a Waters 1515–2410 gel permeation chromatograph (GPC) with tertahydrofuran (1.0 mL/min) as the mobile phase and polystyrene as the standard sample. The viscosity of the UV curable coatings was measured using a NDJ-79 rotational viscometer with a testing temperature of 25 °C and a shear rate of 1000 s−1. A D-type Shore hardness tester was used to test the hardness of a specimen with a size of 40mm × 40mm × 5mm (L×W×H). The test was repeated three times to determine the average value of the results. The water contact angle test of the coating was carried out using a DSA20 contact angle measuring instrument, which was applied dropwise at 25 °C using a micro-syringe and tested at three different positions, and the results were averaged. Paint film scribing instrument in accordance with GB/T 1720-1979 standard test coating adhesion, each sample test three groups, and take the average value of the results. The thermogravimetric analysis of the samples was carried out with an HCT-1 thermal analyzer (Beijing Hengjiu Experimental Equipment Co., LTD.) in an N2 atmosphere from room temperature to 800 °C, with a heating rate of 10 °C/min. A MY44TRA10 light transmittance meter was used to test the coating transmittance and haze. A cylindrical bending apparatus was used to test the flexibility of the coating, and the diameter of the shaft rod was the value of the flexibility of the coating at this time. The abrasion resistance of the coating was tested using a Martindale abrasion tester with a test weight of 500 g. The coating was aged using a UV aging machine (ATLCSCI4000) with a 12 hour cycle, comprising an 8 hour dry UV irradiation phase and a 4 h UV-free cooling phase. The UV intensity was 0.76 W·m−2, and the temperature was maintained at 50 °C. The degree of yellowing was assessed every 12 h in accordance with GB/T 1766-2008. The infrared spectrometer determine the coatings in the 1648~1589 cm−1 absorption peak, and the integral area was recorded as A0. It has been placed in the ultraviolet light for a test, with irradiation every 10 seconds. During this time, the sample has been stored in a light-protected desiccator. The integral area of the coating under the same band after ultraviolet irradiation has been recorded as A1, the double-bond conversion rate is calculated as C% = (A0-A1)/A0 × 100%. About 1.0 g of oligomer has been taken, and 10 mL of DMF has been added to dissolve it. Then, 10 mL of di-n-butylamine-toluene solution was added to react for 20 min. After that, bromocresol green indicator has been added, and the solution has been titrated with hydrochloric acid standard solution until it turns light yellow. The same steps have been followed for the blank test. The calculation of the −NCO content is shown in Eq. (1):
where V0 and V1 are the volumes of hydrochloric acid standard solution (mL) consumed in the blank test and sample test, c is the concentration of hydrochloric acid standard solution used (mol/L), and m is the sample mass (g).
Results and discussion
FT-IR analysis of HPUA
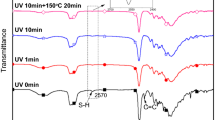
Figure 2 shows the infrared spectra of the reactants, HPUA oligomer, and UV coatings. As shown in the spectra of the raw material, Boltorn™H2004 and PNA have a strong characteristic absorption peak at 3436 cm−1, which can be ascribed to the −OH characteristic absorption peak. The characteristic absorption peak of IPDI at 2270 cm−1 is the −NCO characteristic absorption peak. The characteristic absorption peaks of −N−H and −C=O appear at 3360 cm−1 and 1726 cm−1 for HPUA and UV coatings, respectively, and there is no characteristic absorption peak of −NCO at 2270 cm−1. This means that the −NHCOO− group is generated by the reaction, while the −NCO in IPDI is completely reacted. The appearance of the −CH=CH2 characteristic absorption peak at 1635 cm−1 indicates that the −CH=CH2 group of PETA is successfully grafted onto the HPUA polymer chain, generating the HPUA oligomer with the expected structure.
