Abstract
The isothermal dissolution equilibrium method was applied for the solid–liquid equilibria experiments of the reciprocal aqueous quaternary system Li+, Rb+//Cl−, borate–H2O at 323.2 K, and the relevant diagrams (phase diagram, water content diagram, density/refractive index against composition diagram) were plotted. It was found that there are two commensurate type quaternary invariant points with three-salt cosaturated, five univariant curves, and four crystallization fields corresponding to four single salts, rubidium chloride (RbCl), lithium chloride monohydrate (LiCl·H2O), lithium tetraborate trihydrate (Li2B4O7·3H2O), and rubidium pentaborate tetrahydrate (RbB5O8·4H2O), respectively. LiCl·H2O has a salting out effect on other coexisting salts and the size of the LiCl·H2O crystallization field is the smallest. Li2B4O7·3H2O has the largest crystallization field, which demonstrates that Li2B4O7·3H2O can be more easily separated from the solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Salt lakes and underground brines contain valuable resources. Comprehensive resource assessment and utilization have been performed for salt lakes in the US, Chile, Bolivia, China, Israel and Jordan among others. Salt lake mineral resources are of two types, solid and liquid deposits. Resources in the liquid phase mainly include Na, K, Mg, Li, Rb, Cs, Cl−, Br−, I−, \({\text{SO}}_{4}^{2 - }\), \({\text{CO}}_{3}^{2 - }\)(\({\text{HCO}}_{3}^{ - }\)), and borates [1,2,3]. According to the chemical composition of the brine, the brine can be divided into five types, i.e. chloride type, sulfate type (sodium sulfate sub-type and magnesium sulfate sub-type), carbonate type, nitrate type, and borate type [4]. The statistics showed the amount of boron resource (calculated as H3BO3) in Pingluo underground brine is nearly 2.987 × 107 tons [3]; there are 113 borate deposits in the salt lakes in Qinghai-Tibet Plateau and about 50% of total reserves of China [5]. The utilization of boron resources in brines can meet the increasing demand for boron.
Phase diagrams are widely applied to describe the dissolution and crystallization of salts and for the purpose of separation and purification of salts in chemical industry. Therefore, research on the phase equilibria aimed on borate type brine is necessary for the comprehensive utilization of the borate type brine resources. As for the borate containing system Li–Na–K–Ca–Mg–Rb–Cl−–\({\text{CO}}_{3}^{2 - }\)–\({\text{SO}}_{4}^{2 - }\)–borate–H2O, a series of solubility data for the related subsystems can be found in the open literature as seen in Table 1. Based on the analysis of the above literature, it can be found that boron appears in many polymeric forms, including \({\text{BO}}_{2}^{ - }\), \({\text{B}}_{4} {\text{O}}_{5} \left( {{\text{OH}}} \right)_{4}^{2 - }\) and \({\text{B}}_{5} {\text{O}}_{6} \left( {{\text{OH}}} \right)_{4}^{ - }\), and the crystalline form of boron is related to the coexisting ions and temperature. As for the quaternary system Li+, Rb+//Cl−, borate–H2O, the phase equilibria of three ternary subsystems Li+, Rb+//borate–H2O [12], Rb+//Cl−, borate–H2O [13], Li+//Cl−, borate–H2O [19] were investigated at 323.2 K, research results show that all three ternary subsystems belong to the simple type, without double salt or solid solution formation. Nevertheless, there is almost no literature about the phase equilibria for the quaternary system Li+, Rb+//Cl−, borate–H2O system at 323.2 K; whether there is a new salt crystalline form or crystalline law in the quaternary system depends on phase equilibrium research. In view of this, the phase equilibrium of the quaternary Li+, Rb+//Cl−, borate–H2O system at 323.2 K is presented here in detail, including the solubilities, densities and refractive indices of this quaternary system.
