Abstract
The present work reports surface tension and viscosity studies of the cationic surfactant, DTAB (dodecyltrimethylammonium bromide) and the anionic surfactant, SDS (sodium dodecylsulphate) in 0.01 mol‧kg−1 aqueous solutions of the ionic liquids (ILs) tetraalkylammonium bromide (R4NBr), tetraalkylammonium nitrate (R4NNO3) and tetraalkylammonium acetate (R4NOAc) where alkyl (R) is propyl (Pr), butyl (Bu) or pentyl (Pen). Experimentally determined values of surface tension have been further analysed in terms of surface-active (interfacial) parameters including the surface excess at the air–water interface (\(\Gamma_{\max }\)), minimum area per surfactant molecule (\(A_{\min }\)), surface pressure at the CMC (\(\pi_{{{\text{cmc}}}}\)), efficiency of surfactant in reducing surface tension (\(pC_{20}\)) and adsorption at air/water interface relation to micellization (\({{CMC} \mathord{\left/ {\vphantom {{CMC} {C_{20} }}} \right. \kern-\nulldelimiterspace} {C_{20} }}\)). The thermodynamic parameters of micellization and adsorption viz. change in standard Gibbs free energy of adsorption (\(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\)), change in standard free energy of micellization (\(\Delta G_{{\text{m}}}^{{\text{o}}}\)) and change in standard free energy of transfer (\(\Delta G_{{{\text{tr}}}}^{{\text{o}}}\)) for both DTAB and SDS have also been calculated. The viscometric data have been utilized to compute relative viscosity (\(\eta_{r}\)) and viscous relaxation time (\(\tau\)). All these parameters afford insight into structural rearrangement of amphiphilic molecules at the interface and the relative involvement of hydrophobic and electrostatic interactions between surfactant and IL molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The substitution of toxic and volatile organic solvents with eco-friendly alternatives has been key area of current research in order to reduce environmental pollution and risks to human beings. The incredible and beneficial properties of ionic liquids (ILs), such as wide operating temperature range, negligible vapour pressure, high chemical and thermal stability, high ionic conductivity, large electrochemical window and easily adjustable thermo-physical properties, by the appropriate choice of ions, make them suitable greener contenders for this purpose. Ionic liquids comprise a new class of solvents where the molecules are composed of ions (organic cation and inorganic/organic anion); co-ordinated weakly by electrostatic interactions, due to which most of these are liquids at or near the room temperature. Owing to their properties, these have been used for a broad range of applications in the fields of catalysis, separations, electrochemistry, novel extraction, reaction solvents and organic synthesis [1,2,3,4,5,6,7].
In recent years, the applicability of ILs as solvents for amphiphilic assemblies has been substantially increased owing to their zero vapour pressure, non-flammability and potency to alter self-aggregation behaviour of amphiphiles. Surfactant–IL interactions have been mainly investigated by techniques like surface tension [8,9,10,11,12], conductivity [12,13,14,15], viscosity [13, 16, 17], density and speed of sound measurements [18, 19]. The association of surfactant monomers with IL molecules has also been monitored using fluorescence [10,11,12,13], UV–Visible [8, 15], NMR [8, 12], FT-IR [8, 19], cyclic voltammeter measurements [14, 15], etc. The knowledge of thermo-physical properties of (IL–amphiphilic) mixtures, particularly surface tension and viscosity is vital to understand the nature of interactions between the components of the system and for the modulation of chemical and industrial processes involving the flow of liquids. Surface tension is an essential fundamental thermodynamic property of gas/liquid interfaces, which provides information related to the Gibbs free energy of formation at the interface, intermolecular interactions and shapes of the surface. On the other hand, viscosity is a commonly studied transport property of surfactant solutions, which represents index of inner friction of the liquids. The interactions of solvents with hydrophobic as well as hydrophilic parts of the surfactants result in changes in the viscous flow as well as surface tension of the liquid. Therefore, study of these properties is crucial for the elucidation of solute–solvent interactions and to describe the properties of micro-emulsions and liquid crystals with respect to micellar solution of surfactant system. For instance, Abezgauz et al. have employed surface tension and viscosity methods to investigate the influence of monovalent anions of the lyotropic series on micellization of cationic surfactant N-cetylpyridinium chloride (CPyCl) [16]. The methods have also been successfully used to get information about the physico-chemical properties of mixtures of (mixed zwitterionic surface-active ionic liquids (SAILs) + anionic surfactant) [11] and (SAILs and cationic/gemini surfactants) [12]. Brown et al. [20] have carried out a surface tension study for SAILs based, on organic surfactant anions with substituted quaternary ammonium cations, and found a weak dependence of aggregation and adsorption parameters on tetraalkylammonium structure and a very low surface tension at the \(CMC\) (γ ≈25 mN‧m−1) for triple-chain anions coupled with the tetrapropylammonium cation.