Molecular weight and viscosity testing
The molecular weight and viscosity of HPUA oligomers are important factors that affect the construction and application of coatings. Suitable molecular weight, which ensure the best physical and chemical properties, such as adhesion and crosslink density, and low viscosity of polymer coatings, ensure convenient construction and application. The molecular weight of HPUA resin was tested by gel permeation chromatography (GPC), and the number average molecular weight is measured to be 8142, the weight average molecular weight is 13060, and the polymer dispersion index is 1.604. The viscosity of several coatings is tested by a rotational viscometer, and the viscosity of UV-1 was measured to be only 2700 mPa·s, which is due to the hyperbranching structure of the resin molecules that makes it have a lower viscosity, and the viscosities of UV-2 to UV-4 were 2400 mPa·s, which was due to the dilution effect of the addition of silane coupling agent to the system.
Kinetic analysis of UV curable coating
It is necessary for the coating on the coiled optical PET film to have fast curing and processing speeds, which influences the practical production and application of PET film. The curing kinetics of UV curable coatings are analyzed by calculating the double bond conversion ration with curing time. The final result is shown in Fig. 3. Throughout the whole UV curing process, there is a high rate of light polymerization at an early stage. The conversion ration can reach more than 80%. during the UV irradiation for 10 seconds. The photopolymerization rate decreases with the increase of light time, and the conversion of the double bond is about 87% after 60 seconds. The lowest UV-1 double-bond conversion rate in the first period may be caused by the fact that the system does not add silane coupling agents, the viscosity is relatively large, and the diffusion ability of the active radicals is weak. UV-4 has the highest double bond conversion rate in the early stage, which can be attributed to the molecular structure of KH-570 with a double bond. Due to the high density of the double bond in the system, the early reaction is faster. Overall speaking, the faster reaction rate makes the formation of a dense three-dimensional crosslinked mesh structure faster, with the light curing. The cage effect is caused when the structure is formed, surrounding −CH=CH2 which is not involved in the reaction. After that, the double bonds are difficult to collide with the active radicals, which results in a slower reaction rate of light curing at the later stage of the reaction and a relatively low double bond conversion rate.
Optical properties of UV curable coating
Since it is a UV curable PET optical coating, the optical properties of the coating are very important, and a light transmittance meter is used to test the light transmittance and haze of the coating. As shown in Fig. 4, compared with UV-1, although a small amount of hollow nano-SiO2 particles and silane coupling agent were added to UV-2, UV-3, and UV-4, the effect on the light transmittance and haze of the coating was very small. It can be attributed to two reasons, firstly, the silane coupling agent is used as bridge bond between organic coating and nanoparticles to improve the nano-SiO2 dispersion. Especially, KH570 provides a polymerizable double bond, which can crosslink with the coating film. Secondly, in order to avoid the influence of nanoparticles on the UV curing speed via absorbing some UV light, the hollow structure of nano-SiO2 is chose, and the hollow structure can extremely decrease the loss of UV light intensity, therefore, it can provide the superhardness for UV curable coating and fast curing speed.
Physical properties test for UV curable coating
In addition to the most important optical properties, adhesion, hardness, abrasion resistance, and flexibility also have a significant impact on the coating. The related physical properties of different coating are listed in Table 1. As shown in Table 1, the adhesion of several coating can reach level 0. This is due to the high double bond functionality component of the coating, a large number of double bonds are opened to form a high cross-linking density during the curing process. And the volume shrinkage caused by the high cross-linking density can be partially offset by the polyurethane structure and nano-SiO2, and a large number of urethane bonds in the molecular chains of the oligomers can form a hydrogen bond with the surface of the substrate, which further enhances the adhesion. Compared with UV-1, the hardness and abrasion resistance of the coating with the addition of hollow nano-SiO2 and silane coupling agent are significantly improved because the hollow nano-SiO2 dispersed on the surface of the coating improves the surface hardness of the coating after curing. At the same time, the hollow nano-SiO2 inside the coating as well as the silane coupling agent make the cross-linking between the organic molecules more homogeneous, and the stress transfer between the organic molecules is more homogeneous when the coating surface is subjected to external forces; therefore, the hardness can be further improved. The increase in hardness is accompanied by a simultaneous increase in abrasion resistance. The hardness and abrasion resistance of UV-2 are better than those of UV-3 because the amine group in KH-550 has higher activity in generating a three-dimensional crosslinked structure. UV-4 is the best, with a hardness of up to 89.4 HD and an abrasion resistance of up to 350 times, because the carbon-carbon double bond contained in KH-570 undergoes a chain reaction during the curing process, which further enhances the crosslinking density. The increase in hardness leads to a decrease in flexibility from 2 to 3 mm, but the difference in flexibility from UV-2 to UV-4 is not so big that it is difficult to make a numerical difference.