2 Experimental
2.1 Reagents
Double-deionized water with κ ≤ 5.5 × 10−6 S·m−1 was used in the phase equilibria experiments. The chemicals LiCl (CAS No. 7447-41-8) and Li2B4O7 (CAS No. 12007-60-2) with a purity over 99.0% were purchased from the Chengdu Chron Chemicals Co., Ltd; RbCl (CAS No. 7791-11-9) and Rb2CO3 (CAS No. 584-09-8) with a purity over 99.5% were obtained from JiangXi Dongpeng New Materials Co., Ltd; H3BO3 (CAS No. 10043-35-3) with a purity over 99.8% was from Sinopharm Chmical Reagent Co., Ltd; RbB5O8·4H2O used in this experiment was synthesized in our laboratory from an aqueous solution of Rb2CO3 and H3BO3 [26]. All chemicals mentioned above were dried at 383.2 K about 5–8 h before they were used for experiments.
2.2 Apparatus and Procedure
An isothermal dissolution method was used to carry out the phase equilibria experiments for the quaternary system Li+, Rb+//Cl−, borate–H2O at 323.2 K. First, an initial solution containing two salts and water was prepared according to the compositions of the invariant points of the four corresponding ternary subsystems at 323.2 K, using a BSA124S type analytical balance (the precision is ± 0.0001 g). Then, a third salt was added gradually to the ternary saturated solution at 323.2 K. All samples were shaken by using an oscillation and placed in the thermostatic bath (the precision is ± 0.2 K) at 323.2 K, the system can be considered to reach equilibrium when the analytical relative deviation between two adjacent samplings was less than 0.3%. Experimental results show that the time for reached equilibria is about 5 weeks with stirring. After equilibration, the clarified liquid phases of the samples was taken out to analyze the composition for each component. Also, the density (ρ) was measured using a specific gravity bottle (the uncertainty is ± 2.0 × 10−4 g·cm−3) and the refractive index (nD) was measured by an WYA type Abbe refractometer (the precision is ± 1.0 × 10−4). The wet residue was filtered from the equilibrium solution and dried at 323.2 K, a DX-2700 type X-ray powder diffraction with Cu Kα radiation, operating conditions 40 kV and 30 mA, and scanning angle 10–70° was used to analyze the crystalline form of solid phases at the quinary invariant points.
The analytical methods [27, 28] of Li+, Rb+, Cl− and borate are listed as below:
-
Cl−: AgNO3 gravimetric method, standard uncertainty ur(w(Cl−)) = 0.0027;
-
Rb+: sodium tetraphenylborate–cetyltrimethylammonium bromide titration using titan yellow as an indicator, standard uncertainty ur(w(Rb+)) = 0.0050;
-
Li+: ICP-OES, PerkinElmer, 5300 V-type, standard uncertainty ur(w(Li+)) = 0.0050;
-
borate: neutralization titration in the presence of mannitol, standard uncertainty ur(w(\({\text{B}}_{4} {\text{O}}_{7}^{2 - }\))) = 0.0030.
3 Results and Discussion
The values of solubilities, refractive indices, densities and composition of equilibrated solid phases in the quaternary system Li+, Rb+//Cl−, borate–H2O are presented in Table 2. To simplify the calculation, the traditional stoichiometric form \({\text{B}}_{4} {\text{O}}_{7}^{2 - }\) was selected for description of the concentration of boron in solution. The mass fraction w and the Jänecke index J were used for the expression of the concentration of each solution component in Table 2. The Jänecke index, which is the mole percentage, can be calculated according to the following,
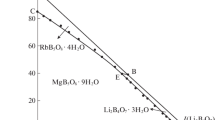
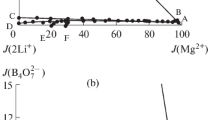
With the Jänecke indices in Table 2, the planar projection diagram is presented in Fig. 1 as square coordinates; each vertex corresponds to a pure component, the points on the side correspond to the components of ternary systems, the points inside the square characterize the compositions of quaternary mixtures.
