Abstract
The interaction of SDS/SDBS in aqueous gelatin solutions is studied above the gelation temperature by viscosity and circular dichroism (CD) measurements. The steep rise observed in the relative viscosity can be due the structural transitions leading to micellar growth of higher order. Circular dichroism spectra indicated that gelatin helped in inducing the sphere→rod transition, without suffering any conformational changes within it. The findings are particularly significant in terms of the head group contribution, hydrophobic interaction and the formation of formulated complexes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interactions between surfactant molecules and synthetic or natural polymers have been studied extensively over the past several decades. These interactions have received considerable attention because of their ability to impart significant changes to the interfacial, rheological and physico-chemical properties of polymer systems with important implications in various applications [1–8]. Surfactant–polymer interactions involve various modes of association facilitated by dipole–dipole or ion–ion forces. Six possible types of association involving either individual surfactant molecule or surfactant clusters (micelles) were discussed by Nagarjan and Kalpaki [9]. NMR and neutron scattering studies on mixtures of non-ionic polymers with ionic surfactants showed a systematic drop in the critical micellar concentration (cmc) and a moderate increase in viscosity suggest the existence of polymer–micelle complexes. When both the surfactant and the polymer are charged the interactions are dominated by strong Coulombic forces. Generally the interaction of a surfactant with an oppositely charged polyelectrolyte results in precipitation [10]. Solubility of the polymer is, however, possible at lower concentrations of the surfactant where complexation is not extensive. At higher concentrations the complex is solubilised by excess of surfactant.
Because of the unique electrolytic character of proteins, phenomena involving protein–surfactant interactions are especially intriguing. The ability of surfactants to denature and precipitate globular proteins and the disinfecting action of the cationic detergents on bacteria is reported [11, 12]. However, it is not possible to generalize about the consequences of interactions of proteins with surfactants due to the diversity of polypeptide structures. The proteins have a net positive charge below the isoelectric point (IEP) and can be considered as cationic polymers. The interactions with anionic surfactant are dominated by precipitation phenomena. Above the IEP, interactions lead to the formation of fully solubilized stable complexes and this can lead to drastic changes in the protein molecule in solution.
In this study, interactions were explored between gelatin, a well-characterized denatured protein in varying percentage of compositions with anionic surfactants, SDS and SDBS over wide range of concentrations. Viscosity is used as a main tool, which is convenient and particularly effective in probing morphological transitions (sphere→higher orders). The particular interest in this work (gelatin-SDS/SDBS system) is the use of gelatin for the production of microcapsules in o/w emulsions using a coacervation process, which consists principally of the precipitation of the protein by addition of an oppositely charged surfactant. Emphasizing the solubilization–precipitation transition, studies have explored the dependence on pH and surfactant/gelatin ratio, establishing a general concept of making microcapsules by forming an insoluble protein surfactant layer around the oil droplets. Thinking about new encapsulation processes, the layer by layer construction of shell about nanoparticles, it is necessary to establish more clearly the surfactant/protein ratio range where the complex adsorbs well on to the substrates because of its amphiphilic properties, but does not precipitate. The measurement of the equilibrium between bound and free surfactant concentrations is also important, because the latter part may unfavorably interact with further introduced molecules.
Experimental Procedures
Sample Preparation
Gelatin, which is denatured collagen, is a cheap and readily available polypeptide. An alkali processed deionized bone gelatin (LOBA Chemie for bacteriology, India) was used as received. Gelatin solutions were prepared by weighing the required amount of gelatin flakes and soaking in hot water (~40 °C) with stirring. The concentrations quoted here are in weight percentage of gelatin. SDS (Merck, India) and SDBS (Tokyo Kasei Kogyo Co., Ltd., Japan) were used as supplied. The surfactant solutions were prepared by weighing out the requisite amount each time and dissolving it in a freshly prepared gelatin solution. The sample solutions after proper mixing were left for equilibration for 24 h. Deionized distilled water from Millipore (Milli-Q; 18 MΩ) was used for all the experiments.