In the literature, focus on self-assembling behaviour of surfactants in the presence of imidazolium cation-based ionic liquids is prominent. But recent studies suggest some drawbacks related to imidazolium ILs, including their cost, harmful preparatory materials, toxicity and decomposition in the reactions involving basic salts and active metals [9, 21, 22]; therefore, some new classes of ILs need to be explored. One of such class is based on relatively less toxic quaternary ammonium cations [23, 24]. In this context, in our previous works, we have reported synthesis and characterization of ionic liquids containing tetraalkylammonium cations and inorganic anions. Further, the micellar properties of DTAB and SDS have been investigated in the aqueous solutions of these ILs using techniques such as conductivity, density and speed of sound, fluorescence and UV–Visible measurements. The antimicrobial activities of these (surfactant-IL) systems have also been tested by measuring the zone of inhibition and minimum inhibitory concentration (MIC) [25,26,27,28]. These ILs have significantly altered the physico-chemical properties of surfactants by interacting with different parts of surfactants via electrostatic and hydrohobic interactions. In continuation to this, the present paper deals with tensiometric and viscometric behaviour of ionic surfactants, DTAB and SDS in the presence of these synthesized ILs at temperatures ranging from 293.15 to 318.15 K at intervals of 5 K. The surface tension data have been utilized to calculate critical micelle concentration (\(CMC\)), interfacial and thermodynamic parameters of micellization and adsorption for (surfactant-IL) systems. The variation of various viscometric parameters viz. relative viscosity (\(\eta_{r}\)) and viscous relaxation time (\(\tau\)) has been deployed to form an opinion about intermolecular interactions prevailing in these mixtures.
2 Experimental
2.1 Materials
The deionized distilled water with a conductivity of 1 to 2 × 10−6 S‧cm−1 and pH of 6.8 to 7.0 (at 298.15 K) was obtained from a Millipore–Elix system and was used for all the experiments. The specification of the chemicals used is provided in Table 1. All the chemicals and reagents have been dried at ~ 333.15 K for 24 h in a vacuum oven before use. The synthesis and characterization of ionic liquids (ILs) tetraalkylammonium nitrate ([R4N+] [\({\text{NO}}_{3}^{ - }\)]) and tetraalkylammonium acetate ([R4N+] [OAc−]) and where R = propyl (Pr), butyl (Bu) and pentyl (Pen) has been reported in our previous study [28].
2.2 Methodology
Surface tensions of (surfactant-IL) mixtures have been measured by drop weight method using Man Singh Survismeter supplied by Spectro Lab Equipments Pvt. Ltd [28]. The survismeter has been subjected to calibration before use at 298.15 K by DMSO and MeOH having γ values 43.33 and 22.41 mN‧m−1 which were in good agreement with those reported in the literature [29, 30]. The instrument has been washed periodically with chromic acid, water and then with ethyl alcohol and dried for 3–4 h in an oven. Then it is filled with the experimental solution, clamped in a high precision water thermostat (± 0.01 K) provided by NSW Pvt. Ltd., New Delhi. The reproducibility for the surface tension measurements comes out to be in the range ± 0.10 mN‧m−1.
Viscometric measurements were carried out using a jacketed Ostwald viscometer which was subjected to calibration before use at 298.15 K using water, dioxane and DMSO as solvents [31,32,33]. The temperature of the viscometer filled with experimental solutions has been kept constant by circulating water from thermostat (± 0.01 K). The efflux time of flow has been measured by a digital stop watch with a precision of ± 0.01 s. The experimental uncertainty in viscosity measurements is found to be 0.01 mPa·s.
3 Results and Discussion
3.1 Tensiometry
3.1.1 Effect of Additive on Surface Tension
The effect of ILs on the micellar behaviour and interfacial properties of surfactants, DTAB and SDS have been examined by measuring the surface tension of (surfactant–IL) system in the temperature range (293.15–318.15) K using the formula
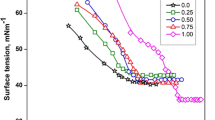
where \(n_{{\text{o}}}\) and \(n\) are the number of drops of solvent and solution respectively. Similarly, \(\rho_{{\text{o}}}\) and \(\rho\) refer to the density of solvent and solution, respectively, and \(\gamma_{{\text{o}}}\) is the surface tension of solvent. The values of \(\gamma_{{}}\) are reported in Tables S1–S3 of Supplementary data and the representative plot of surface tension (\(\gamma\)) vs. log10 [surfactant] for DTAB and SDS in 0.01 mol‧kg−1 aqueous solution of Bu4NOAc has been given in Fig. 1. It has been observed that the surface tension initially decreases as the surfactant concentration increases up to a certain point, after which the surface tension remains roughly constant. This is common behaviour shown by surfactant solutions [33,34,35] and is used to determine their critical micelle concentrations (\(CMC\)). The decrease in surface tension for aqueous surfactant system may be due to the presence of hydrophobic effect causing amphiphilic molecules to adsorb at interfaces, even at low amphiphilic concentrations. Various interactive forces like electrostatic interactions, van der Waals interactions, hydrogen bonding, and/or solvation and de-solvation of adsorbate and adsorbent species are responsible for the process of adsorption. The results show that the value of surface tension of both DTAB and SDS decreases on the addition of ILs. For a particular anion of IL, the surface tension of surfactant varies as
which is in accordance with the hydrophobic character of the cation. The presence of hydrophobic cations may be more effective in screening electrostatic repulsions between surfactant head groups at air–water interface, thereby reducing the free energy of micellization [36]. Hence, the surfactant molecules accumulate on the solvent surface, decreasing the surface tension. On the other hand, \(\gamma\) follows the order:
for common cation. This trend is in compliance with the polarizability of the anion. Anions with higher polarizability may interact strongly with electric field at the interface, which further enhance the binding of anions to the micellar aggregate, eventually lead to the decrease in the electrostatic repulsions between the charged head groups [37]. Moreover, surface tension of both the surfactants show decline in the values with temperature, which may be due to the fact that thermal motion cause the establishment of a dynamic equilibrium between adsorption and desorption making amphiphilic concentration higher at surface as compared to that in the bulk.
