Abstract
The isothermal dissolution method was used to study the stable phase equilibria in the quinary system Li+,Na+,Mg2+//Cl–,B4\({\text{O}}_{7}^{{2 - }}\)–H2O at 273 K. The dry salt diagram, sodium diagram and water diagram in the quinary system were plotted respectively when Sodium chloride (NaCl) is saturated. The experimental results show that a complex salt lithium carnallite (LiCl·MgCl2·7H2O) is formed in the system. The dry base diagram of this system saturated with NaCl consists of four invariant points, nine univariate curves, and six crystalline regions. The crystalline regions are lithium borate trihydrate (Li2B4O7·3H2O), sodium borate decahydrate (Na2B4O7·10H2O), magnesium borate nonahydrate (MgB4O7·9H2O), lithium chloride dihydrate (LiCl·2H2O), magnesium chloride hexahydrate (MgCl2·6H2O), and the double salts lithium carnallite (LiCl·MgCl2·7H2O), among which sodium borate decahydrate (Na2B4O7·10H2O) has the largest crystalline area that shows that Na2B4O7·10H2O is the easiest to precipitate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
With the rapid growth of the world economy, human development has entered a period of scarce resources [1]. The regeneration of various resources and their comprehensive utilization have become a hot topic in current research. Qinghai region is one of the main concentration areas of salt lakes in China. The mineral resources in these salt lakes, such as lithium, potassium, magnesium and boron, are extremely rich. The rational exploitation and utilization of mineral resources in the salt lake not only has a very obvious effect on stimulating local economic growth, but also has a certain positive effect on solving China’s long-term resource shortage [2]. To utilize the salt lake resources of Qinghai region in China, systematic research about phase equilibria at multi-temperatures is necessary.
The focus of stable phase equilibria research is to determine a series of solubilities of salts and corresponding solid phase composition in the phase relationship and the phase diagram of multicomponent systems are plotted. Furthermore, physicochemical properties such as pH, density and refractive index of the liquid phase are also significant data. When the system reaches an equilibrium state, and then draws its isothermal phase diagram to analyze its phase transition law. The earliest study started on the genesis of Stastfort potassium salt mine by Vant’Hoff. The experimental research method is usually isothermal dissolution method. It is well known that solid-liquid phase equilibria of salt–water system at different temperatures play an important role in resource exploitation and utilization and the phase equilibrium basis of research can reveal the origin of evaporite deposit. For a long time, the phase equilibria of sea water system Na+,K+,Mg2+,Ca2+//Cl–,\({\text{SO}}_{4}^{{2 - }}\)–H2O have been studied with systematic research, and the investigated temperature range is from low temperature –60°C to high temperature 250°C [3–5]. These results provide large amounts of important thermodynamic data for the exploitation of seawater resources. More significant studies about phase diagrams in ternary systems and quaternary systems are reported by relevant literatures. However, phase equilibria diagrams in quinary systems are very complicated, comparatively speaking, the studies are reported less. Particularly, the phase equilibrium of quinary systems containing boron and lithium are few reported.
According to the status quo of water-salt system phase equilibrium research, a large amount of research work has focused on Li+,Na+,K+,Rb+,Cs+,Mg2+,Ca2+,Sr2+//Cl–,Br–,\({\text{NO}}_{3}^{{2 - }}\), \({\text{CO}}_{3}^{{2 - }}\),\({\text{SO}}_{4}^{{2 - }}\),B4\({\text{O}}_{7}^{{2 - }}\)–H2O in the ternary, quaternary, and some quinary and hexanary systems, the temperatures range from the low temperature of 273 K existing in salt lakes to the high temperature of 373 K in brines [6]. In the study of the quinary system Na+,K+,Mg2+//Cl–,Br––H2O at 313 K, the physicochemical properties (density, pH, refractive index, viscosity) of the corresponding equilibrium liquid phase were measured, and it was found that if chlorine and bromine were present in the system, a solid solution was formed [7, 8]. Many researchers of Qinghai Institute of Salt Lakes have systematically studied the stable phase relations and solution physicochemical properties of Li+,K+,Mg2+//Cl–,\({\text{SO}}_{4}^{{2 - }}\)–H2O in ternary, quaternary and quinary systems at 298 K [9–12]. Yin et al. took the lead in performing phase equilibrium studies of borate systems in China [13], and carried out specific research on the quaternary system K2CO3–K2SO4–K2B4O7–H2O as well as related ternary subsystems. It is also of great importance to study quinary systems for improving the development and utilization of salt lakes. To date, a series of studies on the equilibria in quinary systems have been carried out. For instance, the Na–K–Mg–Ca–Cl–SO4–H2O system from zero to high concentration at 298.15 K [14], the Li+,K+,Mg2+//Cl–,\({\text{SO}}_{4}^{{2 - }}\)–H2O system at 298.15 K [15], the quinary system Li2B4O7–Na2B4O7–K2B4O7–Li2SO4–Na2SO4–K2SO4–H2O at 273.15 K [16] and Li+,K+,Rb+,Mg2+//borate–H2O at T = 348.15 K [17].
