Abstract
Through first-principles investigation, two new Mn2-based full-Heusler alloys, Mn2HfSi and Mn2HfGe, have been studied using the full-potential linearized augmented plane wave (FP-LAPW) approach as implemented in the WIEN2k code. Our study focuses on these compound’s stability (thermodynamic, dynamic, and mechanical), electronic, and magnetic properties. The L21 (Cu2MnAl-type) structure is shown to be more energetically favorable than the XA (Hg2CuTi-type) structure for both compounds, Mn2HfX (X = Si and Ge). We demonstrate that Mn2HfX (X = Si and Ge) are stable through the calculations of cohesive, formation energies, phonon dispersion curves, and the elastic constants, with the illustration of 3D and 2D bulk and Young’s moduli. Using GGA and GGA-mBJ calculations show that Mn2HfX (X = Si and Ge) are half-metallic ferrimagnets (HMFs), with indirect band gap through the altered Becke-Johnson (mBJ), GGA approximation: 0.689 eV for Mn2HfSi and 0.520 eV for Mn2HfGe. Furthermore, the electrons at the Fermi level (EF) were fully spin-polarized. The total magnetic moment in these two compounds was found to have an integer value of 2 µB per formula, which complies with the Slater-Pauling rule Mtot = Ztot − 24. These compounds are favorable materials for spintronic applications. It was revealed that Mn2HfSi and Mn2HfGe maintained their half-metallicity for lattice constants in the range of 5.7–6.2 Å and 5.75–6.2 Å, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heusler alloys (HAs) have a more comprehensive range of applications, such as spintronic application, optoelectronic, topological insulator, and thermoelectric applications [1,2,3]. Among the essential characteristics of HAs, which have attracted much care in the last years, is the behavior of half-metallic [4]. At Fermi level (EF), we find that one spin direction displays a gap, while the other displays metallic behavior, leading to full spin polarization. Alloys with half-metallic behavior are much required in the spintronic field, which is one of the most reliable and promising emerging technologies in magnetic (multilayer) films [5]. The half-metallic property is not exclusive to Heusler alloys but relatively recently appeared distinctively in two-dimensional compounds [6,7,8]. As an example of the application of this field; tunnel magnetoresistance (TMR) and giant magnetoresistance (GMR) [2, 9, 10]. Following the Slater-Pauling (SP) rule [11], it is also possible to predict a feature of a perfect half-metal in HAs by possessing an integer magnetic moment. In the L21 full-Heusler compounds, as in our case, the SP relation becomes Mtot = Ztot − 24 [12], where Mtot is the total spin magnetic in the unit cell scales and Ztot is the total number of valence electrons per unit cell, while for the half-Heusler compounds, this relation becomes Mtot = Ztot − 18 [13].
The HAs with the form X2YZ, crystallizing in the L21 structure with prototype Cu2MnAl, and the inverse Heusler XA structure with prototype Hg2CuTi are members of the Heusler alloy family. Half-Heusler alloys (ternary and quaternary) have the XYZ (XX′YZ) formula. When one of the two X atoms is replaced by a different transition metal, X′, a quaternary compound with the formula XX′YZ is formed. Here, X represents a high-valent transition or noble metal atom, Y is a low-valent transition metal atom, and Z is an sp element.
Regarding the Mn2-based complete Heusler, the Mn atom’s position in the periodic table provided her the option to crystallize in either the regular or inverse Heusler. These configurational selections are made based on the valence electrons and electronegativity of the X and Y atoms. For more precise information about the Mn2YZ, we discuss all possibilities. Firstly, we started with literature and the known variety of spin alignments [14, 15]. The compounds with X = Mn and Y atoms with a valence lower than Mn have Ferro magnetically (FM—all parallel spins) coupled Mn atoms or are nonmagnetic (NM) (Mn2MoGe, Mn2MoSn, Mn2WGa, Mn2WIn, and Mn2WSb) in the regular stable phase [12]. The current trend that we highlighted in our study is that Mn2YZ have ferrimagnetically (FIM-several spins up and some down) coupled Mn atoms with a low magnetic moment. Among the HAs in the half-metallic ferrimagnets (HMFIs) order, full Heusler based on Mn2 that contains Y from a 3d, 4d, or 5d transition element and Z being an sp element is of particular importance. In contrast to the literature, which declares that the majority of Mn2-based full Heusler are ferromagnetic (FM) in regular phases and the ferrimagnetic found in inverse phases, previous studies have discovered some compounds in the regular phase with ferrimagnetic states, such as Mn2ZrSi and Mn2ZrGe [16]. The anti-parallel alignment of two Mn magnetic moments in a ferrimagnetic structure HMFIs is a fascinating family of HAs that have gotten a lot of greater significance because this is due mainly to the system’s low total magnetic moment, which provides extra benefits [17]. They do not generate a lot of stray fields in electronics, are less affected by external magnetic fields, and have demonstrated that particular materials can exhibit ferrimagnetism. Mn2YZ Heusler alloys are a potential material in future spintronic devices like efficient spin-transfer torque [18], because of strong electron-spin polarization with high Tc [19].
Ruben Weht and Warren E. Pickett theoretically and experimentally explored one of the HMFIs, Mn2Val [19, 20], and then K. Ozdogan and I. Galanakis demonstrated the HMFIs behavior of Mn2VZ (Z = Al, Ga, In, Si, Ge, Sn) with several sp elements [21]. In this regard, Hongzhi et al. predicted half-metallic characteristics for the HAs Mn2CrZ (Z = Al, Ga, Si, Ge, Sb) [22] and added more compounds to the list of HMFIs. Subsequently, Li et al. and Wei et al. [23, 24] examined the electronic structure and magnetism of Mn2CuAl and Mn2CuGe. Further, lately, Meinert et al. [17] researched several Mn2TiZ compounds using simulation studies that illustrated the behavior of HMFIs. While Liu et al. investigated the Mn2NbSi full-Heusler combination [25], new HMFIs Mn2ZrSi and Mn2ZrGe were investigated by Abada et al. [16]. In addition, Benaddi et al. studied the rise of the HMFIs full-Heusler combination in Mn2IrGe [26]; in addition, new Heusler alloys Mn2ScZ (Z = Si, Ge, Sn) were investigated by Ram et al. [27]. Also, Ram et al. studied structural and mechanical properties in the inverse Heusler alloy Mn2ZnSi(1-x) Gex [28]. Sokolovskiy et al. have demonstrated the ferrimagnetic nature of Mn2NiZ (Z = Ga, In, Sn, Sb) alloys [29]. Predictions of the half-metallic, mechanical, and acoustic properties of Hg2CuTi-type in Mn2LiZ (Z = As, Sb) compounds were investigated by Jiang et al. [30] as well as the electronic structure, magnetism, and thermoelectricity of new Mn2(Y, Zn)Sn Heusler compounds by Yousuf and Gupta [31]. Huijun et al. studied the structural, elastic, electrical, and magnetic characteristics of the new full-Heusler Mn2MgGe alloy [32], while Maizia et al. researched the new full-Heusler Mn2OsGe HMFIs alloy [33].