3.2 IL Influence on the Micellization of DTAB and SDS
The impact of ILs on the micellization of the surfactant, which has been found to be more prominent in comparison to that of simple electrolytes, may be due to the amphiphilic nature of ILs. In general, the main interactions in (IL–surfactant-water) system are electrostatic as and hydrophobic, which are in turn dependent on the type of head group, counter-ion and hydrophobic chain length of both surfactant and IL molecules.
The \(CMC\) values have been determined from the intersection of two straight lines in low and high surfactant concentration in (\(\gamma\)–log10 [surfactant]) profiles and are recorded in Table 2. It has been found that the \(CMC\) values of both the surfactants show a significant reduction in the values on addition of ILs. The short hydrophobic alkyl chains of ILs may penetrate into the hydrophobic core of surfactant micelle, thereby acting as spacers between the head group resulting in the shrinkage of micelle. These hydrophobic interactions between the hydrocarbon parts of the surfactant and IL favour the micellization of surfactant. Thus, for a particular anion, the \(CMC\) of both SDS and DTAB follows the order:
This decrease in \(CMC\) values can be correlated to the hydrophobic chain length of IL. The increased ionic size from [Pr4N+] to [Pen4N+] enhances the hydrophobic interactions between the hydrophobic chains of IL and surfactant micelles. This further reduces the repulsions between ionic head groups of the surfactants, resulting in promoted micellization. However, the additional electrostatic interactions between the anionic micelles and cationic counter-ions neutralize the effective charge on the head groups of the surfactant in (SDS–IL) system thereby reducing the repulsions between polar head groups and stabilize the micelles to greater extent [38,39,40,41,42].
On the other hand, anions of ILs interact well with the surfactant’s head groups, increasing their tendency to aggregate. The effective size as well as the polarizability of the anion influences this process of micellization. According to Hedin et al. [43], small anions don’t lose their water of hydration and hence interact feebly with the cationic micelles, whereas anions of large size form water-insoluble ion pairs with micellar structures. In the present work, \(CMC\) of DTAB follows the order:
The observed trend for \(CMC\) is in agreement with the effective size of the ions. The larger anion is less hydrated and thus effectively interact with the cationic micelles, neutralizing its charge to some extent [16, 46, 47]. The ionic charge density is another factor that significantly alters the micelle formation of surfactants. Since the bigger ions have lesser charge density, they have weaker potential to bind. In case of SDS, the competition between anion adsorption and electrostatic repulsions between anion and head group of anionic micelles results in following inclination in \(CMC\):
The effect of temperature on the \(CMC\) values of the surfactants has been plotted in Fig. 2. It has been observed that \(CMC\) of both the surfactants first decreases and then increases with increase in temperature, displaying a typical parabolic plot with a broad minimum at around 298.15 K. In general, the variation of \(CMC\) with temperature in aqueous surfactant solution is complex and can be seen as interplay of hydrophilic and hydrophobic hydrations of surfactant monomers in aqueous solution [25, 27]. The gradual decrease of \(CMC\) values at lower temperature and gradual increase of \(CMC\) values at higher temperature may be due to the dominating effect of first and second factors, respectively.
3.2.1 Surface-Activity Parameters
To gain insight into the structural rearrangement of amphiphilic molecules at the interface and the relative involvement of hydrophobic and electrostatic interactions between surfactant and IL, the surface tension data have been further analysed in terms of surface-active (interfacial) parameters including the surface excess at the air–water interface (\(\Gamma_{\max }\)), minimum area per surfactant molecule (\(A_{\min }\)), surface pressure at the CMC (\(\pi_{{{\text{cmc}}}}\)), efficiency of surfactant in reducing surface tension (\(pC_{20}\)) and relationship of adsorption at air/water interface to micellization (\({{CMC} \mathord{\left/ {\vphantom {{CMC} {C_{20} }}} \right. \kern-\nulldelimiterspace} {C_{20} }}\)). All these parameters have been documented in Tables S4, S5 of the Supplementary data.
A quantitative idea about the surfactant adsorption at the solution surface can be obtained in terms of surface excess concentration at the interface, \(\Gamma_{\max }\) which is the maximum value that adsorption can attain and has been calculated using the Gibbs adsorption equation [48,49,50]:
where (\({{\partial \gamma } \mathord{\left/ {\vphantom {{\partial \gamma } {\partial \log_{10} [C])_{T,P} }}} \right. \kern-\nulldelimiterspace} {\partial \log_{10} [C])_{T,P} }}\)) is the slope calculated from the plot between \(\gamma\) versus log10[surfactant], R is the universal gas constant, T is the absolute temperature and n is the number of species formed in the solution by the dissociation of surfactant molecules. The value of n is taken as 2 for the conventional surfactant and 3 for a dimeric surfactant (a divalent surfactant ion and two univalent counter-ions in the absence of an electrolyte).
Using the value of \(\Gamma_{\max }\), the minimum area occupied by each surfactant molecule at saturated air–liquid interface can easily be evaluated according to the following equation [51, 52]:
where No is Avogadro’s number and the factor 1018 arises as a conversion factor of area from m2 to nm2.