Besides, our research group members also conducted a great number of quinary phase diagram studies in different systems. These cases in point are that the quinary system Na+,Mg2+,K+//\({\text{SO}}_{4}^{{2 - }}\),B4\({\text{O}}_{7}^{{2 - }}\)–H2O at 288 K [18], Na+,K+//Cl–,\({\text{SO}}_{4}^{{2 - }}\),B4\({\text{O}}_{7}^{{2 - }}\)–H2O at 323 K [19], Li+,Na+//\({\text{CO}}_{3}^{{2 - }}\),\({\text{SO}}_{4}^{{2 - }}\),B4\({\text{O}}_{7}^{{2 - }}\)–H2O at 288 K [20], the quinary system NaBr–KBr–MgBr2–SrBr2–H2O at T = 323 K [21] and NaBr–KBr–MgBr2–SrBr2–H2O at 298 K [22].
Aiming at the abundant salt lake resources of Qinghai-Tibet Plateau in China, the phase equilibrium studies are of significance at the low temperature. Accordingly, the research on the phase equilibria of water- salt system is conducive to promoting the development and utilization of salt lake resources. Therefore, a phase equilibrium study of the quinary system Li+,Na+,Mg2+//Cl–,B4\({\text{O}}_{7}^{{2 - }}\)–H2O at 273 K will be conducted for the complex multi-component system in the salt lake of the Qaidam Basin, which is not only a supplement to the water-salt system phase equilibria research, but also provides a scientific basis for the rational development and utilization of resources in salt lakes.
EXPERIMENTAL
Reagents and instruments. The experimental reagents used in the experiment are listed in Table 1. The deionized water was used at pH 6.6 and the conductivity was less than 1.0 × 10–5 S m–1. CO2 was removed by boiling before preparing the samples. MgB4O7·9H2O (99.0%, wt %) is synthesized in the laboratory according to literature method [23], and its purity of the synthesized MgB4O7·9H2O can satisfy the experimental research.
The experimental instruments used include AL104 type electronic balance produced by MettlerToledo Company in the United States, with an accuracy of 0.0001 g; a UPT-II-20T ultrapure water machine produced by Sichuan Youpu Ultrapure Technology Co., Ltd.; a SHH-250 constant temperature biochemical incubator produced by Chongqing Inbo Experimental Instrument Co., Ltd., temperature range: –15…60°C, with an accuracy of 0.1°C; an HY-5 type rotary oscillator produced by Jintan Scientific Analysis Co., Ltd. Company; an DX-2700 X-ray powder crystal diffractometer; a 1010-AB electric blast drying oven and Optima 5300 ICP-OES.
Procedure. The experiment will use the isothermal dissolution equilibrium method to study the stable phase equilibrium. The specific method is to add another inorganic salt to a certain ratio according to the data of the invariant point of the next-level system under a constant temperature condition, and then add a fixed amount of distilled water to the experimental bottle containing the mixed salt, later seal its mouth well. The prepared sample is placed in a circulating shaker, and then placed in a constant temperature biochemical incubator together with the shaker, and the temperature of the incubator is adjusted to 273 K, so that the sample is sufficiently shaken under constant temperature conditions to accelerate the equilibrium cycle. After a period of time, turn off the oscillator, wait for the sample in the experiment bottle to stand sufficiently to completely separate the solid and liquid phases, and then take the supernatant of the experiment bottle for quantitative analysis of the liquid phase content composition, and wait for the content composition of each substance to remain unchanged which will as a criterion for the system to reach equilibrium. After confirming that the system has reached equilibrium, use a pipette to quantitatively take the supernatant into a volumetric flask, and then make a volumetric analysis of each substance’s content and composition. At the same time, take out a certain sedimentary solid phase. After suction filtration, washing, and sufficient grinding, solid phase identification was performed.
