Abstract
The stable phase equilibria, densities, and refractive indices of the aqueous quaternary system Li+,Rb+,Mg2+//borate–H2O at 323 K were determined by the isothermal dissolution method. It was found that the phase diagram consists of one quaternary invariant point, two isothermal dissolution curves, and three fields of crystallization corresponding to three single salts Li2B4O7 · 3H2O, MgB4O7 · 9H2O, and RbB5O8 · 4H2O, respectively. The size of crystallization regions of salt decreased in the order MgB4O7 · 9H2O > RbB5O8 · 4H2O > Li2B4O7 · 3H2O. The comparison of phase diagrams of the system at 298–348 K shown that the crystalline form of salts lithium borate, magnesium borate, and rubidium borate did not change at 298–348 K. The crystalline area of MgB4O7 · 9H2O is the largest than that of 298 and 348 K. The crystalline area of RbB5O8 · 4H2O is the smallest than that of 298 and 348 K, while the crystalline are of Li2B4O7 · 3H2O is larger than that of 298 K, and smaller than that of 348 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Phase equilibrium of borate containing system is important because there are various forms of boron such as B(OH)3, \({\text{BO}}_{2}^{ - }\), \({\text{B}}({\text{OH}})_{4}^{ - }\), \({{{\text{B}}}_{{\text{4}}}}{{{\text{O}}}_{{\text{5}}}}({\text{OH}})_{4}^{{2 - }}\) appeared in the solution [1]. Therefore the solubility isotherms and thermodynamics properties of the borates in pure liquids within multiple temperatures are of significant importance for extracting borates from salt lake and underground brines. For this reason, the phase equilibria of the aqueous systems or subsystems composed of lithium, potassium, sodium, magnesium, rubidium, and borate were done by previous research [2–11].
As for the system Li+,Rb+,Mg2+//borate–H2O studied in this article, the corresponding phase diagrams at 298 and 348 K have been completed by our research group [12, 13], while the solid-liquid equilibrium of system Li+,Rb+,Mg2+//borate–H2O at 323 K has not been carried out. Consequently, on the basis of results for ternary subsystems, the phase equilibrium of system Li+,Rb+,Mg2+//borate–H2O at 323 K is reported in detail.
2 EXPERIMENTAL
2.1 Materials
All solutions were prepared using the doubly deionized water, which was obtained using a Millipore water system with an electrical conductivity less than 5.5 × 10–6 S m–1. Lithium tetraborate anhydrous (Li2B4O7), magnesium oxide (MgO), and boric acid (H3BO3) were obtained from Chengdu Kelong Chemical Reagent Plant with purity of 99.5%. Rubidium carbonate (Rb2CO3) was obtained from Jiangxi Dongpeng New Materials Co., Ltd. with purity of 99.5%. Rubidium pentaborate (RbB5O6(OH)4 · 2H2O) and Hungtsaoite (MgB4O5(OH)4 · 7H2O) with purity higher than 99.0% were synthesized in our laboratory [14, 15].
2.2 Methods and Apparatus
The isothermal dissolution method was applied to the phase equilibrium experiments [2]. A series of samples with different amounts of Li2B4O7, MgB4O7 · 9H2O, RbB5O8 · 4H2O, and H2O were put into 100 mL tightly sealed bottles, immersed in the THZ-82 type thermostatic shaker with 120 rpm speed at (323 ± 0.2) K, once the content of liquid phase became constant, it indicated that the equilibrium had been reached. And then, the liquid and solid phases were separated by filtration at 323 K. The densities of the solution at equilibrium were determined by the gravity bottle method [16], and the refractive indices of the equilibrated solution were measured with the Abbe refractometer (WYA type, Shanghai Precision & Scientific Instrument Co., Ltd.); meanwhile a certain amount solution was diluted to a final volume of 250 mL for analysis the compositions. The wet solids were dried at 323 K and characterized using X-ray diffraction (DX-2700 type, Dandong Fangyuan Instrument Co., Ltd.).
3 ANALYTICAL METHODS [17, 18]
Li+: ICP-OES, with standard uncertainty of 0.50%;
Rb+: sodium tetraphenylborate (STPB)—hexadecyltrimethylammonium bromide (C19H42BrN) back titration method, with standard uncertainty of 0.50%;
Borate: neutralization titration in the presence of mannitol with standard uncertainty of 0.30%;
Mg2+: titration with EDTA standard solution, standard uncertainty of 0.50%.
4 RESULTS AND DISCUSSION
The experimental data for the aqueous quaternary system Li+,Rb+,Mg2+//borate–H2O at 323 K are given in Table 1. Commonly, \({{{\text{B}}}_{{\text{4}}}}{\text{O}}_{7}^{{2 - }}\) represents all kinds of possibly existing forms of boron ions in the solution. Thus, the crystalline form of salt was described as RbB5O8 · 4H2O and \({{{\text{B}}}_{{\text{4}}}}{\text{O}}_{7}^{{2 - }}\) is used to express the different boric species in solution, therefore, Rb2B4O7 was indicated in Table 1.
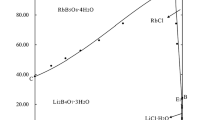
The phase diagram of system Li+,Rb+,Mg2+//borate–H2O at 323 K was plotted as shown in Fig. 1. The phase diagram consists of one quaternary invariant point, three isothermal dissolution curves, and three crystalline zones. The composition of the solid phase in point E was analyzed by the X-ray diffraction method and the XRD pattern was given in Fig. 2. The identification is performed by comparison of the diffraction pattern to databases showing that salts Li2B4O7 · 3H2O (PDF no. 50-0564), RbB5O8 · 4H2O (PDF no. 43-0415), and MgB4O7 · 4H2O (PDF no. 34-1288) coexist in the invariant point E. The liquid composition of point E is w(Li2B4O7) = 3.63%, w(Rb2B4O7) = 4.87%, w(MgB4O7) = 0.25%, and w(H2O) = 91.25%.