The value of \(\Gamma_{\max }\) mainly depends upon the hydrophobic chain length of the surfactant and temperature. It is measure of amount of species adsorbed at air–water interface and is important parameter to provide information regarding the intermolecular interactions prevailing among the components of the systems under consideration [36]. In general, the magnitude of \(\Gamma_{\max }\) has been considered as the combined effect of (i) the attractive interactions between the hydrophobic parts of surfactant and IL molecules in the monolayer formed at the air–liquid interface of the solution and (ii) steric hindrance due to the bulky hydrophobic tails of surfactant molecules. By the inspection of data in the tables, it can be concluded that \(\Gamma_{\max }\) values shift towards lower magnitude with the temperature as well as addition of IL. Rise in temperature causes an increase in thermal motion of the molecules, which, in turn, leads to poorer packing of adsorbed surfactant molecules at the interface, thereby decreasing the surface excess concentration (\(\Gamma_{\max }\)) [53]. Further, the addition of IL gives rise to a significant decline in the values of \(\Gamma_{\max }\) probably due to increased hydrophobicity of the system. The bulkier and hydrophobic ion pairs formed between the tetraalkylammonium ions and hydrophobic parts of the surfactant may be adsorbed on air–water interface with the result in lowering \(\Gamma_{\max }\) [15]. Therefore, for the particular anion of the IL, the observed trend for surface excess concentration is as follows:
However, for the common cation, the values follow the order:
\(A_{\min }\) is the minimum area occupied by the surfactant molecule at the interface and depends upon various factors such as
-
(i)
The changed structure around the head group due to surfactant hydrophilic group–additive interaction;
-
(ii)
The changed nature of water in the presence of additives; and
-
(iii)
The presence of additives at the air/water interface.
A perusal of data shows that the \(A_{\min }\) values increase with rise in temperature due to increased thermal motion of the molecules leading to poorer packing at the interface. Moreover, inclusion of IL allows some of the IL molecules to occupy the interface, therefore the total number of surfactant molecules at the interface decreases and \(A_{\min }\) increases [15]. The trends for \(A_{\min }\) are opposite to those for \(\Gamma_{\max }\) values (Eq. 3).
From the surface tension data, three additional parameters: the effectiveness of surface tension reduction (\(\pi_{{{\text{cmc}}}}\)), adsorption efficiency (\(pC_{20}\)) and adsorption at air/water interface relation to micellization (\({{CMC} \mathord{\left/ {\vphantom {{CMC} {C_{20} }}} \right. \kern-\nulldelimiterspace} {C_{20} }}\)) have also been evaluated. The lowering of the surface tension can be expressed in terms of the surface film pressure (\(\pi_{{{\text{cmc}}}}\)) [51] which is the difference in the surface tension of the pure solvent (\(\gamma_{{\text{o}}}\)) and surface tension at the \(CMC\) (\(\gamma_{{{\text{cmc}}}}\)) and can be calculated using the relation;
where \(\gamma_{{\text{o}}}\) and \(\gamma_{{{\text{cmc}}}}\) are the surface tension of the solvent and of the micellar solution at the \(CMC\) , respectively. The parameter \(\pi_{{{\text{cmc}}}}\) is a measure of effectiveness of surfactant molecules to reduce the surface tension at the solvent surface. The values of \(\pi_{{{\text{cmc}}}}\) have been found to be lower in the aqueous solutions of ILs as compared to those in water. The surface pressure, \(\pi_{{{\text{cmc}}}}\), however, decreases with increase in temperature.
On the other hand, \(pC_{20}\) is an important parameter related to the efficiency of adsorption of surfactant molecules at the air–liquid interface. It is defined as the negative logarithm of \(C_{20}\), where \(C_{20}\) is the concentration required to reduce the surface tension of the solvent by 20 mN‧m−1.
Higher values of \(pC_{20}\) indicate a high absorption efficiency of the surfactant and hence greater reduction in the surface tension [36, 54]. \(pC_{20}\) values of aqueous solutions of both DTAB and SDS have been found to be independent of the type of IL and temperature. However, the values are higher for SDS in comparison with DTAB indicating better adsorption efficiency of former at air–water interface.
The \({{CMC} \mathord{\left/ {\vphantom {{CMC} {C_{20} }}} \right. \kern-\nulldelimiterspace} {C_{20} }}\) ratio is an index of adsorption onto air–liquid interface to the micellization in the bulk of surfactant solution. Inspection of data in Tables S4, S5 reveals that the values increase with the hydrophobic character of the ionic liquid. Thus the results emphasis on the conclusion that with the enhancement in the hydrocarbon chain length of the IL, surfactant molecules prefer to adsorb at the air–solvent interface rather than undergoing micellization [55].
3.3 Thermodynamics of Micellization and Adsorption
The thermodynamic parameters of micellization and adsorption viz. change in standard Gibbs free energy of adsorption (\(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\)), change in standard free energy of micellization (\(\Delta G_{m}^{{\text{o}}}\)) and change in standard free energy of transfer (\(\Delta G_{{{\text{tr}}}}^{{\text{o}}}\)) for both DTAB and SDS have been calculated and the values are documented in Table 3.
The change in standard Gibbs free energy of adsorption (\(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\)) values have been evaluated using following relation [36]:
where, \(\Delta G_{m}^{{\text{o}}}\) is known as the change in standard Gibbs free energy of micellization and calculated as \(\Delta G_{m}^{{\text{o}}} = RT\ln (X_{{{\text{cmc}}}} )\).