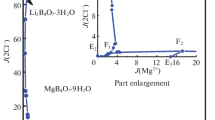
There are two quantrnary invariant points (E1 and E2), five isothermal dissolution curves, and four crystallization fields in the phase diagram as shown in Fig. 1. The invariant point of the quaternary system is cosaturated with three salts and an equilibrated solution. The results of X-ray diffraction analysis of point E1 (Fig. 2) show that salts RbCl, LiCl·H2O, and Li2B4O7·3H2O coexist. The corresponding mass fraction composition of the equilibrated solution at the invariant point E1 is w(Li+) = 5.33%, w(Rb+) = 20.26%, w(Cl−) = 35.58%, w(B4O72−) = 0.13%, and w(H2O) = 38.70%. The results of X-ray diffraction analysis of point E2 (Fig. 3) show that salts RbB5O8·4H2O, RbCl, and Li2B4O7·3H2O coexist. The corresponding mass fraction composition of point E2 is w(Li+) = 0.16%, w(Rb+) = 34.83%, w(Cl−) = 14.58%, w(B4O72−) = 1.45%, and w(H2O) = 48.98%.
For the invariant points in the reciprocal quaternary system, the quaternary invariant points can be divided into two types, incommensurate and commensurate, by the judgement of whether the invariant point lies in a triangle formed by corresponding cosaturated salts or not. The commensurate invariant point is located in the triangle, whereas the incommensurate invariant point lies out the triangle. In Fig. 2, point E1 lies in the triangle formed by its cosaturated salts LiCl·H2O, RbCl, and Li2B4O7·3H2O, point E2 lies in the triangle formed by its cosaturated salts RbCl, RbB5O8·4H2O, and Li2B4O7·3H2O, thus E1 and E2 are both commensurate invariant points.
The five isothermal dissolution curves, namely curves AE1, BE1, CE2, DE2 and E1E2, are cosaturated with two salts and an equilibrated solution. The cosaturated salts for each univariant curve are listed below.
-
AE1: saturated with LiCl·H2O + Li2B4O7·3H2O
-
BE1: saturated with RbCl + LiCl·H2O
-
CE2: saturated with Li2B4O7·3H2O + RbB5O8·4H2O
-
DE2: saturated with RbCl + RbB5O8·4H2O
-
E1E2: saturated with RbCl + Li2B4O7·3H2O
The water content diagram is plotted in Fig. 4. The water content at curves AE1, CE2, and DE2 decreases with \(J\!\left( {{\text{Rb}}_{2}^{2 + } } \right)\) increase, while at curves BE1 and E1E2, the water content changes in the opposite way, with the increase of \(J\!\left( {{\text{Rb}}_{2}^{2 + } } \right)\), the water content increases. The relationships between density or refractive index and composition are presented in Figs. 5 and 6. Besides the curve E1E2, the values of density and refractive index of solution at equilibrium have the same change rule: with the increase of \(J\!\left( {{\text{Rb}}_{2}^{2 + } } \right)\) at AE1, BE1, and CE2, the values of density and refractive index increase; while at curve DE2, with the \(J\!\left( {{\text{Rb}}_{2}^{2 + } } \right)\) increase, the values of density and refractive index decrease. The density of the system reaches its maximum value at point E2, the refractive index value reaches the maximum at point E1, and the water content of the system reaches the minimum value at point E1.
4 Conclusions
-
(1)
The solid–liquid equilibrium of reciprocal aqueous quaternary system Li+, Rb+//Cl−, borate–H2O at 323.2 K has been studied using the isothermal dissolution method. The system belongs to the simple cosaturation type, without double salt or solid solution formed.
-
(2)
The stable phase diagram consists of two quaternary commensurate type invariant points, five isothermal dissolution curves, and four crystallization zones. The salt Li2B4O7·3H2O has the largest field of crystallization, and it is easily precipitated from the solution composed of lithium, rubidium, chloride, and borate at 323.2 K.
-
(3)
The density of the system reaches its maximum value at point E2, the refractive index value reaches its maximum at point E1, and the water content of the system reaches its minimum value at point E1.