Analytical methods. Lithium ion (Li+) and sodium ion (Na+) concentration was analyzed by the ICP-OES method and subtraction method (with a precision of 0.5 wt %). Magnesium ion (Mg2+) concentration was analyzed by the EDTA volume method (with a precision of 0.5 wt %)—with chrome black T as indicator. The concentration of borate ion (B4\({\text{O}}_{7}^{{2 - }}\)) was analyzed by basic titration with a precision of 0.3 wt %. Chloride ion (Cl–) concentration was determined by Mohr’s method using a silver nitrate standard solution (with a precision of 0.3 wt %).
RESULTS AND DISCUSSION
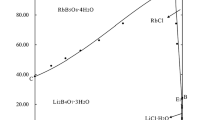
The isothermal dissolution method was used to study the quinary system. When the system reached equilibrium, samples were taken for analyzing. Table 2 lists the equilibrium liquid phase composition, dry salt composition data of the system at 273 K. The two factors of temperature and water are omitted in the isothermal dry base diagram. At this time, there are still three degrees of freedom. In order to simplify and facilitate the phase diagram to guide the actual production, NaCl is omitted, and its phase diagram, water diagram, and sodium diagram are drawn when NaCl is saturated in this system. Each diagram is analyzed and discussed accordingly. After omitting NaCl in the process of drawing the phase diagram, three kinds of ions Li+,Mg2+,B4\({\text{O}}_{7}^{{2 - }}\) are left, and the number of moles of 2Li+,Mg2+,B4\({\text{O}}_{7}^{{2 - }}\) is specified as 100 mol. J(2Li+), J(Mg2+), J(B4\({\text{O}}_{7}^{{2 - }}\)) is the Jӓnecke index of the corresponding ion. Figure 1 plots the dry-base phase diagram and a partially enlarged diagram when NaCl is saturated; Fig. 2 is a sodium diagram; Fig. 3 is a diagram of the water content of the system when NaCl is saturated; the equilibrium solid phase at the invariant point was also measured and analyzed briefly. Figures 4, 5 and 6 show the X-ray powder crystal diffraction patterns of the invariant points K1, K2, and K3, respectively. By identifying the equilibrium solid phase at the invariant point, it was found that the X-ray powder crystal diffraction pattern of the solid phase was in good agreement with the peak intensity and position in the standard card.
According to Table 2 and Fig. 1, it can be seen that the system has double salt lithium carnallite (LiCl· MgCl2·7H2O) formed when sodium chloride (NaCl) is saturated. The dry phase diagram of the system at equilibrium is composed of four invariant points, nine univariate curves and six crystalline regions. Among them, A, B, C, D, E and F respectively indicate the invariant points of the quaternary sub-systems in the quinary system, and the four invariant points of the quinary system are marked as K1, K2, K3 and K4, respectively. The composition of the liquid phase at each invariant point and the corresponding equilibrium solid phase are listed: The equilibrium liquid composition of the invariant point K1 is w(Li+) = 0.31%, w(Na+) = 0.61%, w(Mg2+) = 8.10%, w(Cl–) = 25.59%, w(B4\({\text{O}}_{7}^{{2 - }}\)) = 1.22%, the corresponding equilibrium solid phase is Na2B4O7·10H2O + MgB4O7· 9H2O + MgCl2·6H2O + NaCl. The equilibrium liquid composition of the invariant point K2 is w(Li+) = 0.86%, w(Na+) = 0.53%, w(Mg2+) = 