Abstract
The iron (Fe) diffusion in superconducting MgB2 bulk samples has been studied for sintering time durations of 15 min, 30 min, 1 h, 2 h, and 4 h at 900∘C. Fe coating bulk polycrstalline superconducting MgB2 samples for Fe coating were prepared by pelletizing and used in the diffusion experiments with initial sintering at 800∘C for 1 h. A thin layer of Fe was coated on MgB2 pellets by evaporation in vacuum. Effects of Fe diffusion on the structural, electrical, and superconducting properties of MgB2 have been investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), infrared spectroscopy (IR), energy-dispersive X-ray spectroscopy (EDS), and resistivity measurements. Fe diffused samples have slightly increased critical transition temperatures and have larger lattice parameter c values, in comparison with bare samples. Fe diffusion coefficients were calculated from depth profiles of c parameter and room temperature resistivity values. Depth profiles were obtained by successive removal of thin layers from Fe diffused surfaces of the samples. Our results have shown that the Fe diffusion coefficient decreases with increasing sintering time and resistivity measurements can be utilized for determination of diffusion coefficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
MgB2 superconductors has relatively higher critical temperature (T c) compared to other binary superconductors [1] and possibility of their technological applications at temperatures in the range of 20–30 K is rapidly increasing. Diffusion process is important to elucidate the superconducting mechanism and to produce materials with better superconducting properties.
The diffusion technique opens new possibilities of preparation of superconductors with improved properties. Experimental data on the diffusion parameters are useful to control the development of crystalline structure, resistivity at room temperature, and the properties of superconductors [2, 3]. The diffusion is used in processing of superconductors in two essential ways; (i) mass transport during sintering process which results in consolidation and (ii) diffusion between superconductors and sheaths or substrates [4]. In the literature, Fe diffusion in high temperature superconductors has been reported with calculations of diffusion coefficient [5,6,7], but very limited data is present on the diffusion coefficient of Fe in MgB2 bulk samples. Altin and coworkers have studied the MgB2 fabrication by diffusion-controlled three-layered (B-Mg-B) technique [8]. They determined the diffusion coefficient using Fick’s law and EDX data and they have reported the coefficient diffusion of B in Mg matrix and that of Mg in B matrix was calculated as 1.66 × 10−7 and 3.14 × 10−8 cm2 s −1, respectively. The highest T c value (39.4 K) of MgB2 material was achieved at 800∘C/1 h. According to Terzioglu and his coworkers [9], microstructural, superconducting, and mechanical properties of the Cu diffused bulk MgB2 superconductors depend strongly on the annealing time, and they have calculated the maximum diffusion coefficient to be about 2.58 × 10−7 cm2/s.
Samuely et al. studied electron doping and scattering effects via aluminum and carbon substitution in MgB2. They have proved that, the decline in diffusion coefficient at the π indicates in the carbon doping, while the Al substitution leaves the sample in the clean limit [10].
The powder in tube method is generally used to fabricate MgB2 wires in various metal tubes including soft iron or stainless steel [11,12,13,14]. When Fe or stainless steel sheathing material comes into contact with superconducting MgB2 core, Fe diffusion becomes an important issue. In order to deal with this problem, barrier material is placed between MgB2 and metal sheath [15, 16]. Nevertheless, the characterization of the interaction between metal sheath and MgB2 core is not well illustrated.
In this paper, we have studied the effect of Fe diffusion on the microstructural and superconducting properties of MgB2 bulk superconductors. Moreover, depth profiles of the lattice parameter c and room temperature resistivity (ρ 300 K) enabled us to find the diffusion coefficient (D) of Fe in MgB2 and to study time dependence of diffusion for the first time.
2 Experimental Details
All MgB2 bulk samples were fabricated by standard solid state reaction technique using ex situ powders with %99.95 purity and these powders were further ball-milled in argon (Ar) for 3 h to obtain better homogeneity. A number of bulk rectangular bar samples with dimensions of 25 × 4 × 2 mm3 were pelletized in a hardened steel die under a pressure of 400 MPa at room temperature. Coating is applied on bulk pellets by evaporation of Fe metal using EDWARDS-AUTO 306 in 10−4 Pa vacuum. A thin coating layer of Fe was deposited by the process of Fe evaporation only on one of the wide faces (25 × 4 mm2) of the pelletized samples. For each evaporation processes, a 15-cm long iron wire with a diameter of 0.1 mm (0.01 g) was used (Alfa Easer, purity 99.99%). All sintering process were carried out under 5–10 bar Ar pressures in a tube furnace for 15 min, 30 min, 1 h, 2 h, and 4 h at 900∘C. The heating and cooling rates for every sintering process were adjusted to be 5∘C/minute.