Until recently, the bulk of Mn2YZ Heusler alloys that have been studied had 4d transition metal components in the Y position. Much study has not been done on Mn2-based Heusler compounds containing 5d transition metal components. This study’s main objective is to utilize first-principles calculations to compute the electronic structures and magnetic characteristics of Mn2HfSi and Mn2HfGe Heusler compounds with AlCu2Mn-type structures and employ them to forecast new HMFIs compounds. There are no comparable experimental studies or theoretical data to our knowledge.
2 Calculation Method
The structural, electronic, and magnetic properties of full-Heusler Mn2HfX (X = Si, Ge) have been investigated using first-principles FP-LAPW (full-potential linearized augmented plane wave) [33] as employed in the WIEN2k code [34]. We have adopted the generalized gradient approximation in the Perdew-Burke-Ernzerhof form (GGA-PBE) [35, 36]. The threshold energy between core states and valence states was − 7.0 Ry. The RmtKmax is chosen equal to 9, which determines the matrix size (convergence), where Kmax is the plane wave cut-off, Rmt is the smallest of all atomic sphere radii, and the Gmax = 14 (a.u-1). The choice of muffin-tin radii (RMT) is made so that the interstitial region between the different spheres is the smallest to ensure the accuracy of our calculations. Selected RMT values are 2.30 for Mn and 2.38 for Hf, and for Si and Ge, 2.16 and 2.25. The irreducible Brillouin zone (IBZ) k-point number has been set at 4000. The Mn:(Ar) 3d54s2, Hf:(Xe) 5d26s2, Si:(Ne) 3s23p2, and finally, Ge:(Ne)3s23p2 electrons are presented as valence electrons. Also, Tran and Blaha-modified Becke-Johnson (TB-mBJ) potential was used to predict the band gap results with more accuracy [37]. The convergence criteria for the total energy have been taken as 10−4 eV. Phonon spectra are calculated by the pseudo-potential-based Quantum Espresso package [38] within the framework of GGA-PBE.
3 Results and Discussion
3.1 Structural and Thermodynamic Properties
3.1.1 Structural Properties
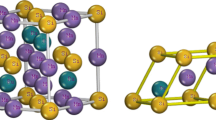
Full-Heusler alloys have two possibilities to crystalize first in the L21 with Cu2MnAl prototype (space group: \(Fm\overline{3 }m\) N°:225) or in the XA with Hg2CuTi prototype (space group: \(F\overline{4 }3m\) N°:216) structure. Four interpenetrating fcc sub-lattices make up the cubic L21 structure. X atoms occupy two of them at Wyckoff position 8c (0.25, 0.25, 0.25), while Y and Z atoms occupy the other two at places 4b (0.5, 0.5, 0.5) and 4a (0, 0, 0), respectively. However, it was employed in all four locations for the XA structure: 4a (0, 0, 0), 4b (0.25, 0.25, 0.25), 4c (0.5, 0.5, 0.5), and 4d (0.5, 0.5, 0.5) (0.75, 0.75, 0.75). Mn atoms occupy sites B (0.25, 0.25, 0.25) and D (0.75, 0.75, 0.75) in the L21 structure, while Hf/Si-Ge atoms occupy sites C (0.5, 0.5, 0.5) and A (0, 0, 0), respectively (Table 1). Together, inequivalent Mn atoms (as named Mn1/Mn2) occupy A (0, 0, 0) and B (0.25, 0.25, 0.25), while Hf/Si-Ge occupy C (0.5, 0.5, 0.5) and D (0.75, 0.75, 0.75) sites in the XA structure (see Fig. 1).
First, we study the structural stability to find the most stable geometry because all the physical parameters depend on its crystal structure. It is best characterized for use in the industry based on experimental studies that validate what we found in the calculation. Since the Hf atom is less electronegative than Mn and Hafnium has fewer valence electrons than manganese atoms, the L21 AlCu2Mn-type structure is projected to be more advantageous than the XA Hg2CuTi-type structure for the two compounds Mn2HfSi and Mn2HfGe. In 5d transition metals, it has been discovered that elements with more valence electrons are more likely to occupy the Wyckoff position 8c (0.25, 0.25, 0.25) in the L21 structure. In contrast, those with less are more likely to occupy 4b (0.5, 0.5, 0.5) and 4a (0, 0, 0), respectively. In both Cu2MnAl- and Hg2CuTi-type structures of the full-Heusler alloys Mn2HfSi and Mn2HfGe, we estimated the total energy as a function of the cell volume per formula unit for the nonmagnetic (NM) and ferromagnetic (FM) states (see Fig. 2a–b).
Secondly, we notice that our results concern the magnetic stability for the Mn2HfX since X = Si-Ge. The results show that the FM phases have lower energies than the NM phases in these systems, according to our findings. As a result, Mn atoms prefer to occupy the C sites, whereas Hf and Si-Ge atoms prefer to occupy the B sites and A sites, respectively.
By fitting the total energy as a function of volume to the Birch-Murnaghan equation of state, the equilibrium lattice constants a0, bulk modulus B, and its pressure derivative B are achieved [39]. The projected results are shown in Table 2. Mn2HfSi and Mn2HfGe have equilibrium lattice constants of 5.9853 Å and 6.0685 Å, respectively, in L21 AlCu2Mn-type. Due to the lower atomic radius of Si than that of Ge, the estimated lattice constant of Mn2HfSi is less than that of Mn2HfGe. There are no experimental or theoretical data available for comparison to our knowledge.