Viscosity Measurements
The viscosities of the solutions were measured using an Ostwald viscometer thermostated at 35 ± 2 °C under Newtonian conditions. The solutions were kept at this temperature for at least 30 min to attain thermal equilibrium. At this temperature, gelatin is characterized macroscopically as a random coil in dilute solutions with a length of ~20 Å [13–15].
Circular Dichroism (CD)
The CD spectra are expressed in terms of molar ellipticity [16] for the pure aqueous solution of gelatin solution and samples equilibrated with alcohols were analyzed at 35 °C using a Jasco spectropolarimeter. A scan speed of 20 nm min−1 was used with an average of five scans per sample. A slit width of 1 nm and a time constant of 1 s were used in a 1 mm cell for the samples.
Results and Discussion
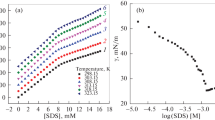
Figures 1 and 2 provide a global view of the relative viscosities of SDBS and SDS at different percentage compositions of gelatin (0, 0.5, 1.0, 1.5 and 2% wt) at 35 ± 2 °C. These curves trace the relative viscosity of the surfactant doped gelatin (Sg) versus surfactant concentration, Cs where the relative viscosity is defined as ratio of viscosity of the surfactant doped gelatin solution to that of bulk gelatin solution. A regular increase in viscosity with increase in concentration of SDS and an abrupt change in case of SDBS were noticed. Minimum concentrations of SDS and SDBS were sufficient for the viscosity rise, with an increase in the percentage composition of the gelatin.
Gelatin, the biopolymer, is a polypeptide with the representation (Gly-X-Pro) n , where X stands for amino acid residues. On interaction with SDS, it interacts electrostatically with the charged sites of the gelatin. This may help in the reduction of electrostatic repulsions within the biopolymer and can result in coiling. The hydrophobic parts of the surfactants are affected by this coiling and they come closer resulting in the formation of aggregates denoted by the marginal increase (region I).
Surfactants do not present any CD signal in the spectral range 190–260 nm and thus the observed CD is for the biopolymer alone. At low surfactant concentrations [Fig. 3 (2)] little change in the ellipticity is observed and it increases with the increase in surfactant concentration [Fig. 3 (3)]. The CD data (Fig. 3) support this presumption that gelatin induces the micellar aggregation without having any structural changes within it. Preferential adsorption on the biopolymer is believed to lead to the formation of micellar aggregates. DLS studies [13] of the system also support the contraction of the coil size leading to the formation of a more compact coil in a surfactant concentration up to 8 mM. In addition, the radius of the SDS–gelatin complex is found to increase continuously. The increase in viscosity can be an onset of an association of the polymer with SDS. The micelles at this stage act as transient cross links, i.e., inter polymer association is possible. The influence of repulsive interactions between the negative charges carried by the excess SDS molecules correspondingly makes the complex strongly hydrophilic and therefore it is displaced from the interface by free SDS molecules.
For constant gelatin concentration and varying SDBS concentration, the relative viscosity shows a regular and steep increase (Fig. 2). The large changes in viscosity over the concentration range studied underlie the strong molecular interactions in the gelatin–SDBS system and its dependence on (SDBS). This can be because of the extra benzene ring in the SDBS monomer. The head groups cannot come closer because of the repulsive interactions of the π-electron cloud of the ring present near the surface of the micelle. A marginal change in viscosity (growth I) leading to an abrupt increase (growth II), were identified from the plot, but the growth in the two regions were observed to be at a different rate. Here the spherical micelles present originally might have grown in size (region I) which changes to higher order aggregates (region II) giving rise to viscous solutions at a higher range.
CD spectra with SDBS–gelatin (Fig. 4) showed an increase in ellipticity, suggesting that the micelle can either bind intra molecularly, i.e., within a gelatin molecule, or it can bind cooperatively with two or more molecules to form a large complex. Because of the highly cooperative nature of the binding process, saturation can be achieved only with a large excess of binding sites, i.e., high gelatin concentration, Cg with no screening. At higher (SDBS), growth increases with Cg indicates that accessible binding sites are depleted. If (gelatin) is not sufficiently high, the complexation process will not be complete, i.e., some molecules will remain unbound and the complex will contain a lower number of gelatin chains. CD measurements in the presence of concentrated SDBS solutions were not performed due to high scattering in the entire wavelength region.