The change in standard Gibbs free energy of transfer (\(\Delta G_{{{\text{tr}}}}^{{\text{o}}}\)) of surfactants has been obtained from \(\Gamma_{\max }\) values using following equation:
The value of \(\Delta G_{m}^{{\text{o}}}\) signifies the work required for the transfer of surfactant molecules from monomeric form to the micellar phase, whereas \(\Delta G_{{{\text{tr}}}}^{{\text{o}}}\) is an index that represents the shifting of molecular species from the bulk to micellar interior. From thermodynamic data of micellization (Table 3), \(\Delta G_{m}^{{\text{o}}}\) and \(\Delta G_{{{\text{tr}}}}^{{\text{o}}}\) values have been observed to be more negative for SDS–IL systems as compared to DTAB–IL systems. However, the values merely show dependence on temperature and nature of IL.
Negative values of \(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\) indicate that the adsorption of surfactant molecules at air–liquid interface is Gibbs free energy favourable. \(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\) is the change in standard free energy of transfer of one mole of the surfactant in the solution to the interface. Moreover, the absolute values are greater than absolute \(\pi_{{{\text{cmc}}}}\) values suggesting that the surfactant molecules preferably adsorb at the interface, rather than undergo micelle formation until the full surface coverage. This may be due to the greater freedom of motion of hydrocarbon chains at air–liquid interface than in the interior of the micelle, because of minimal repulsions between hydrophobic phases and aqueous solution at interface [56]. After that, the molecules diffuse to the bulk of the solution to form micelles. The absolute values of \(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\) for SDS are greater than those for DTAB in aqueous solutions of ILs. Thus, the micellization as well as adsorption of the surfactant molecules seems to be governed by various thermodynamic aspects.
3.4 Viscometry
We have also performed the viscometric studies of cationic surfactant, DTAB, and anionic surfactant, SDS, in the presence of 0.01 mol‧kg−1 aqueous solutions of ILs in the temperature range (293.15–318.15 K) to explore the intermolecular forces prevailing between the different components in ternary (surfactant–IL–water) system. The viscosity data have been analysed in the form of relative viscosity; \(\eta_{r} = \eta /\eta_{{\text{o}}}\)[57] and viscous relaxation time; \(\tau = 4\eta /3\rho u^{2}\)[58] where \(\eta\) is the viscosity of solution and \(\eta_{{\text{o}}}\) is the viscosity of the pure solvent.
In the present case, viscosity of both the surfactants decreases with rise in temperature (Tables 4–6). Increase in temperature increases the kinetic energy of molecules and ions present in the solution. This increase in random motion of the species intrinsically decreases the forces of attraction, which the moving solute and solvent molecules and ions have to overcome. Such a decrease in interactions seems to be responsible for the decrease in viscosity with increase in temperature [59,60,61]. Increasing the surfactant concentration or addition of IL may result in structural transitions and micellar growth in the surfactant solutions that may enhance viscosity of the system. For a particular anion, the viscosity of both the surfactants varied in the sequence:
which is exactly the same as expected from the hydrophobic character of these cations. Higher the hydrophobicity of the cation, greater will be its interactions with the micellar core of the surfactant and hence higher will be the viscosity of the system [16]. On the other hand, if we compare the viscosity of surfactants in R4NOAc, R4NNO3, R4NBr and water, the values have been found to decrease in the order:
However, the viscosity data have been further investigated in the form of relative viscosity (\(\eta_{r}\)). An increase in \(\eta_{r}\) values (Tables S6–S8 of Supplementary data) demonstrates the presence of enhanced electrostriction effects. This is probably due to the insertion of tetraalkylammonium cations into the micelles of surfactant, owing to the steric compulsions, which in turn results in structural changes. This will expose the tetraalkylammonium head groups to the water continuum thereby increasing the electrostriction [62]. Noteworthy is that the shape of \(\eta_{r}\) vs. surfactant concentration curves is qualitatively the same as is generally observed in the case of surfactant solutions [63, 64] (Fig. 3).
Representative plots of relative viscosity, \(\eta_{r}\) vs. concentration of a DTAB and b SDS in 0.01 mol‧kg−1 aqueous solution of Pr4NBr at 293.15 K (filled square), 298.15 K (filled circle), 303.15 K (filled triangle), 308.15 K (down-filled triangle), 313.15 K (left-filled triangle) and 318.15 K (right-filled triangle)
Similar observations can be made from the behaviour of viscous relaxation time (\(\tau\)) values (Tables S9–S11 of Supplementary data). Relaxation time may be defined as the time taken for the excitation energy to appear as translational energy, which is affected by the presence of any impurity and temperature. It is directly proportional to viscosity and inversely related to isentropic compressibility of the solution. In the present work, gradual increase in \(\tau\) values has been found with increase in concentration of these surfactants, but the same decrease with rise in temperature, mainly owing to the structural relaxation processes occurring in the system because of the rearrangement of the molecules [65]. This observation confirms the existence of intermolecular interactions in present ternary (surfactant + IL + water) system (Table 5).