3.1.2 Thermodynamic Properties
Third, two energy parameters—cohesive energy and formation enthalpy—are used to evaluate the chemical and thermodynamic stabilities of all the compounds. To ascertain chemical stability and investigate if it is possible to create these half-metals in the experiment, these two energy values are defined in Eqs. (1) and (2):
We start by the cohesive energy Ecoh, where Etotal refers to the total energy of Mn2HfX since the X = Si-Ge compound of one formula unit at equilibrium volume, and Eiso(Mn), Eiso(Hf), and Eiso(X) are the total energy of isolated atoms. ΔHr (Mn2HfX) is the formation enthalpy of Mn2HfX since X = Si-Ge compound; Ecoh(Mn2HfX) is the cohesive energy of Mn2HfX since X = Si-Ge per formula; Ecoh(Mn), Ecoh(Hf), and Ecoh(X) are the cohesive energy of Mn, Hf, and X = (Si and Ge). The calculated cohesion energy and formation enthalpy of all the compounds in the cubic phase are given in Table 3. The negative values of Ecoh(Mn2HfX) and ΔHr (Mn2HfX) ensure the chemical and thermodynamic stability of Mn2HfX since X = Si-Ge compounds. As a result, these compounds, Mn2HfSi and Mn2HfGe, could be synthesized experimentally.
3.2 Electronic Properties
3.2.1 Band Structure and Density of States
In the AlCu2Mn-type compounds Mn2HfSi and Mn2HfGe, we provide the spin-polarized band structures at equilibrium lattice constants in the L21 phase, along the high-symmetry directions of the first Brillouin zone. To predict the band gap results with more accuracy with the experimental, GGA + U and TB-mBJ are well positioned. The researchers’ investigation revealed that GGA-mBJ functional possesses similar accuracy to GGA + U, with small detail’s differences [40, 41]. For this reason, our study is focused on GGA-mBJ methods.
We agree with some studies [42, 43] that spin–orbit coupling (SOC) can be of crucial importance for the half-metallic property of some 3d materials, and also, 2d materials are affected by the SOC [44, 45]. For Heusler alloys, the spin–orbit coupling can induce states within the gap, but the alloys keep a very high degree of spin polarization at the Fermi level. Galanakis et al. [46] confirm in their calculation of spin (mspin) and orbital (morb) magnetic moments for some half- and full-Heusler compounds that the orbital moments in Heusler alloys are expected to be small. For this reason, this study has not considered the effect of spin–orbit coupling (SOC).
It is obviously apparent in Fig. 3a–c. The majority-spin channel has an energy gap GMaj at the Fermi level (EF), suggesting semiconducting property, as opposed to the minority-spin channel, which is metallic and has intersections at the Fermi level (EF) (Fig. 3b–d). The band structures of the two materials are similar, as can be seen for these compounds. The indirect band gaps for Mn2HfSi and Mn2HfGe, respectively, are 0.689 eV and 0.520 eV at approximately EF along the G-X symmetry, according to Fig. 3a–c, in the majority-spin channel. These gaps result in an equilibrium-state HM character due to 100% spin polarization at EF caused by these gaps. Additionally, we can observe that each compound’s spin-up and spin-down band structures are not superposed, indicating that they are magnetic, with a value determined by the difference between the spin-up and the spin-down densities (see the density of state).
In both majority- and minority-spin channels, it is noteworthy that, as shown in Fig. 3a–c, three energy bands of p electrons from Si or Ge atoms cover the majority of the energy region between − 6.2 and − 3.8 eV. The s band is well separated from other bands and has a very low energy level, so we do not display it. The Si (Ge) s and p electronic states that give rise to the low-lying bands have the same characteristics in both directions. Si (Ge) atoms give s-p states that may be hybridized with transition metal d electrons to control how occupied the p-d orbitals are. This hybridization affects on the energy gap’s breadth.
According to Galanakis et al. [12], hybridization occurs in the L21 structure, between the Mn 3d orbitals and their second nearest neighbors, Mn 3d orbitals, and between the Mn 3d orbitals and their first nearest neighbors, Hf 5d orbitals or Si (Ge) sp states; the hybridization between these atoms is qualitatively very important. The five d orbitals are divided into two doubly degenerate states, d4 and d5 (z2, x2 − y2), and three triply degenerate states, d1, d2, and d3 (xy, yz, zx) (Fig. 5a). The eg orbitals (the t2g orbitals) can only couples with the eg orbitals (the t2g orbitals) of the other Mn atom forming bonding hybrids, denoted by eg (or t2g) and antibonding orbitals, denoted by eu ( or t1u).
In the second step, we consider the hybridization of the Mn–Mn orbitals with the d orbitals of Hf (Fig. 5b). The doubly degenerate eg orbitals hybridize with the d4 and d5 orbitals of Hf, which transform according to the same irreducible representation of the symmetry group. They create doubly degenerate bonding states (eg), which are very low in energy, and antibonding states unoccupied above the Fermi level. The 3t2g orbitals of Mn couple with d1, d2, and d3 of Hf create six new orbitals, of which three are bonding, and the other three are high energy antibonding. Finally, the two eu and three t1u orbitals of Mn cannot couples with any of the d orbitals of Hf since these do not transform with the u representations, and they are orthogonal to the eu and t1u states of Mn.
Therefore, these states are antibonding concerning the Hf atoms and the Si (or Ge) atoms. The t1u states are occupied below the Fermi level, while the eu states are just above the Fermi level. The absence of hybridization between the eu and t1u states of Mn and the d orbitals of the Hf atoms will result in splitting. The energy gap in the majority spin states is attributed to this eu − t1u difference. Thus, there are eight filled minority d bands.
To further demonstrate the nature of electronic structures, as a function of energy for the lattice constant of Mn2HfSi and Mn2HfGe, and understand the band structure, we have shown the spin-polarized band structures, total density of states (TDOS), and the partial density of states (PDOS) of 3d-Mn, 5d-Hf, and 3p-Si and Ge. According to the approximations of GGA-mBJ as shown in Fig. 6a–b, we have also represented the variation of deg-Mn, dt2g-Mn, deg-Hf, and dt2g-Hf; where the eg states have lower energies than the t2g states in the two compounds. The dashed line represents the Fermi level.