Aging and yellowing resistance test for UV curable coating
As a protective coating for PET optical films, its resistance to aging and yellowing is equally important, so it is treated with a UV aging machine to test its flexibility, hardness, and adhesion, and its yellowing performance is observed and recorded every 12 hours. The results are shown in Table 2, indicate that the adhesion of the coating decreases after aging treatment, but all except UV-3 are able to reach level 2. The change in hardness is small, while the coating becomes brittle and the flexibility decreases. In terms of yellowing grade, the UV-2 and UV-3 show poor resistance to yellowing, possibly due to the amino groups in KH-550 and the epoxy groups in KH-560. On the other hand, UV-1 and UV-4 demonstrate excellent resistance to yellowing.
Hydrophobicity test for UV curable coating
UV curable PET optical coating not only have high transmittance and excellent film properties, but also exhibit excellent hydrophobicity, greatly improving the coating's ability to release stains, which is investigated by a water contact angle test. As shown in Fig. 5, the water contact angle of UV-1 is 91.8°, and the water contact angles of UV-2, UV-3, and UV-4 increased to different degrees compared with UV-1, measuring 100.3°, 100.9°, and 101.8°, respectively. This is because the water contact angle is mainly affected by the molecular structure of the internal molecules and the surface roughness. The UV-1 coating surface is relatively smooth, and the existence of urethane bonds within the coating makes it hydrophilic to a certain extent. After the addition of hollow nano-SiO2 and the introduction of silane coupling agents, the curing process of the coating's internal crosslinking density increases. However, the molecular gap becomes smaller, thus making the coating has excellent water resistance. Moreover, due to the presence of hollow nano-SiO2, the surface of the coating has a greater nano-roughness, similar to the rough structure of the surface of the lotus leaf, which greatly enhances the water contact angle of the coating surface [27]. In conclusion, the results show that the coating with the addition of hollow nano-SiO2 and silane coupling agents exhibit obvious hydrophobicity to water.
Coating performance after abrasion testing
After the abrasion test of the coating, the light transmission, haze, and water contact angle of the coating are tested again. As shown in Table 3, the light transmission and haze of the coating are basically unaffected, and the water contact angle of the coating is slightly decreased. This is because the endpoint of the coating wear test is when the coating surface just starts to show scratches, so there is no effect on the optical properties of the coating, whereas the abrasion test may damage the microscopic surface of the coating, making the coating water contact angle decrease. Overall, the coating shows excellent overall performance, even after the abrasion resistance test.
Thermal stability for UV curable coating
The thermal stability of materials under high temperature conditions can be determined by observing the mass change of the sample with temperature. Figure 6a, b and Table 4 show the TGA curves, DTG curves and thermal weight loss parameters of the four coating, respectively. It can be seen that the initial decomposition temperature of UV-1 is 244.79 °C, the thermal decomposition temperature at 50% weight loss is 454.12 °C, the maximum weight loss rate temperature is 467.25 °C, the maximum weight loss rate is 14.80%/min, and the residual carbon rate at 800 °C is 6.39%. Compared with UV-1, the initial decomposition temperature, thermal decomposition temperature at 50% weight loss, maximum weight loss rate temperature, and residual carbon rate at 800 °C of UV-2, UV-3, and UV-4 are all increased to different degrees, while the maximum weight loss rate is reduced to different degrees.