4 Conclusion
The work presents the effect of tetraalkylammonium cation-based ionic liquids (ILs) on the surface and viscometric properties of two conventional surface-active agents, DTAB (a cationic surfactant), and SDS (an anionic surfactant). The ionic liquids have significantly altered the surface-active properties and viscometric parameters of these surfactants. The tensiometric studies have revealed that CMC values of the surfactants decrease on the addition of ILs, which can be explained on the basis of hydrophobic as well as electrostatic interactions between the components of ternary (surfactant + IL + water) system. The processes of both micellization and adsorption are favourable as shown by their negative magnitudes, but the \(\Delta G_{{{\text{ad}}}}^{{\text{o}}}\) values are more negative than \(\Delta G_{m}^{{\text{o}}}\), demonstrating the preferable adsorption of surfactant molecules over their micelle formation until the surface is fully covered. The viscometric measurements have also established the presence of enhanced electrostriction effects and structural transitions and micellar growth due to rearrangement of molecules in this system.
References
Parmentier, D., Metz, S.J., Kroon, M.C.: Tetraalkylammonium oleate and linoleate based ionic liquids: promising extractants for metal salt. Green Chem. 15, 205–209 (2013)
Rios, A.P., Fernandez, F.J.H., Lozano, L.J., Sanchez, S., Moreno, J.I., Godınez, C.: Removal of metal ions from aqueous solutions by extraction with ionic liquids. J. Chem. Eng. Data 55, 605–608 (2010)
Han, X., Armstrong, D.W.: Ionic liquids in separations. Acc. Chem. Res. 40, 1079–1086 (2007)
Fukumoto, K., Yoshizawa, M., Ohno, H.: Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 127, 2398–2399 (2005)
Inoue, T., Ebina, H., Dong, B., Zheng, L.: Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J. Colloid Interfac. Sci. 314, 236–241 (2007)
Wang, G., Li, P., Du, Z., Wang, W., Li, G.: Surface activity and aggregation behavior of siloxane-based ionic liquids in aqueous solution. Langmuir 31, 8235–8242 (2015)
Quinn, B.M., Ding, Z., Moulton, R., Bard, A.J.: Novel electrochemical studies of ionic liquids. Langmuir 18, 1734–1742 (2002)
Sinha, S., Bahadur, P.: Effect of organic counter-ions on the surface activity, micellar formation and dye solubilization behaviour of cationic surfactants. Indian J. Chem. 41, 914–920 (2002)
Li, N., Zhang, S., Ma, H., Zheng, L.: Role of solubilized water in micelles formed by Triton X-100 in 1-butyl-3-methylimidazolium ionic liquid. Langmuir 26, 9315–9320 (2010)
Shi, L., Li, N., Zheng, L.: Aggregation behavior of long-chain N-aryl imidazolium bromide in a room temperature ionic liquid. J. Phys. Chem. C 115, 18295–18301 (2011)
Wang, X., Wang, R., Zheng, Y., Sun, L., Yu, L., Jiao, J., Wang, R.: Interaction between zwitterionic surface activity ionic liquid and anionic surfactant: Na+-driven wormlike micelles. J. Phys. Chem. B 117, 1886–1895 (2013)
Sharma, R., Mahajan, S., Mahajan, R.K.: Surface adsorption and mixed micelle formation of surface active ionic liquid in cationic surfactants: conductivity, surface tension, fluorescence and NMR studies. Colloids Surf. A 427, 62–75 (2013)
Rao, V.G., Ghatak, C., Ghosh, S., Pramanik, R., Sarkar, S., Mandal, S., Sarkar, N.: Ionic liquid-induced changes in properties of aqueous cetyltrimethylammonium bromide: a comparative study of two protic ionic liquids with different anions. J. Phys. Chem. B 115, 3828–3837 (2011)
Javadian, S., Ruhi, V., Shahir, A.A., Heydari, A., Akbari, J.: Imidazolium-based ionic liquids as modulators of physicochemical properties and nanostructures of CTAB in aqueous solution: the effect of alkyl chain length, hydrogen bonding capacity, and anion type. Ind. Eng. Chem. Res. 52, 15838–15846 (2013)
Javadian, S., Nasiri, F., Heydari, A., Yousefi, A., Shahir, A.A.: Modifying effect of imidazolium-based ionic liquids on surface activity and self-assembled nanostructures of sodium dodecyl sulfate. J. Phys. Chem. B 118, 4140–4150 (2014)
Abezgauz, L., Kuperkar, K., Hassan, P.A., Ramon, O., Bahadur, P., Danino, D.: Effect of Hofmeister anions on micellization and micellar growth of the surfactant cetylpyridinium chloride. J. Colloid Interfac. Sci. 342, 83–92 (2010)
Mahajan, R.K., Sharma, R.: Analysis of interfacial and micellar behavior of sodium dioctyl sulphosuccinate salt (AOT) with zwitterionic surfactants in aqueous media. J. Colloid Interfac. Sci. 363, 275–283 (2011)
Pal, A., Chaudhary, S.: Ionic liquid induced alterations in the physicochemical properties of aqueous solutions of sodium dodecylsulfate (SDS). Colloids Surf. A 4305, 8–64 (2013)