From Fig. 6a and b, the partial DOSs of the 3d orbitals of Mn for the two materials show the same behavior. One can also notice that the DOS around and below EF is mainly associated with the 3d states of Mn, which confirms that the bonding states come mostly from the transition metal of higher valence (the Mn atom), while the partial DOSs of the 5d orbitals of Hf is mostly found above the Fermi level, i.e., the unoccupied antibonding bands are due mainly to the lower valence transition metal (the Hf atom) [13]. According to the partial DOSs, we notice that the gap, at the Fermi level, between the Hf atoms and the sp atoms is somewhat larger than that of the Mn atom. The peaks above EF are due to the t2g states. The actual gap is determined by the Mn–Mn interaction or, more precisely, by the t1u − eu splitting. This also explains why the gaps are relatively small for the Mn2HfSi and Mn2HfGe compounds (Table 4).
It is clear that the majority states in these alloys have no DOS at EF, but the minority states have crossings at the Fermi level, indicating that Mn2HfSi and Mn2HfGe compounds are real HMFIs. The range of energy from − 6 to − 3 eV is related to the p bands of the Si or Ge atoms, as seen in Fig. 6a–b. As can be seen, the partial DOSs of Mn 3d orbitals in the two materials exhibit similar conformation. We see that the bonding states predominately reside at the higher valence transition metal Mn atom and that the DOS around and below EF is connected mainly with the Mn 3d states.
In the inverse XA (Hg2CuTi) types for Mn2HfX (X = Si, Ge) (Fig. 4a-d), we have shown the the spin-polarized band structures total density of states (TDOS) and the partial density of states (PDOS) of 3d-Mn, 5d-Hf, and 3p-Si and Ge according to the approximations mBJ-GGA Fig. 7a–b. According to the last figure, it is clear that the metallic behavior in the two channels of spin has intersections at the Fermi level (EF) in two directions, even though the total magnetic moment per cell remains approximately integer (see Table 4). According to the density morphological results, the electronic structures depend on the atoms’ L21 and XA ordering rather than the GGA or GGA-mBJ method.
3.3 Magnetic Properties
At the equilibrium lattice constants of two compounds, the calculated total magnetic moment is 2.00 µB per unit cell. The HM character of these alloys is revealed by the integer value of the magnetic moment. For Mn2HfSi and Mn2HfGe, Table 4 displays the computed total, atom-resolved magnetic moments, to which is the contribution of the interstitial regions in the unit cell (interstitial moments).
The Mn atoms contribute the majority of the overall magnetic moment. The significant exchange splitting between the majority and minority spin states of the Mn atom is to blame for this. We also observed that the Hf and sp atoms’ partial moments were anti-parallel to the Mn moments in two systems, which results in the ferrimagnetic character.
It is recalled that for full-Heusler alloys, the total magnetic moment of spin satisfies rule 24, which is the analog of the Slater-Pauling rule. In this rule, the total magnetic moment Mtot, per formula unit, is related to the total number Ztot of valence electrons in the unit cell by:
However, for half-metallic in full-Heusler alloys with less than 24 electrons per unit cell, such as the alloys studied in this work, the energy gap is in the majority spin band and not in the minority spin band. In this case, the Slater-Pauling role given by Eq. (3) has been generalized by Skaftouros et al. for inverse Heusler alloys [15], even for full-Heusler alloys containing less than 24 electrons, such as Mn2ZrSi and Mn2ZrGe [16]. Therefore, the appropriate (adequate) Slater-Pauling rule for Mn2HfSi and Mn2HfGe is Mtot = Ztot − 24.
Indeed, the total spin magnetic moment Mtot is just the number of uncompensated spin magnetic moments, so:
Zmaj and Zmin are the numbers of occupied majority and minority spin states, respectively, with Ztot = Zmaj + Zmin. In both, Mn2HfSi and Mn2HfGe, the majority-spin bands are occupied by a total of twelve electrons per unit cell [47, 48]: 1 s, 3p, 2, 3t2g, and 3t1u (see Fig. 3a–c). Then, with Zmaj, the appropriate relation for such compounds is:
According to this rule, the total magnetic moment of Mn2HfSi and Mn2HfGe alloys with 22 valence electrons should be around 2.00 µB. Abada et al. derived the same relationship for Mn2ZrZ (Z = Si, Ge) [16].
Calculations were approved for lattice parameters ranging from 5.6 to 6.3 Å for this determination in phase L21. Figure 8a–b shows the half-metallicity character above the constant lattice values of 5.98 Å and 6.07 Å for Mn2HfSi and Mn2HfGe, respectively. Ferrimagnetic behavior results in both compounds over 5.7–6.2 for Mn2HfSi and 5.75–6.2 for Mn2HfGe. The total magnetic moments for both compounds have integer values. The atoms of Mn have positive magnetic moments, while the two atoms of Hf and Si-Ge have negative magnetic moments. In addition, our compounds have a significant area of half-metallicity, due to their stability at both positive and negative pressures. When the lattice constants are modified in this interval, as we mentioned before, Mn2HfSi and Mn2HfGe keep their half-metallicity. This study demonstrates that the half-metallicity is more stable in constant lattice fluctuation.
Table 4 shows no difference in the magnetic moment between the two mentioned approximations: GGA-mBJ and GGA-PBE, and they give identical values of 2.00 µB. This integer number (Mtot = 2 µB) for total magnetic moment confirms that Mn2HfSi and Mn2HfGe compounds follow the Slater-Pauling rule Mtot = Ztot − 24 [49, 50]. Furthermore, our full-Heusler alloys Mn2HfSi and Mn2HfGe are distinguished by 100% spin polarization at the Fermi level, as determined by a simple calculation based on the polarization rule [51]:
where n↑(EF) and n↓(EF) are the state densities for spins up and down, and before all this, they exhibit a half-metallicity (see Fig. 6a–b). All these results reveal that these compounds are potential materials for spintronic applications. We can see that Mn contributes the majority of the overall magnetic moment, while interstitial magnetic moments (Minter) and sp element (MX) are neglected.
3.4 Mechanical Properties
3.4.1 Elastic Properties
We studied the mechanical stability of the alloys Mn2HfSi and Mn2HfGe to confirm this stability by calculating the elastic constants, which describe many features such as the crystal’s response to applied forces, mechanical properties, structural stability, anisotropy, and bond indexes of solids. The elastic constants for cubic structures are simplified to three independent constants, C11, C12, and C44 because of the symmetry of this system. Due to this system’s symmetry and for cubic structures, the elastic constants C11, C12, and C44 are simplified to three independent constants. So we have calculated the elastic constants C11, C12, and C44 by fitting the total energy of the strained crystal. The results obtained are given in Table 5. The determined elastic constants in the L21 phase are favorable and support the stability criterion, demonstrating the mechanical stability of the two compounds, Mn2HfSi and Mn2HfGe:
Phase L21 (see Table 5) shows the results collected until now.