X-ray powder diffraction patterns of these samples were obtained with a Rigaku Multiflex + XRD using CuK α radiation (λ = 1.5418 Å) in the range 2𝜃 = 10 − 90∘ at a scan speed of 5∘/minutes and step increment of 0.02∘ at room temperature. Determination of the chemical phases in the samples and lattice parameters of MgB2 were achieved using the XRD patterns.
Infrared spectroscopy (Perkin Elmer Spectrum Two) is a very useful instrument that principally evaluate the individual frequencies of atomic bonds between 1012 and 1013 Hz. We executed IR spectroscopy measurements on Fe diffused bulk samples for detecting impurities and indicating presence of iron borides in Fe coated/diffused samples.
DC-electrical resistivity of the prepared materials was carried out by a standard four-probe technique. High conductivity silver paint is used for the contacts and R-T curves of the samples were measured in a closed cycle cryostat in the temperature range from 10 to 45 K. A Keithley 220 programmable current source and a Keithley 2182A nanovoltmeter system were used. All the data are recorded by Labview software. \(T_{\mathrm {c}}^{\text {offset}}\) and \(T_{\mathrm {c}}^{\text {onset}}\) are defined as zero resistivity temperature and the temperature at which resistivity starts to decline, respectively. T c is determined by the maximum of the first derivative of resistivity as a function of temperature, and the ρ 300 K values of all samples were calculated from I–V curves measured at room temperature.
Further, the surface morphology and grain connectivity of the bare and Fe coated samples are analyzed by SEM (JEOL 6390-LV), operated at 20 kV. The elemental compositions and distributions in the samples are investigated using EDS.
The ρ 300 K of Fe coated samples were measured by four-probe dc resistivity technique applying 1.5 N weight to ensure a firm contact. Use of silver paint was avoided not to affect resistivity measurements since silver paint would be absorbed by the bulk of MgB2 samples. These measurements were performed by successive removals of thin layers (∼25 μ m) from the Fe coated/diffused surface of the sample.
Removal of thin layers form Fe diffused surface was achieved by rubbing against an unused fine grinding paper (P2000) under 1.5 N constant weight. Removals of thin layers were done in 8 steps corresponding to a depth of 200 μ m. XRD and ρ 300 K were made after each step. Ground surface was cleaned after every grinding process with pressurized air stream before XRD and resistivity measurements to eliminate the effect of loose particles coming from MgB2 or sandpaper.
The Fe coated MgB2 bulk samples were sintered for durations of 15 min, 30 min, 1 h, 2 h, and 4 h at 900∘C, herein after denoted as SF 1590, SF 3090, SF 190, SF 290, and SF 490, respectively. Likewise, the bare samples sintered at 900∘C for these same durations are presented as SP 1590, SP 3090, SP 190, SP 290, and SP 490.
3 Result and Discussion
3.1 XRD Analyses
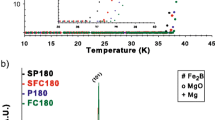
Figure 1a illustrates the XRD patterns between two theta angles of 10∘ and 90∘ for non-sintered MgB2 ex situ powder (black line), sintered MgB2 pellet (red line), Fe coated — not sintered MgB2 (blue line), and Fe coated — sintered MgB2 (green line) samples. Although a considerable amount of free Mg element is detected together with MgB2 phase in MgB2 ex situ powder, we have observed barely noticeable amount of MgO phase before heat treatment.
Intensity of the XRD peaks belonging to MgO phase is increased upon sintering process. XRD patterns of Fe coated — not sintered MgB2 sample has a peak belonging to Fe, while Fe coated — sintered MgB2 samples contain either Fe2B or FeB peak. We can say that the intensity of the Fe2B increases with sintering process and B atoms, either un-reacted or allocated from the structure of MgB2, can form compound with Fe.
Figure 1b gives the XRD patterns for the Fe coated MgB2 and bare (SB 1590) samples prepared in this work. Miller indices belonging to MgB2 phase and peaks belonging to MgO and Fe2B phases are indicated in these patterns, which is in general agreement with other reports [17, 18]. We used the Debye-Scherrer method to calculate lattice parameters (a and c) of all pellets and the values of lattice parameter c are tabulated in Table 1.
The lattice parameter a of coated and bare samples sintered for all times are approximately around 3.08 Å with some small arbitrary variations; on the other hand, the lattice parameter c of these samples varies between 3.5220 and 3.5483 Å, Fe-coated samples having larger c parameter. It is most likely that Fe atoms migrate into MgB2 through defects [19] and replace Mg atoms in the structure causing c parameter to enlarge. There is also a possibility that Fe atoms may occupy interstitial positions, if the geometry of MgB2 unit cell and the diameter of Fe ions are considered [20].