Bansal, S., Kaur, N., Chaudhary, G.R., Mehta, S.K., Ahluwalia, A.S.: Physiochemical properties of new formulations of 1-ethyl-3- methylimidazolium bis(trifluoromethylsulfonyl)imide with Tritons. J. Chem. Eng. Data 59, 3988–3999 (2014)
Brown, P., Butts, C., Dyer, R., Eastoe, J., Grillo, I., Guittard, F., Rogers, S., Heenan, R.: Anionic surfactants and surfactant ionic liquids with quaternary ammonium counterions. Langmuir 27, 4563–4571 (2011)
Wei, Z., Wei, X., Wang, X., Wang, Z., Liu, J.: Ionic liquid crystals of quaternary ammonium salts with a 2-hydroxypropoxy insertion group. J. Mater. Chem. 21, 6875–6882 (2011)
Docherty, K.M., Kulpa, C.F.: Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 7, 185–189 (2005)
Eike, D.M., Brennecke, J.F., Maginn, E.: Predicting melting points of quaternary ammonium ionic liquids. Green Chem. 5, 323–328 (2003)
Pernak, J., Smiglak, M., Griffin, S.T., Hough, W.L., Wilson, T.B., Pernak, A., Matejuk, J.Z., Fojutowski, A., Kita, K., Rogers, R.D.: Long alkyl chain quaternary ammonium-based ionic liquids and potential applications. Green Chem. 8, 798–806 (2006)
Chauhan, S., Kaur, M., Kumar, K., Chauhan, M.S.: Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J. Chem. Thermodyn. 78, 175–181 (2014)
Chauhan, S., Kaur, M., Rana, D.S., Chauhan, M.S.: Volumetric analysis of structural changes of cationic micelles in the presence of quaternary ammonium salts. J. Chem. Eng. Data 61, 3770–3778 (2016)
Chauhan, S., Kaur, M.: Modulation of aggregation behaviour of anionic surfactant in the presence of aqueous quaternary ammonium salts. J. Surf. Deterg. 20, 599–607 (2017)
Chauhan, S., Kaur, M., Singh, K., Chauhan, M.S., Kohli, P.: Micellar and antimicrobial activities of ionic surfactants in aqueous solutions of synthesized tetraalkylammonium based ionic liquids. Colloids Surf. A 535, 232–241 (2017)
Singh, M.: A simple instrument for measuring the surface tension and viscosity of liquids. Instrum. Exp. Tech. 48, 270–271 (2005)
Stairs, R.A., Rispin, W.T., Makhija, R.C.: Surface tension of some non-aqueous salt solutions. Can. J. Chem. 48, 2755–2762 (1970)
Lebel, R.G., Goring, D.A.I.: Density, viscosity, refractive index, and hygroscopicity of mixtures of water and dimethyl sulfoxide. J. Chem. Eng. Data 7, 100–101 (1962)
Korson, L., Hansen, W.D., Miller, F.J.: Viscosity of water at various temperatures. J. Phys. Chem. 73, 34–39 (1969)
Besbes, R., Ouerfelli, N., Latrous, H.: Density, dynamic viscosity, and derived properties of binary mixtures of 1,4 dioxane with water at T = 298.15 K. J. Mol. Liq. 145, 1–4 (2009)
Naqvi, A.Z., Al–dahbali, G.A., Akram, M.: Adsorption and micellization behavior of cationic surfactants (gemini and conventional)—amphiphilic drug systems. J. Solution Chem. 42, 172–189 (2013)
Rub, M.A., Sheikh, M.S., Asiri, A.M., Azum, N., Khan, A., Khan, A.A.P., Khan, S.B.: Aggregation behavior of amphiphilic drug and bile salt mixtures at different compositions and temperatures. J. Chem. Thermodyn. 64, 28–39 (2013)
Chauhan, S., Sharma, V., Singh, K., Chauhan, M.S., Singh, K.S.: Influence of lactose on the micellar behavior and surface activity of bile salts as revealed through fluorescence and surface tension studies at varying temperatures. J. Mol. Liq. 222, 67–76 (2016)
Rao, K.S., Gehlot, P.S., Gupta, H., Drechsler, M., Kumar, A.: Sodium bromide induced micelle to vesicle transitions of newly synthesized anionic surface active ionic liquids based on dodecylbenzenesulfonate. J. Phys. Chem. B 119, 4263–4274 (2015)
Sadeghi, R., Golabiazar, R.: Surface and micellar properties of ionic liquid 1-Dodecyl-3-methylimidazolium bromide in aqueous solution in the absence and presence of a series of organic electrolytes. J. Chem. Eng. Data 60, 1063–1071 (2015)
Patel, J., Varade, D., Bahadur, P.: Effect of tetraalkylammonium bromide on the micellar behavior of ionic and non-ionic surfactants. Indian J. Chem. 43, 715–721 (2004)
Das, C., Das, B.: Effect of tetraalkylammonium salts on the micellar behavior of lithium dodecyl sulfate: a conductometric and tensiometric study. J. Mol. Liq. 137, 152–158 (2008)
Behera, K., Pandey, S.: Modulating properties of aqueous sodium dodecyl sulfate by adding hydrophobic ionic liquid. J. Colloid Interfac. Sci. 316, 803–814 (2007)
Ali, A., Ansari, N.H.: Studies on the effect of amino acids/peptide on micellization of sds at different temperatures. J. Surf. Deterg. 13, 441–449 (2010)
Hedin, N., Furo, I., Eriksson, P.O.: Fast diffusion of the Cl− ion in the headgroup region of an oppositely charged micelle. A 35Cl NMR spin relaxation study. J. Phys. Chem. B 104, 8544–8547 (2000)
Das, D., Ismail, K.: Aggregation and adsorption properties of sodium dodecyl sulfate in water–acetamide mixtures. J. Colloid Interfac. Sci. 327, 198–203 (2008)