References
Zheng, M.P., Deng, T.L., Oren, A.: Introduction to Salt Lake Sciences. Science Press, Beijing (2018)
Zheng, M.P.: Saline Lakes and Salt Basin Deposits in China. Science Press, Beijing (2014)
Li, W., Dong, Y.P., Song, P.S.: The Development and Utilization of Salt Lake Brine Resource. Chemical Industry Press, Beijing (2012)
Gao, S.Y., Song, P.S., Xia, S.P., Zheng, M.P.: Salt Lake Chemistry: New type Boron and Lithium Salt Lake. Science Press, Beijing (2007)
Lin, Y.J., Zheng, M.P., Liu, X.F.: Boron resource of salt lakes in Qinghai-Tibet Plateau. Sci. Technol. Rev. 35, 77–82 (2017)
Wang, S.Q., Guo, Y.F., Liu, W.J., Deng, T.L.: Phase equilibria in the aqueous ternary system (LiBO2 + CaB2O4 + H2O) at 288.15 and 298.15 K. J. Solution Chem. 44, 1545–1554 (2015)
Wang, S.Q., Yang, J., Shi, C.C., Zhao, D., Guo, Y.F., Deng, T.L.: Solubilities, densities, and refractive indices in the ternary systems (LiBO2 + NaBO2 + H2O) and (LiBO2 + KBO2 + H2O) at 298.15 K and 0.1 MPa. J. Chem. Eng. Data 64, 3122–3127 (2019)
Yu, Y., Zhao, K.Y., Guo, Y.F., Wang, X., Deng, T.L.: Phase equilibria and phase diagrams for the ternary systems (KCl/K2SO4 + KB5O8 + H2O) at 298.15 K and 101.325 kPa. J. Solution Chem. 48, 1135–1146 (2019)
Sun, K.R., Zhao, K.Y., Li, L., Guo, Y.F., Li, M.L., Duo, J., Deng, T.L.: Solid–liquid phase equilibria in the ternary aqueous systems (NaB5O8 + KB5O8 + H2O) and (LiB5O8 + KB5O8 + H2O) at 298.15 K and 101.325 kPa. J. Solution Chem. 48, 1105–1118 (2019)
Zhao, X.P., Zhang, X.P., Sang, S.H.: The phase and density diagrams of the systems MgCl2–MgB4O7–H2O and KCl–K2B4O7–H2O at 273 K. Russ. J. Phys. Chem. A 91, 1932–1938 (2017)
Yang, Y.Y., Zhang, X.P., Wang, D., Sang, S.H.: Phase equilibria of two ternary systems: Li2SO4–Li2B4O7–H2O and K2B4O7–K2SO4–H2O at 273 K. J. Chem. Eng. Data 62, 2123–2127 (2017)
Feng, S., Yu, X.D., Cheng, X.L., Zeng, Y.: Phase diagrams and physicochemical properties of Li+, K+(Rb+)//borate–H2O systems at 323 K. Russ. J. Phys. Chem. A 91, 2149–2156 (2017)
Huang, Q., Li, M.L., Wang, L., Yu, X.D., Zeng, Y.: The phase and physicochemical properties diagrams of systems Rb+(Mg2+)//Cl− and borate–H2O at 323 K. Russ. J. Phys. Chem. A 93, 211–217 (2019)
Yu, X.D., Liu, M., Wang, L., Cheng, X.L., Zeng, Y.: Phase equilibria of potassium borate + rubidium borate + H2O and rubidium borate + magnesium borate + H2O aqueous ternary systems at 323 K. J. Chem. Eng. Chin. Univ. 32, 514–521 (2018)
Wang, M.X., Lei, L.Y., Guo, Y.F., Meng, L.Z., Wang, S.Q., Deng, T.L.: Phase equilibria of the reciprocal quaternary system (Na+, Ca2+ // Cl–, borate–H2O) at 288.15 K and 0.1 MPa. J. Chem. Eng. Data 63, 4005–4011 (2018)
Yang, L., Li, X.P., Gao, Y.Y., Zhang, W.Y., Sang, S.H.: Stable phase equilibria in quaternary LiCl–NaCl–MgCl2–H2O and NaCl–Na2B4O7–MgCl2–MgB4O7–H2O systems at 273 K. J. Chem. Eng. Jpn. 52, 165–170 (2019)