6.98%, w(Cl–) = 24.91%, and w(B4\({\text{O}}_{7}^{{2 - }}\)) = 1.44%, its corresponding equilibrium solid phase is Li2B4O7·3H2O + MgB4O7· 9H2O + MgCl2·6H2O + NaCl. The equilibrium liquid composition of the invariant point K3 is w(Li+) = 3.19%, w(Na+) = 0.29%, w(Mg2+) = 2.55%, w(Cl–) = 23.45%, w(B4\({\text{O}}_{7}^{{2 - }}\)) = 1.60%, its corresponding equilibrium solid phase is Li2B4O7·3H2O + LiCl·MgCl2· 7H2O + MgCl2·6H2O + NaCl. The equilibrium liquid composition of the invariant point K4 is w(Li+) = 3.36%, w(Na+) = 0.27%, w(Mg2+) = 1.90%, w(Cl–) = 22.55%, and w(B4\({\text{O}}_{7}^{{2 - }}\)) = 1.25%, its corresponding equilibrium solid phase is Li2B4O7·3H2O + LiCl·2H2O + LiCl·MgCl2· 7H2O + NaCl. The nine univariate curves are K1A, K1B, K1K2, K2C, K2K3, K3K4, K3F, K4D, and K4E. K1A represents MgCl2·6H2O + MgB4O7·9H2O + NaCl co-saturated liquid phase line, K1B represents Na2B4O7·10H2O + MgB4O7·9H2O + NaCl co-saturated liquid phase line, K1K2 represents Na2B4O7· 10H2O + MgCl2·6H2O + NaCl co-saturated liquid phase line, K2C represents Li2B4O7·3H2O + Na2B4O7· 10H2O + NaCl co-saturated liquid phase line, K2K3 indicates Li2B4O7·3H2O + MgCl2·6H2O + NaCl co-saturated liquidus, K3K4 indicates Li2B4O7·3H2O + LiCl·MgCl2·7H2O + NaCl co-saturated liquidus, K3F indicates MgCl2·6H2O + LiCl·MgCl2·7H2O + NaCl saturated liquidus, K4D represents Li2B4O7·3H2O + LiCl·2H2O + NaCl co-saturated liquidus, K4E represents LiCl·2H2O + LiCl·MgCl2·7H2O + NaCl cosaturated liquidus.
The six crystalline regions correspond to the single salt Li2B4O7·3H2O, Na2B4O7·10H2O, MgB4O7·9H2O, LiCl·2H2O, MgCl2·6H2O and the double salt LiCl· MgCl2·7H2O. It can be seen from the phase region size in the figure that MgB4O7·9H2O has the smallest crystalline region and Na2B4O7·10H2O has the largest crystalline region, which can be inferred that the solubility of Na2B4O7·10H2O is the smallest in this system and it can first be separated from the solution.
It can be obtained from Fig. 2 that the change of J(2Na+) is most obvious on the K2C curve, the content is highest at C, and its content decreases sharply with the increase of J(Mg2+). The changes of J(2Na+) on other univariate curves are not significant. As can be seen from the composition chart of water content in Fig. 4, the curve K2C has the most obvious change. The water content of the system is the largest at point C. At this time, the total salt content of the system is small. However, with the addition of MgCl2, the salt content of the system has increased rapidly. The content decreases sharply, reaching a minimum at the point of invariant point K2.
CONCLUSIONS
The isothermal dissolution method was used to study the phase equilibrium of the quinary system Li+,Na+,Mg2+//Cl−,B4\({\text{O}}_{7}^{{2 - }}\)−H2O (when NaCl is saturated) at 273 K, it is found that the dry phase diagram consists of four invariant points, nine univariate curves, and six crystalline regions. Its crystalline regions are Li2B4O7·3H2O, Na2B4O7·10H2O, MgB4O7· 9H2O, LiCl·2H2O, MgCl2·6H2O, and complex salt LiCl·MgCl2·7H2O. Among the crystalline regions, the phase region of Na2B4O7·10H2O is the largest, it is also easiest to crystallize and precipitate at low temperature. The complex salt LiCl·MgCl2·7H2O was formed in the quinary system at 273 K.