Among the three crystalline regions, the crystallization areas of the salts are in the following order: MgB4O7 · 9H2O > RbB5O8 · 4H2O > Li2B4O7 · 3H2O, which means that the solubility of Li2B4O7 is the largest.
The comparison of the phase diagram (Fig. 3) of system Li+,Rb+,Mg2+//borate–H2O between 298 [12], 323 and 348 K [13] shows that (1) the crystalline form of salts lithium borate, magnesium borate, and rubidium borate did not change at 298–348 K; (2) the crystalline of MgB4O7 · 9H2O is the largest than that of 298 and 348 K, the crystalline area of RbB5O8 · 4H2O is the smallest than that of 298 and 348 K, while the crystalline are of Li2B4O7 · 3H2O is larger than that of 298 K, while smaller than that of 348 K.
Figures 4–6 were constructed with J(Rb2B4O7) as the abscissa and J(H2O), the density, or the refractive index of the solution at equilibrium as the ordinate. As Fig. 4 shows, the water content decreases on the AE and BE curve with J(Rb2B4O7) incrementally until it reaches the smallest value at point E; while, on the curve CE, the water content reduces with J(Rb2B4O7) decrement until it reaches the smallest value at point E. Figures 5 and 6 show that on the monovariant curve AE, the values of density and refractive index of the solution at equilibrium are positively correlated with J(Rb2B4O7), the refractive indices increase along with J(Rb2B4O7) decrease on the curves BE and CE, and reach the maximum values at invariant point E.
5 CONCLUSIONS
The solubilities and physicochemical properties (density and refractive index) of the aqueous quaternary system Li+,Rb+,Mg2+//borate–H2O at 323 K were investigated by using the isothermal dissolution method. The solid phases of the quaternary system were Li2B4O7 · 3H2O, MgB4O7 · 9H2O, and RbB5O8 · 4H2O. It is found that MgB4O7 · 9H2O salt contains almost of the crystallization field. Comparisons between the stable phase diagrams at 298, 323, and 348 K show that the crystalline form of three salts was not affected by temperature from 298 to 348 K, when the temperature is 323 K, the crystalline are of MgB4O7 · 9H2O is the largest than that of 298 and 348 K, the crystalline area of RbB5O8 · 4H2O is the smallest, the crystalline are of Li2B4O7 · 3H2O is larger than that of 298 K, while smaller than that of 348 K.
REFERENCES
J. Li and S. Y. Gao, J. Salt Lake Sci. 1, 62 (1993).
S. Feng, X. D. Yu, X. L. Cheng, and Y. Zeng, Russ. J. Phys. Chem. A 91, 2149 (2017).
X. D. Yu, M. Liu, L. Wang, X. L. Cheng, and Y. Zeng, J. Chem. Eng. Chin. Univ. 32, 514 (2018).
A. V. Churikov, K. V. Zapsis, V. V. Khramkov, M. A. Churikov, and I. M. Gamayunova, J. Chem. Eng. Data 56, 383 (2011).
L. Li, Y. F. Guo, S. S. Zhang, M. M. Shen, and T. L. Deng, Fluid Phase Equilib. 436, 13 (2017).
L. Z. Meng and D. Li, Braz. J. Chem. Eng. 31, 251 (2014).
X. D. Yu, Y. L. Luo, L. T. Wu, X. L. Cheng, and Y. Zeng, J. Chem. Eng. Data 61, 3311 (2016).
S. S. Guo, X. D. Yu, and Y. Zeng, J. Chem. Eng. Data 61, 1566 (2016).
X. D. Yu, Y. Zeng, P. J. Chen, and L. G. Li, J. Chem. Eng. Data 63, 3125 (2018).
S. Turesunbadalov and L. Soliev, J. Chem. Eng. Data 63, 598 (2018).
X. D. Yu, Y. Zeng, S. S. Guo, and Y. J. Zhang, J. Chem. Eng. Data 61, 1246 (2016).
X. Duan, Y. Zeng, J. Luo, Y. Tao, and X. D. Yu, J. Chem. Eng. Jpn. 50, 470 (2017).
H. B. Li, L. Liu, X. D. Yu, Y. J. Zhang, Z. Q. Li, and Y. Zeng, Russ. J. Phys. Chem. A 89, 1572 (2015).
Y. Zeng, X. D. Yu, L. L. Liu, and Q. H. Yin, CN Patent No. 103172078 A (2013).
Y. Jing, Sea-Lake Salt Chem. Ind. 29, 24 (2000).
H. Y. Cheng and H. Cheng, Chemical Reagent-General Methods for the Determination of Density (China Standards Press, Beijing, 2007).
H. Z. Yuan, Y. J. Zhu, L. P. Wu, and X. Zhang, Rock Miner. Anal. 30, 87 (2011).
Inst. of Qinghai Salt-Lake, Chin. Acad. Sci., Analytical Methods of Brines and Salts (Science Technol. Press, Beijing, 1984).
ACKNOWLEDGMENTS
Supported by the National Natural Science Foundation of China (U1507111, 41473059), the Research Fund from the Science and Technology Department of Sichuan Province (2017JY0191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xudong Yu, Li, M., Zheng, Q. et al. Solid–Liquid Phase Equilibrium in Aqueous Quaternary System Li+,Rb+,Mg2+//Borate–H2O at T = 323 K. Russ. J. Phys. Chem. 93, 2197–2202 (2019). https://doi.org/10.1134/S0036024419110359
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419110359







 323 K,
323 K, 