Mata, J., Varade, D., Bahadur, P.: Aggregation behavior of quaternary salt based cationic surfactants. Thermochim Acta. 428, 147–155 (2005)
Maiti, K., Mitra, D., Guha, S., Moulik, S.P.: Salt effect on self-aggregation of sodium dodecylsulfate (SDS) and tetradecyltrimethylammonium bromide (TTAB): physicochemical correlation and assessment in the light of Hofmeister (lyotropic) effect. J. Mol. Liq. 146, 44–51 (2009)
Luczak, J., Markiewicz, M., Thoming, J., Hupka, J., Jumgnickel, C.: Influence of the Hofmeister anions on self-organization of 1-decyl-3-methylimidazolium chloride in aqueous solutions. J. Colloid Interfac. Sci. 362, 415–422 (2011)
Zhou, Q., Rosen, M.: Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir 19, 4555–4562 (2003)
Chauhan, S., Sharma, V.: Sharma, K: Maltodextrin–SDS interactions: volumetric, viscometric and surface tension study. Fluid Phase. Equilib. 354, 236–244 (2013)
Kumar, K., Chauhan, S.: Surface tension and UV–visible investigations of aggregation and adsorption behavior of NaC and NaDC in water–amino acid mixtures. Fluid Phase Equilib. 394, 165–174 (2015)
Ruiz, C.C., Hierrezuelo, J.M., Molina Boliva, J.A.: Effect of glycine on the surface activity and micellar properties of N-decanoyl-N-methylglucamide. Colloid Polym. Sci. 286, 1281–1289 (2008)
Chakraborty, T., Ghosh, S., Moulik, S.P.: Micellization and related behavior of binary and ternary surfactant mixtures in aqueous medium: Cetyl Pyridinium chloride (CPC), cetyl trimethyl ammonium bromide (CTAB), and polyoxyethylene (10) cetyl ether (Brij-56) derived system. J. Phys. Chem. B 109, 14813–14823 (2005)
Aiad, I., El-Sukkary, M.M., Soliman, E.A., El-Awady, M.Y., Shaban, S.M.: Characterization, surface properties and biological activity of new prepared cationic surfactants. J. Ind. Eng. Chem. 20, 1633–1640 (2014)
Jiao, J., Zhang, Y., Fang, L., Yu, L., Sun, L., Wang, R., Cheng, N.: Electrolyte effect on the aggregation behavior of 1-butyl-3-methylimidazolium dodecylsulfate in aqueous solution. J. Colloid Interfac. Sci. 402, 139–145 (2013)
Wang, X., Yan, F., Li, Z., Zhang, L., Zhao, S., Ana, J., Yu, J.: Synthesis and surface properties of several nonionic–anionic surfactants with straight chain alkyl-benzyl hydrophobic group. Colloids Surf. A 302, 532–539 (2007)
Patial, P., Shaheen, A., Ahmad, I.: Synthesis, surface active and thermal properties of novel imidazolium cationic monomeric surfactants. J. Ind. Eng. Chem. 20, 4267–4275 (2014)
Dhondge, S.S., Zodape, S.P., Parwate, D.V.: Volumetric and viscometric studies of some drugs in aqueous solutions at different temperatures. J. Chem. Thermodyn. 48, 207–212 (2012)
Gill, D.S., Singh, J., Singh, P., Rehani, S.K., Khajura, R.: Shear relaxation times of some binary liquid systems and electrolyte solutions. Indian J. Chem. A 37, 45–48 (1998)
Iqbal, M.J., Chaudhary, M.A.: Volumetric and viscometric studies of antidepressant drugs in aqueous medium at different temperatures. J. Chem. Eng. Data 54, 2772–2776 (2009)
Kaur, I., Kumar, H.: Viscometric measurements of L-serine with antibacterial drugs ampicillin and amoxicillin at different temperatures: (30515 to 31515) K. J. Mol. Liq. 177, 49–53 (2012)
Naik, A.B.: Densities, viscosities, speed of sound and some acoustical parameter studies of substituted pyrazoline compounds at different temperatures. Indian J. Pure Appl. Phys. 53, 27–34 (2015)
Bakshi, M.S., Kaur, I.: Head-group-modification-controlled mixing behavior of binary cationic surfactants: conductometric, viscometric, and NMR studies. Colloid Polym. Sci. 281, 935–944 (2003)
Sharma, K., Chauhan, S., Priya, B.: Extended studies on molecular interactions of SDBS and DTAB in aqueous solutions of amino acid at T = 293.15–313.15 K. J. Mol. Liq. 222, 407–414 (2016)
George, J., Nair, S.M., Sreejith, L.: Interactions of sodium dodecyl benzene sulfonate and sodium dodecyl sulfate with gelatin: a comparison. J. Surfact. Deterg. 11, 29–32 (2008)
Chauhan, S., Chaudhary, P., Sharma, K., Kumar, K.: Kiran: Temperature-dependent volumetric and viscometric properties of amino acids in aqueous solutions of an antibiotic drug. Chem. Pap. 67, 1442–1452 (2013)
Acknowledgements
Maninder Kaur thanks UGC, New Delhi, for the award of Senior Research Fellowship (No. F.17-40/2008(SA-1) dated on 31.07.2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chauhan, S., Kaur, M. Interactions of Ionic Surfactants with Aqueous Solutions of Tetraalkylammonium Cation-Based Ionic Liquids: Tensiometric and Viscometric Measurements. J Solution Chem 51, 1483–1507 (2022). https://doi.org/10.1007/s10953-022-01203-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01203-w