The structures of Mn2HfSi and Mn2HfGe have positive values for the Cauchy pressure (CP), defined as the difference between C12 and C44 that indicates a ductile nature, while the negative value indicates brittleness. Additionally, the material is described as ductile if the Pugh’s ratio (PR), also known as the B/G ratio, is larger than 1.75 and brittle if the PR is lower than 1.75. [52]. Our PR value demonstrates that Mn2HfSi and Mn2HfGe are ductile. Furthermore, the Poisson ratio (ʋ) confirmed these conclusions. Brittle materials have a value of ʋ ˂ 0.26, while ductile materials have a value of ʋ > 0.26 [53].
3.4.2 Polycrystalline Elastic Moduli
The terms “bulk modulus,” “shear modulus,” and “Young’s modulus” indicate the materials’ resistance to volume change, their tolerance for shape change, and their stiffness, respectively.
We employed the widely known Voigt-Reuss-Hill (VRH) [54]. The isotropic bulk modulus B and the shear modulus G are defined as follows in this approximation:
where V and R subscript denotes Voigt and Reuss bound, respectively.
Young’s modulus is a mathematical term that refers to the modulus of the ratio of linear stress to strain, which is defined as E.
A material with a high B and G value implies a high resistance to the relevant changes, but a stiffer material has a higher E value.
For both compounds, the shear modulus G is lower than the bulk modulus B; therefore, we deduce that they are more resistive to hydrostatic pressure than shear. Young’s E and shear modulus G of Mn2HfSi are larger than Mn2HfGe, so Mn2HfSi is more tensile (stiffer) than Mn2HfGe. Furthermore, a perfect isotropic material has an anisotropic factor A = 1, but any other value of A causes the material to be anisotropic. As a result, the Zener anisotropy factor A is 1.20 for Mn2HfSi and 0.83 for Mn2HfGe. Based on these values, our compounds are closer to the isotropic materials.
Our compounds are ductile, as evidenced by the predicted B/G ratios of 2.71 for Mn2HfSi and 2.76 for Mn2HfGe. In addition, the alloy Mn2HfSi displays a CP of 83.54 GPa and 63.67 GPa for Mn2HfGe. The positive value of this measure validates the alloy’s ductility, resulting in an appropriate B/G ratio. Finally, the Poisson’s ratio is 0.33 for Mn2HfSi and 0.34 for Mn2HfGe. Lewandowski et al. [55] examined a very different class of materials. They concluded that a drastic change in failure behavior correlates with the change in Poisson’s ratio, and the apparent signal change occurs somewhere between ν = 0.26 and ν = 0.31. Higher values of ν signal ductility and lower values signify brittle behavior.
So based on this result, the conclusion, demonstrating that our materials have the behavior of metallic ductility in their equilibrium states, this result shows that these two compounds have a promising future in spintronics, especially in terms of the hardness of the slices.
3.4.3 Debye Temperature
The Debye temperature, which is linked to several physical characteristics of solids, including specific heat, elastic constants, and melting temperature, is a crucial metric in the study of material properties. A more considerable Debye temperature means a bigger bonding strength, and vice versa [56]. From Table 5, one can note that the studied compounds Mn2HfX (X = Si, Ge) possess a relatively low Debye temperature (Figs. 9 and 10). At low temperatures (below ΘD), when T < ΘD, only low-frequency vibrations are excited [57, 58]. That is what we can see in the phonon dispersion curves (Fig. 11a–b), whereas at higher temperatures (above ΘD), the movement is more disordered, and all vibration modes are excited.
Based on the calculated density and elastic constants data, we can calculate the Debye temperature ΘD using the following formula [59]:
h is the Planck constant, k is Boltzmann’s constant, n is the number of atoms in the molecule, NA is the Avogadro number, ρ is the density, M is the molar mass, and vm is the average sound velocity, which can be calculated as follows:
Here, vt and vl are the transverse and longitudinal elastic wave velocity, respectively, obtained using the bulk modulus B and the shear modulus G in the following equations:
3.4.4 Anisotropy of Mechanical Behavior
To illustrate the anisotropy of the polycrystalline bulk (B) and Young modulus (E) is to plot them on the two-dimensional surface (and/or three-dimensional) as a function of direction. We plotted Young’s (E) and bulk (B) modulus in different directions using spherical coordinates for Mn2HfX (X = Si, Ge). Regarding the cubic crystal class, the directional dependence of Young modulus (E) and bulk modulus (B) can be expressed as follows [60]:
In the equations above, Sij represents the compliance matrix, and l1, l2, and l3 are the direction cosines, which are given as l1 = sinθ cosϕ, l2 = sinθ sinϕ, and l3 = cosθ in the spherical coordinates.
2D directional dependence would give rise to a circle shape for an isotropic system, which we see for the bulk and Young modulus in the two compounds Mn2HfX (X = Si, Ge), except for the Young’s modulus for Mn2HfSi, which present a small degree of anisotropy due to the deviation degree from the circle shape.
Since the system is cubic, the 3D illustration plot and the projections on XY (XY = XZ = YZ) planes of the bulk and Young’s modulus are shown in Figs. 9a–d and 10a–b. This figure shows that along the X-, Y-, and Z-axis, the compression is the same for all studied compounds. For Young’s modulus, we note that the maximum values occur when the stress is applied along the crystallographic bisector direction axe in the XY coordinate plane. In contrast, the minimum values are realized when the stress is applied along the X (X = Y = Z) crystallographic axe.
3.5 Dynamical Properties
Finally, to ensure the stability of Mn2HfX (X = Si and Ge) compounds in the cubic structure L21- with prototype Cu2MnAl, we need to compute the dynamic stability through the phonon dispersion curves. So the characteristics of phonons have significant importance in studying crystalline materials; they show the material’s response to atomic vibration. Various physical properties of materials can be determined directly or indirectly from the phonon dispersion spectra and phonon DOS [22]. With the help of phonon dispersion spectra (PDS), the structural stability, the phase transition, and the contribution of vibrations in a material’s thermal and charge transport properties can be explained [23]. The phonon dispersion curves of Mn2HfX (X = Si and Ge) in the ground state have been calculated by the pseudo-potential-based Quantum Espresso package within the framework of GGA-PBE [5].