It can be seen that the thermal stability of the coating is effectively improved by the addition of hollow nano-SiO2 and silane coupling agents. This is due to improvements in the local heat transfer capacity of the coating with hollow nano-SiO2, as well as a three-dimensional network formation by connecting hollow nano-SiO2 and silane coupling agents. During the curing process, the thermal decomposition rate of the molecular chains inside the coating is impeded. Therefore, the reason for the increased thermal stability of the material is elucidated after the above analysis.
Effect of modified SiO2 on coating properties
The best overall performance of the coating is achieved when the silane coupling agent is KH-570. 5.00 g of hollow nano SiO2 is added into a four-mouth bottle containing 200 mL of ethyl acetate, dispersed by an ultrasonic wave for 1 hour, then added to 25.00 g of KH-570 and 1.00 g of deionized water, heated to 75 °C, and stirred for 10 h. After cooling, it was washed with excess ethyl acetate and dried, noted as SiO2-KH-570. 6 wt% of SiO2-KH-570 particles are added to UV-1 and stirred for 30 min away from light, noted as UV-5. As shown in Table 5, compared with UV-4, the coating transmittance as well as the haze of UV-5 deteriorates significantly, while other properties are not significantly enhanced, which may be attributed to the low grafting rate of SiO2 on the surface. With the addition of SiO2-KH-570 with the same mass as that of UV-4, the particulate matter increases significantly, thus leading to a decrease in optical properties. Therefore, the modification of SiO2 followed by its addition does not significantly enhance the overall performance of the coating.
Conclusion
In this study, a hyperbranched polyurethane acrylate oligomer is successfully synthesized with hyperbranched polyol as the core. The structures of these oligomers are characterized by Fourier transform infrared spectroscopy (FT-IR). The analysis confirmed that the expected HPUA oligomers are generated, and subsequently, a series of UV curable coatings were prepared and characterized. It can be concluded that the addition of hollow nano-SiO2 and silane coupling agents does not affect the light transmittance of the coating, while the surface properties and heat resistance are improved. When the hollow nano-SiO2 is added and the silane coupling agent is KH-570, excellent aging and yellowing resistance, water contact angle of 101.8°, the hardness reaches 89.4 HD, the flexibility reaches 3 mm, and the abrasion resistance reaches 500 g/350 times. The cured coating also shows excellent adhesion on the PET film.
Data availability
Data will be made available on request.
References
Jiao Z, Yang Q, Wang X et al (2017) UV-curable hyperbranched urethane acrylate oligomers modified with different fatty acids. Polym Bull 74:5049–5063
Liu C, Li T, Zhang J et al (2016) Preparation and properties of phosphorous-nitrogen containing UV-curable polymeric coatings based on thiol-ene click reaction. Prog Org Coat 90:21–27
Sagong H, Seo H, Kim T et al (2020) Decomposition of the PET film by MHETase using Exo-PETase function. ACS Catal 10(8):4805–4812
Szczurek A, Tran T, Kubacki J et al (2023) Polyethylene terephthalate (PET) optical properties deterioration induced by temperature and protective effect of organically modified SiO2–TiO2 coating. Mater Chem Phys 306:128016
Chang C, Oyang T, Hwang F et al (2012) Preparation of polymer/silica hybrid hard coatings with enhanced hydrophobicity on plastic substrates. J Non Cryst Solids 358:72–76
Xu J, Pang W, Shi W (2006) Synthesis of UV-curable organic-inorganic hybrid urethane acrylates and properties of cured films. Thin Solid Films 514:69–75
Miao X, Li Y, Zhang Q et al (2012) Low shrinkage light curable dental nanocomposites using SiO2 microspheres as fillers. Mater Sci Eng 32(7):2115–2121
Startek K, Szczurek A, Tran T et al (2021) Structural and functional properties of fluorinated silica hybrid barrier layers on flexible polymeric foil. Coatings 11(5):573