These observations underline some important characteristics of the thickening response of gelatin in the presence of the surfactants used. It was further noticed that the extent of thickening is a function of gelatin/SDS(SDBS) concentration and their interaction is governed by Coulombic forces between the anionic group of the surfactant and positive charges along the gelatin molecule. These charges are most likely the protonated amino and guanidine groups in the arginine and lysine amino acids which are present abundantly in gelatin molecule [17]. Thus gelatin promotes the structural transitions of the surfactants without suffering any conformational changes, as confirmed by CD measurements.
References
Valstar A, Brown W, Almgren M (1999) The lysozyme-sodium dodecyl sulfate system studied by dynamic and static light scattering. Langmuir 15:2366
Valstar A, Almgren M, Brown W (2000) The interaction of bovine serum albumin with surfactants studied by light scattering. Langmuir 16:922
Griffiths PC, Roe JA (2000) Fluorescence probe studies of gelatin–SDS interactions. Langmuir 16:8248
Valstar A, Vasilescu M, Vigouroux C, Stilbs P, Almgren M (2001) Photoinduced electron transfer from N,N-dimethylaniline to 7-amino coumarins in protein-surfactant complex: slowing down of electron transfer dynamics compared to micelles. Langmuir 17:3208
Stenstam A, Khan A, Wennerstrom H (2001) The lysozyme-dodecyl sulfate system an example of protein-surfactant aggregation. Langmuir 17:7513
Priev A, Zalipsky S, Cohen R, Barenholz Y (2002) Determination of critical micelle concentration of lipopolymers and other amphiphiles: comparison of sound velocity and fluorescent measurements. Langmuir 18:612
Moore PN, Puvvada S, Blankschtein D (2003) Role of the surfactant polar head structure in protein-surfactant complexation: zein protein solubilization by SDS and by SDS/C12En surfactant solutions. Langmuir 19:1009
Deo N, Jockusch S, Turro NJ, Somasundaran P (2003) Surfactant interactions with zein protein. Langmuir 19:5083
Nagarajan R, Kalpaki E (1985) In: Dubin. P (ed) Micro domains in polymer solutions. Plenum, New York, p 369
Dubin PL (1992) Critical conditions for the binding of polyelectrolyte to small oppositely charged micelles, D W McQuigg, J I Kaplan, P L Dubin, J Phys Chem
Kuhn R, Bielig HJ (1940) Bet Chem Ges 73:1080
Rose PL, Gettins J, Gould C, Hall D, Jobling P, Rassing J, Wyn-Jones E (1980) J Chem Soc Faraday Trans II 76:1535
Saxena A, Antony T, Bohidar HB (1998) Dynamic light scattering study of gelatin-surfactant interactions. J Phys Chem B 102(26):5063
Li Y, Cheng R (2006) Viscometric study of gelatin in dilute aqueous solutions. J Polym Sci B: Polym Phys 44(13):1804
Pezron I, Djabourov M, Leblond J (1991) Conformation of gelatin chains in aqueous solutions I: a light and small-angle neutron scattering study. Polymer 32(17):3201
Piez KA, Sherman MR (1970) Characterization of the product formed by renaturation of al-CB2, a small peptide from collagen. Biochemistry 9:4129
Connelly RW, Greener J (1985) High-shear viscometry with a rotational parallel-disk device. J Rheol 29:209
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
George, J., Nair, S.M. & Sreejith, L. Interactions of Sodium Dodecyl Benzene Sulfonate and Sodium Dodecyl Sulfate with Gelatin: A Comparison. J Surfact Deterg 11, 29–32 (2008). https://doi.org/10.1007/s11743-007-1050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-007-1050-6