Unlike the supercell method, the approach used to calculate the phonon dispersion is based on linear response theory. This fact is used in the linear response density functional calculations to construct the dynamical matrix using Density-Functional Perturbation Theory (DFPT) [61,62,63,64,65,66,67,68,69], which gives access to phonon frequency, group velocity, and phonon interaction.
The phonon dispersion curves (PDC) of Mn2HfX (X = Si and Ge) along the high symmetry directions of the Brillouin zone (BZ) at zero pressure are illustrated in Fig. 11a–b. It can be seen that they are no imaginary modes, which means no negative frequency at the gamma point in PDC. However, the PDC of Mn2HfX (X = Si and Ge) exhibits positive phonon frequencies in the Brillouin zone’s whole region, indicating that the two structures are dynamically stable. The acoustic modes are null at point G in these two compounds and up to 100 cm−1 at point W. There is a clear spectral gap between the acoustic and optical phonon branches in the PDC of Mn2HfX (X = Si and Ge). The formation of this gap is essentially due to the difference in the mass of atoms. Indeed, the phonon vibrational frequencies have a dependence in \(\sqrt{\frac{k}{m}}\), k is the force constants, and m is the masses of atoms. The optical phonons of Mn2HfSi are coupled, on the contrary for Mn2HfGe. So our materials under investigation are dynamically stable.
4 Conclusion
In conclusion, the first-principles research of the new full-Heusler alloys Mn2HfSi and Mn2HfGe was carried out, using GGA and GGA-mBJ in the Density Functional Theory with the code Wien2k. The Cu2MnAl-type and Hg2CuTi-type alloys’ energies in the NM and FM states were calculated using the L21 and XA structures, respectively. We discovered that these compounds are stable with a spin polarization of 100% at EF in the AlCu2Mn-type with L21 structure. Furthermore, the optimized lattice constants in the ideal structure L21 for Mn2HfSi and Mn2HfGe are predicted to be 5.98 Å and 6.07 Å. Additionally, the partial moments of Hf and Si-Ge atoms in two alloys have negative values. They are anti-parallel to the positive moments of Mn atoms, confirming that our compounds, Mn2HfSi and Mn2HfGe, are HMFIs with indirect band gaps that are, respectively, 0.689 eV and 0.520 eV in the majority-spin channel. A total magnetic moment is an integer number of 2.00 µB per formula unit, which follows the Slater-Pauling rule of Mtot = Ztot − 24. It is confirmed that there is no change in magnetic moment between the two specified approximations, GGA-mBJ and GGA-PBE. The half-metallicity of these structures is maintained for lattice constants in the range of 5.70–6.20 Å for Mn2HfSi and 5.75–6.20 Å for Mn2HfGe, respectively, resulting in stability against constant lattice changes due to the vast half-metallicity area in these compounds. According to the elastic constant calculated anisotropic properties, both materials, Mn2HfSi and Mn2HfGe, are almost isotropic.
Data Availability
We confirm that we know the research data policy, and the data are available in this paper and on a request from the corresponding author.
References
Kieven, D., Klenk, R., Naghavi, S., Felser, C., Gruhn, T.: I-II-V half-Heusler compounds for optoelectronics: ab initio calculations. Phys. Rev. B 81, 075208 (2010)
De Boeck, J., Van Roy, W., Das, J., Motsnyi, V., Liu, Z., Lagae, L., Boeve, H., Dessein, K., Borghs, G.: Technology and materials issues in semiconductor-based magnetoelectronics. Semicon. Sci. Technol. 17, 342 (2002)
Kimura, Y., Tamura, Y., Kita, T.: Thermoelectric properties of directionally solidified half-Heusler compound NbCoSn alloys. Appl. Phys. Lett. 92, 012105 (2008)
Jin, Y., Kharel, P., Valloppilly, S., Li, X.-Z., Kim, D., Zhao, G., Chen, T., Choudhary, R., Kashyap, A., Skomski, R.: Half-metallicity in highly L21-ordered CoFeCrAl thin films Appl. Phys. Lett. 109, 142410 (2016)
Wolf, S., Awschalom, D., Buhrman, R., Daughton, J., von Molnár, V.S., Roukes, M., Chtchelkanova, A.Y., Treger, D.: Spintronics: a spin-based electronics vision for the future. Science 294, 1488–95 (2021)
Wang, S., Pratama, F., Ukhtary, M.S., Saito, R.: Independent degrees of freedom in two-dimensional materials. Phys. Rev. B 101, 081414 (2020)
Wang, S.-K., Tian, H.-Y., Yang, Y.-H., Wang, J.: Spin and valley half metal induced by staggered potential and magnetization in silicene. Chin. Phys. B 23, 017203 (2013)
Wang, S., Yu, J.: Magnetic behaviors of 3d transition metal-doped silicane: a first-principle study. J. Superconduct. Nov. Magnet. 31, 2789–95 (2018)
Prinz, G.A.: Magnetoelectron. Sci. 282, 1660–1663 (1998)
Chadov, S., Graf, T., Chadova, K., Dai, X., Casper, F., Fecher, G.H., Felser, C.: Efficient spin injector scheme based on Heusler materials. Phys. Rev. Lett. 107, 047202 (2011)
Faleev, S.V., Ferrante, Y., Jeong, J., Samant, M.G., Jones, B., Parkin, S.S.: Unified explanation of chemical ordering, the Slater-Pauling rule, and half-metallicity in full Heusler compounds. Phys. Rev. B 95, 045140 (2017)
Galanakis, I., Dederichs, P., Papanikolaou, N.: Slater-Pauling behavior and origin of the half-metallicity of the full-Heusler alloys. Phys. Rev. B 66, 174429 (2002)
Galanakis, I., Dederichs, P., Papanikolaou, N.: Origin and properties of the gap in the half-ferromagnetic Heusler alloys. Phys. Rev. B 66, 134428 (2002)