Park S, Thanakkasaranee S, Shin H et al (2020) Preparation and characterization of heat-resistant PET/bio-based polyester blends for hot-filled bottles. Polym Test 91:106823
Zhong W, Yang X, Gao H et al (2021) Oxygen barrier property of synthesized polyacrylate coatings containing inter-chain cross-linking architecture on PET film. J Appl Polym 138(33):50836
Qiu F, Xu H, Wang Y et al (2012) Preparation, characterization and properties of UV−curable waterborne polyurethane acrylate/SiO2 coating. J Coat Technol Res 9(5):503–514
Kim J, Cho B, Kweon J et al (2014) Preparation and properties of UV−curable di−functional sulfur−containing thioacrylate and thiourethane acrylate monomers with high refractive indices. Prog Org Coat 77(11):1695–1700
Takeuchi H, Konno T, Mori H (2017) Synthesis of multifunctional silsesquioxane nanoparticles with hydroxyl and polymerizable groups for UV−curable hybrid coating. React Funct Polym 115:43–52
Yong Q, Pang H, Liao B et al (2018) Preparation and Characterization of Low Gloss Aqueous Coating via Forming Self-roughed Surface Based on Waterborne Polyurethane Acrylate Hybrid Emulsion. Prog Org Coat 115:18–26
Lv C, Hu L, Yang Y et al (2015) Waterborne UV-curable polyurethane acrylate/silica nanocomposites for thermochromic coatings. RSC Adv 5:25730–25737
Zhang Q, Huang C, Wang H et al (2016) UV-curable coating crosslinked by a novel hyperbranched polyurethane acrylate with excellent mechanical properties and hardness. RSC Adv 6:107942–107950
Wang Q, Thomas J, Soucek M (2023) Investigation of UV-curable alkyd coating properties. J Coat Technol Res 20(2):545–557
Mao H, Qiang S, Xu Y et al (2017) Synthesis of polymeric dyes based on UV curable multifunctional waterborne polyurethane for textile coating. New J Chem 41(2):619–627
Yin W, Zeng X, Li H et al (2011) Synthesis, photopolymerization kinetics, and thermal properties of UV-curable waterborne hyperbranched polyurethane acrylate dispersions. J Coat Technol Res 8:577–584
Lin R, Yin X, Liu H et al (2023) Synthesis of xylitol-based hyperbranched polyurethane acrylate and its application in self-matting acrylate coatings. J Coat Technol Res 20:1579–1594
Mishra R, Mishra A, Raju K (2009) Synthesis and property study of UV-curable hyperbranched polyurethane acrylate/ZnO hybrid coatings. Eur Polym J 45:960–966
Yang Z, Wicks D, Hoyle C et al (2009) Newly UV-curable polyurethane coatings prepared by multifunctional thiol- and ene-terminated polyurethane aqueous dispersions mixtures: preparation and characterization. Polymer 50:1717–1722
Killops K, Campos L, Hawker C (2008) Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene “Click” chemistry. J Am Chem Soc 130:5062–5064
Li K, Shen Y, Fei G et al (2015) The effect of PETA/PETTA composite system on the performance of UV curable waterborne polyurethane acrylate. J Appl Polym 132:41262
Wang Y, Wang S, Zhou X et al (2022) A highly stretchable and self-healable hyperbranched polyurethane elastomer with excellent adhesion. React Funct Polym 181:105443
Fu J, Yu H, Wang L et al (2020) Preparation and properties of UV-curable hyperbranched polyurethane acrylate hard coatings. Prog Org Coat 144:10563
Yang Z, Wu J, Ma G et al (2021) Effect of the particle sizes of silica on the properties of UV-curing matting coatings. J Coat Technol Res 18:183–192
Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of Shanxi Province (No. 20210302123094).
Author information
Authors and Affiliations
Contributions
Kexin Zheng: Investigation, experiment, data curation, writing, modification. Lixia Ling: Supervision, funding acquisition, review & editing. Jianbing Wu: Supervision, funding acquisition, review & editing. Baojun Wang: Funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, K., Ling, L., Wu, J. et al. Preparation of hyperbranched polyurethane acrylates and their properties in UV curable nano-SiO2 composite coatings for PET optical film. J Polym Res 31, 70 (2024). https://doi.org/10.1007/s10965-024-03922-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-024-03922-8