REFERENCES
A. I. Rasulov, P. A. Akhmedova, B. Yu. Gamataeva, et al., Russ. J. Inorg. Chem. 64, 135 (2019). https://doi.org/10.1134/S0036023619010169
S. H. Sang, X. P. Li, D. W. Li, et al., Russ. J. Inorg. Chem. 63, 1644 (2018). https://doi.org/10.1134/S0036023618160027
C. E. Harvie, N. Møller, and J. H. Weare, Geochim. Cosmochim. Acta 48, 723 (1984). https://doi.org/10.1016/0016-7037(84)90098-X
N. Møller, Geochim. Cosmochim. Acta 52, 821 (1988). https://doi.org/10.1016/0016-7037(88)90354-7
J. P. Greenberg and N. Møller, Geochim. Cosmochim. Acta 53, 2503 (1989). https://doi.org/10.1016/0016-7037(89)90124-5
J. Zhang, X. W. Shi, and S. L. Zhao, CIESC J. 67, 379 (2016). https://doi.org/10.11949/j.issn.0438-1157.20151172
Y. B. Weng, Y. F. Wang, and J. K. Wang, J. Chem. Eng. Chin. Univ. 21, 695 (2007). https://doi.org/10.1002/cem.1038
Y. B. Weng, Tianjin: Tianjin University, 2008. https://doi.org/10.7666/d.y1531792.
S. Wang, Y. Guo, and T. Deng, Adv. Mater. Res. 549, 437 (2012). https://doi.org/10.4028/www.scientific.net/AMR.549.437
H. X. Li, D. W. Zeng, and Y. Yao, Ind. Eng. Chem. Res. 53, 7579 (2014). https://doi.org/10.1021/ie404445t
C. Li, B. Zhao, S. Wang, et al., Fluid Phase Equilib. 499 (2019). https://doi.org/10.1016/j.fluid.2019.07.001
X. L. Huang, P. S. Song, L. J. Chen, et al., Calphad 32, 188 (2008). https://doi.org/10.1016/j.calphad.2007.05.006
J. Z. Yin, Y. L. Shen, and J. M. Shen, Acta Chimica Sinica, 30 (1964).https://doi.org/10.1007/bf00912335
C. E. Harvie and and J. H. Weare, Geochim. Cosmochim. Acta 44, 981 (1980). https://doi.org/10.1016/0016-7037(80)90287-2
P. S. Song and Y. Yan, Calphad 27, 343 (2003). https://doi.org/10.1016/j.calphad.2004.02.001
Y. Zeng and X. F. Lin, J. Chem. Eng. Data 54, 2054 (2009). https://doi.org/10.1021/je8009013
X. D. Yu, Y. Zeng, and S. S. Guo, J. Chem. Eng. Data 61, 1246 (2016). https://doi.org/10.1021/acs.jced.5b00888
S. H. Sang and J. Peng. Calphad 34, 64 (2010). https://doi.org/10.1016/j.calphad.2009.12.001
S. H. Sang, X. Zhang, and J. J. Zhang, J. Chem. Eng. Data 57, 907 (2012). https://doi.org/10.1021/je201138z
S. H. Sang, S. J. Ni, and H. A. Yin, Chin. J. Inorg. Chem. 26, 1095 (2010).
G. L. Nie, S. H. Sang, and R. Z. Cui, J. Chem. Eng. Data 64, 3436 (2019). https://doi.org/10.1021/acs.jced.9b00225
G. L. Nie, R. Z. Cui, S. H. Sang, et al., J. Mol. Liquids 306, 112635 (2020). https://doi.org/10.1016/j.molliq.2020.112635
Y. Jing, Sea-Lake Salt Chem. Ind. 29, 24 (2000). https://doi.org/CNKI:SUN:HHYH.0.2000-02-008.
ACKNOWLEDGMENTS
Thanks for the scientific research platform provided by Chengdu University of Technology, and thanks my mentor, Professor Sang Shihua, for the guidance and support of the project.
Funding
This project was supported by the National Natural Science Foundation of China (41873071) and the National Natural Science Foundation of China-Qaidam Saline Lake, Chemical Engineering Science Research Joint Fund of Qinghai Provincial People’s Government (U1407108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Guo, X.F., Sang, S.H., Zhang, H.Z. et al. Stable Phase Equilibria in the Quinary System Li+,Na+,Mg2+//Cl–,B4 \({\text{O}}_{7}^{{2 - }}\)–H2O at 273 K. Russ. J. Inorg. Chem. 66, 916–923 (2021). https://doi.org/10.1134/S0036023621060097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621060097