Pickett, W.E., Moodera, J.S.: Half metallic magnets. Physi. Today 54, 39–45 (2001)
Skaftouros, S., Özdoğan, K., Şaşıoğlu, E., Galanakis, I.: Generalized Slater-Pauling rule for the inverse Heusler compounds. Phys. Rev. B 87, 024420 (2013)
Abada, A., Amara, K., Hiadsi, S., Amrani, B.: First principles study of a new half-metallic ferrimagnets Mn2-based full Heusler compounds: Mn2ZrSi and Mn2ZrGe. J. Magnet. Magnetic Mater. 388, 59–67 (2015)
Meinert, M., Schmalhorst, J.-M., Reiss, G.: Ab initio prediction of ferrimagnetism, exchange interactions and Curie temperatures in Mn2TiZ Heusler compounds. J. Phys.: Condens. Matter 23, 036001 (2010)
Gasi, T., Nayak, A.K., Winterlik, J., Ksenofontov, V., Adler, P., Nicklas, M., Felser, C.: Exchange-spring like magnetic behavior of the tetragonal Heusler compound Mn2FeGa as a candidate for spin-transfer torque. Appl. Phys. Lett. 102, 202402 (2013)
Meinert, M., Schmalhorst, J.-M., Reiss, G.: Exchange interactions and Curie temperatures of Mn2CoZ compounds. J. Phys.: Condens. Matter 23, 116005 (2011)
Kubota, T., Kodama, K., Nakamura, T., Sakuraba, Y., Oogane, M., Takanashi, K., Ando, Y.: Ferrimagnetism in epitaxially grown Mn 2 VAl Heusler alloy investigated by means of soft x-ray magnetic circular dichroism. Appl. Phys. Lett. 95, 222503 (2009)
Özdogan, K., Galanakis, I., Şaşioglu, E., Aktaş, B.: Search for half-metallic ferrimagnetism in V-based Heusler alloys Mn2VZ (Z= Al, Ga, In, Si, Ge, Sn). J. Phys.: Condens. Matter 18, 2905 (2006)
Luo, H., Zhu, Z., Liu, G., Xu, S., Wu, G., Liu, H., Qu, J., Li, Y.: Prediction of half-metallic properties for the Heusler alloys Mn2CrZ (Z= Al, Ga, Si, Ge, Sb): a first-principles study. J. Magnet. Magnetic Mater. 320, 421–8 (2008)
Li, S., Ren, Z., Zhang, X., Cao, C.: Electronic structure and magnetism of Mn2CuAl: a first-principles study. Phys. B: Condens. Matter 404, 1965–8 (2009)
Wei, X.-P., Hu, X.-R., Mao, G.-Y., Chu, S.-B., Lei, T., Hu, L.-B., Deng, J.-B.: Half-metallic ferrimagnetism in Mn2CuGe. J. Magnet. Magnetic Mater. 322, 3204–7 (2010)
Liu, L., Hu, L., Liu S., Xiong, J., Liao, Q., Wen, Y.: First‐principles investigations on the ground‐state bulk properties and lattice constant dependent half‐metallic ferrimagnetism of Mn2NbSi full‐Heusler compound. Int. J. Quant. Chem. 121, e26566 (2021)
Benaddi, F., Belkharroubi, F., Ramdani, N., Ameri, M., Haouari, S., Ameri, I., Drici, L., Azzi, S., Al-Douri, Y.: Electronic and magnetic investigation of half-metallic ferrimagnetic full-Heusler Mn2IrGe. Emerg. Mater. 4, 1745–60 (2021)
Ram, M., Saxena, A., Aly, A.E., Shankar, A.: Half-metallicity in new Heusler alloys Mn 2 ScZ (Z= Si, Ge, Sn). RSC Adv. 10, 7661–70 (2020)
Ram, M., Saxena, A., Aly, A.E., Shankar, A.: Study of half metallicity, structural and mechanical properties in inverse Heusler alloy Mn 2 ZnSi (1− x) Ge x and a superlattice. RSC Adv. 9, 36680–9 (2019)
Sokolovskiy, V.V., Zagrebin, M.A., Sokolovskaya, Y., Buchelnikov, V.D.: Structural and magnetic properties of Mn2NiZ (Z= Ga, In, Sn, Sb) Heusler alloys from ab initio calculations. In: Solid State Phenomena: Trans Tech Publ) pp 229–32 (2015)
Jiang, D., Ye, Y., Yao, W., Zeng, D., Zhou, J., Ruan, W., Wen, Y.: First-principles predictions on half-metallic, mechanical, and acoustic properties of CuHg2Ti-type Mn2LiZ (Z= As, Sb) compounds. J. Superconduct. Nov. Magnet. 33, 1065–72 (2020)
Yousuf, S., Gupta, D.: Robustness in spin polarization and thermoelectricity in newly tailored Mn2-based Heusler alloys. Indian J. Phys. 92, 855–64 (2018)
Wan, H., Yao, W., Zeng, D., Zhou, J., Ruan, W., Liu, L., Wen, Y.: Structural, elastic, electronic, and magnetic properties of a new full-Heusler alloy Mn2MgGe: first-principles calculations. J. Superconduct. Nov. Magnet. 32, 3001–8 (2019)
Maizia, A., Belkharroubi, F., Bourdim, M., Khelfaoui, F., Azzi, S., Amara, K.: First-principles study of a half-metallic ferrimagnetic new full-Heusler Mn2OsGe alloy. In: Spin: World Scientific) p 2050026 (2020)
Blaha, P., Schwarz, K., Madsen, G.K., Kvasnicka, D., Luitz, J.: wien2k An augmented plane wave+ local orbitals program for calculating crystal properties 60, (2001)
Perdew, J.P., Burke, K., Ernzerhof, M.: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996)
Perdew, J.P., Burke, K., Wang, Y.: Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 54, 16533 (1996)
Tran, F., Blaha, P.: Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 102, 226401 (2009)
Giannozzi, P., Andreussi, O., Brumme, T., Bunau, O., Nardelli, M.B., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Cococcioni, M.: Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys.: Condens. Matter 29, 465901 (2017)
Murnaghan, F.D.: The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. 30, 244–247 (1944)
Zhou, F., Liu, Y., Wang, J., Kuang, M., Yang, T., Chen, H., Wang, X., Cheng, Z.: Intersecting topological nodal ring and nodal wall states in superhard superconductor FeB 4. Phys. Rev. Mater. 5, 074201 (2021)
Yang, T., Jin, L., Liu, Y., Zhang, X., Wang, X.: Spin-polarized type-II nodal loop and nodal surface states in hexagonal compounds X TiO 2 (X= Li, Na, K, Rb). Phys. Rev. B 103, 235140 (2021)