Yu, X.D., Zeng, Y., Chen, P.J., Li, L.G.: Solid–liquid equilibrium of the quaternary system lithium, potassium, rubidium, and borate at 323 K. J. Chem. Eng. Data 63, 3125–3129 (2018)
Yan, F.P., Yu, X.D., Yin, Q.H., Zhang, Y.J., Zeng, Y.: The solubilities and physicochemical properties of the aqueous quaternary system Li+, K+, Rb+//borate–H2O at 348 K. J. Chem. Eng. Data. 59, 110–115 (2014)
Yu, X.D., Luo, Y.L., Wu, L.T., Cheng, X.L., Zeng, Y.: Solid–liquid equilibrium on the reciprocal aqueous quaternary system Li+, Mg2+//Cl− and borate–H2O at 323 K. J. Chem. Eng. Data 61, 3311–3316 (2016)
Zeng, Y., Xie, G., Wang, C., Yu, X.D.: Stable phase equilibrium in the aqueous quaternary system Rb+, Mg2+//Cl−, borate–H2O at 323 K. J. Chem. Eng. Data 61, 2419–2425 (2016)
Yin, Q.H., Mu, P.T., Tan, Q., Yu, X.D., Li, Z.Q., Zeng, Y.: Phase equilibria for the aqueous reciprocal quaternary system Rb+, Mg2+//Cl−, borate–H2O at 348 K. J. Chem. Eng. Data 59, 2235–2241 (2014)
Yu, X.D., Zheng, M.P., Zeng, Y., Wang, L.: Solid–liquid equilibrium of quinary aqueous solution composed of lithium, potassium, rubidium, magnesium, and borate at 323.15 K. J. Chem. Eng. Data. 64, 5681–5687 (2019)
Yu, X.D., Zeng, Y., Guo, S.S., Zhang, Y.J.: Stable phase equilibrium and phase diagram of the quinary system Li+, K+, Rb+, Mg2+ // borate–H2O at T=348.15 K. J. Chem. Eng. Data 61, 1246–1253 (2016)
Turesunbadalov, S., Soliev, L.: Investigation of phase equilibria in quinary water–salt systems. J. Chem. Eng. Data 63, 598–612 (2018)
Turesunbadalov, S., Soliev, L.: Determination of phase equilibria and construction of comprehensive phase diagram for the quinary Na, K//Cl, SO4, B4O7–H2O system at 25°C. J. Chem. Eng. Data 62, 698–703 (2017)
Zhu, L. X., Yue, T., Gao, S. Y., Xia, S. P.: Thermochemistry of tetrahydrated rubidium pentaborate. J. Chem. Thermodyn. 35, 433–438 (2003)
Institute of Qinghai Salt-lake of Chinese Academy of Sciences: Analytical Methods of Brines and Salts, second edn., Chinese Science Press, Beijing (1988)
Yuan, H.Z., Zhu, Y.J., Wu, L.P., Zhang, X.: Determination of high-content of lithium in natural saturated brines by inductively coupled plasma-atomic emission spectrometry. Rock Miner. Anal. 30, 87–89 (2011)
Acknowledgements
This project was financially supported by the NSFC (U1607121), and Science and Technology Innovation Talent Program of Sichuan Province (2019JDRC0016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhuge, F., Yu, X., Zeng, Y. et al. Phase Equilibria for the Reciprocal Aqueous Quaternary System Li+, Rb+//Cl−, Borate–H2O at 323.2 K. J Solution Chem 49, 1349–1359 (2020). https://doi.org/10.1007/s10953-020-01003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01003-0