Zhou, F., Cui, C., Wang, J., Kuang, M., Yang, T., Yu, Z.-M., Wang, X., Zhang, G., Cheng, Z.: Perovskite-type YRh 3 B with multiple types of nodal point and nodal line states. Phys. Rev. B 103, 245126 (2021)
Wang, J., Yuan, H., Liu, Y., Wang, X., Zhang, G.: Multiple dimensions of spin-gapless semiconducting states in tetragonal Sr 2 CuF 6. Phys. Rev. B 106, L060407 (2022)
Wang, S., Ren, C., Li, Y., Tian, H., Lu, W., Sun, M.: Spin and valley filter across line defect in silicene. Appl. Phys. Express 11, 053004 (2018)
Wang, S., Zhang, P., Ren, C., Tian, H., Pang, J., Song, C., Sun, M.: Valley hall effect and magnetic moment in magnetized silicene. J. Superconduct. Nov. Magnet. 32, 2947–2957 (2019)
Galanakis, I., Mavropoulos, P.: Spin-polarization and electronic properties of half-metallic Heusler alloys calculated from first principles. J. Phys.: Condens. Matter 19, 315213 (2007)
Faid, F., Elchikh, M., Bahlouli, S., Kaddar, K.: Strong correlation effect in ferrimagnetic half-metallic v2CoAl and V2CoGa Heusler compounds. J. Superconduct. Nov. Magnet. 31, 2491–500 (2018)
Galanakis, I., Mavropoulos, P., Dederichs, P.H.: Electronic structure and Slater-Pauling behaviour in half-metallic Heusler alloys calculated from first principles. J. Phys. D Appl. Phys. 39, 765 (2006)
Slater, J.C.: Wave functions in a periodic potential Physical Review 51, 846 (1937)
Pauling, L.: The nature of the interatomic forces in metals Physical Review 54, 899 (1938)
Rai, D., Shankar, A., Ghimire, M., Thapa, R.: Electronic and magnetic properties of a full-Heusler alloy Co2CrGe: a first-principles study. J. Theor. Appl. Phys. 7, 1–6 (2013)
Pugh, S.: XCII Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. The London, Edinburgh, and Dublin Philos. Mag. J. Sci. 45 823–43 (1954)
Frantsevich, I.N.: Elastic constants and elastic moduli of metals and insulators Reference book (1982)
Hill, R.: The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 65, 349 (1952)
Lewandowski, J., Wang, W., Greer, A.: Intrinsic plasticity or brittleness of metallic glasses. Philos. Mag. Lett. 85, 77–87 (2005)
Yan, J., Shou, H., Peng, M., Duan, Y., Cao, Y.: The anisotropic properties and Debye temperatures of Ti-Zn compounds: a first-principles calculation Materials Research Express 6, 116528 (2019)
Alarco, J.A., Mackinnon, I.D.: Phonon dispersions as indicators of dynamic symmetry reduction in superconductors Phonons in Low Dimensional Structures, pp. 75–101. InTech Open, London UK (2018)
Feng, D., Jin, G.: Introduction To Condensed Matter Physics, Volume 1: World Scientific Publishing Company) (2005)
Gueddouh, A.: Magnetic moment collapse induced by high-pressure in semi-borides TM2B (TM= Fe, Co). A first-principles study Chinese Journal of Physics 56, 944–957 (2018)
Rougab, M., Gueddouh, A.: Light doping effects of rare-earth elements: Sc, Y, La and Lu in rockSalt AlN—first-principles study. Appl. Phys. A 127, 1–11 (2021)
Baroni, S., De Gironcoli, S., Dal Corso, A., Giannozzi, P.: Phonons and related crystal properties from density-functional perturbation theory Reviews of modern physics 73, 515 (2001)
Yang, T., Xie, C., Chen, H., Wang, X., Zhang, G.: Phononic nodal points with quadratic dispersion and multifold degeneracy in the cubic compound Ta 3 Sn . Phys. Rev. B 105, 094310 (2022)
Xie, C., Yuan, H., Liu, Y., Wang, X.: Two-nodal surface phonons in solid-state materials. Phys. Rev. B 105, 054307 (2022)
Ding, G., Zhou, F., Zhang, Z., Yu, Z.-M., Wang, X.: Charge-two Weyl phonons with type-III dispersion. Phys. Rev. B 105, 134303 (2022)
Zhong, M., Han, Y., Wang, J., Liu, Y., Wang, X., Zhang, G.: Material realization of double-Weyl phonons and phononic double-helicoid surface arcs with P 2 1 3 space group. Phys. Rev. Mater. 6, 084201 (2022)
Zhou, F., Chen, H., Yu, Z.-M., Zhang, Z., Wang, X.: Realistic cesium fluogermanate: an ideal platform to realize the topologically nodal-box and nodal-chain phonons. Phys. Rev. B 104, 214310 (2021)
Wang, J., Yuan, H., Kuang, M., Yang, T., Yu, Z.-M., Zhang, Z., Wang, X.: Coexistence of zero-, one-, and two-dimensional degeneracy in tetragonal SnO 2 phonons . Phys. Rev. B 104, L041107 (2021)
Zhou, F., Zhang, Z., Chen, H., Kuang, M., Yang, T., Wang, X.: Hybrid-type nodal ring phonons and coexistence of higher-order quadratic nodal line phonons in an AgZr alloy. Phys. Rev. B 104, 174108 (2021)
Zhong, M., Liu, Y., Zhou, F., Kuang, M., Yang, T., Wang, X., Zhang, G.: Coexistence of phononic sixfold, fourfold, and threefold excitations in the ternary antimonide Zr 3 Ni 3 Sb 4. Phys. Rev. B 104, 085118 (2021)
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to the paper as follows:
Mohamed Lamine Belkhir: conceptualization, data collection and design, and investigation. Ahmed Gueddouh: writing—original draft, supervision, investigation, resources, writing—review and editing, and validation. Fares Faid: supervision and validation. Mourad Rougab: design and investigation.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belkhir, M.L., Gueddouh, A., Faid, F. et al. The Structural, Electronic, Magnetic, Mechanical, and Lattice Dynamical Properties of the Novel Full-Heusler Alloys Mn2HfX (X = Si and Ge): Ab Initio Study. J Supercond Nov Magn 36, 131–146 (2023). https://doi.org/10.1007/s10948-022-06431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-022-06431-1