The crystallite size of the samples is found to be within the angstrom scale by using the Scherrer equation [21, 22] is
The average size of a crystal in the broadening region is defined as (1), where d is the thickness of the crystal, k is a constant 0.941 (dimensionless shape factor), β is the full width at half maximum (FWHM) of the Bragg peak corrected using the corresponding peak in micron-sized powder and 𝜃 0 is the angular position of the peak maximum. Also, Warren formula [23] is
where β reference denotes the half width of the standard material in radians and grain size values are displayed in Table 1. It is found that the Fe-coated samples sintered at 900∘C for 4 h had largest grain size (302.08 Å) and the differences between Fe coated and bare samples are apparent. This indicates the suitability of higher time sintering for obtaining better crystallinity with larger grains but this not desirable for applications due to weaker flux pinning. Furthermore, grain size of Fe-coated MgB2 material compared to bare ones is larger indicating that Fe diffusion causes grain size to increase.
The FWHM values decrease with increasing annealing time from 15 min to 4 h at 900∘C, as seen Table 1. On the other hand, the sample SB1590 has highest value of FWHM (0.404∘) and smallest grain size value of about 199.51 Å giving evidence for strain effects. This is probably due to insufficient sintering with too short sintering duration of 15 min.
3.2 Infrared Spectroscopy Analyses (IR)
To support the result of XRD, samples were examined by infrared spectroscopy the results of which can be seen in Fig. 2 where MgB2 ex situ powder (black line), pellet (red line), Fe coated but not sintered MgB2 (blue line), and Fe coated — sintered MgB2 (green line) represent curves of IR spectrum. According to the result of IR, it is found that there is not any significant difference in spectrums of sintered/nonsintered MgB2 bare (no Fe coating) samples and also Fe coated — nonsintered MgB2 sample, while three quite weak broad band are determined at 1100, 1300, and 1700 cm−1 for the Fe coated and sintered MgB2 pellet. It is known that the interaction between Fe and B substantiates between 1000 and 1700 cm−1 for studies related with FeB and Fe2B [24, 25] and the frequency of 400 cm−1 would be preferable to make measurements with better sensitivity.
3.3 Electrical Resistivity Measurements
To investigate the effect of Fe coating on the superconducting properties of MgB2 samples, we performed electrical resistivity measurements in the temperature range of 10–45 K. Figure 3a, b shows the resistivity variation with temperature for different sintering time durations. The insets magnify the resistive transition region, in order to show more details.
The \(T_{\mathrm {c}}^{\text {offset}}\), \(T_{\mathrm {c}}^{\text {onset}}\), T c, and 300 K resistivity of SB and SF groups given in Table 2. A slight increase in T c is observed due to Fe coating, as given Fig. 3b, SF1590 sample had highest critical temperature value (T c = 38.56 K). On the other hand, SB190 and SF490 samples have lower T c values of 37.04 and 37.31 K, respectively.
Normal state resistivity just above the transition is higher for Fe-coated samples, considering that the resistivity of iron at 40 K is lower than bare MgB2 samples, higher resistivity in Fe-coated samples is seemingly due to chemical interactions between Fe and MgB2 superconductor.
Improvement in T c upon Fe coating in our experiments is an important result since 10% Fe doping causes T c reduction [26]. Processing parameters, such as sintering temperature, sintering time, coated material, and chemical interaction at the interface may also affect the T c and \(T_{\mathrm {c}}^{\text {offset}}\) values. Critical temperatures of Fe diffused samples are higher than those of the bare samples, when all other processing parameters are same. \(T_{\mathrm {c}}^{\text {offset}}\) and T c values of Fe-coated samples are higher and the difference widens up to 2 h sintering duration then disappears (as seen Fig. 3b), while \(T_{\mathrm {c}}^{\text {onset}}\) of coated and bare samples sintered for all times are approximately around 39.2 ± 0.5 K. Transition width widens sharply with increasing sintering time for bare samples up to 2 h then surprisingly shrinks while Fe-coated/diffused samples almost remain constant up to 2 h of sintrering then becomes twice as large even larger than the transition width of the bare sample (SB 490).
Fe-diffused MgB2 samples have higher T c and ρ 300 K which can be explained by noting the role of diffused Fe atoms into MgB2 grains. As given in Table 2, Fe-coated samples have higher room temperature resistivity compared to bare samples. When the annealing time is increased from 15 min to 4 h ρ 300 K value of bare and coated samples increases from 39.8 to 63.3 μΩcm and 45.6 – 95.4 μΩcm, respectively. This increase may be attributed to the modified intra-grain and inter-grain properties. Behaviour of the bare and Fe-coated/diffused MgB2 samples may be explained by the improvement of crystallinity with increasing the annealing time, resulting in increasing grain size [27]. Additionally, this improvement might be due to coupling between the Fe-coated superconducting grains [28] and/or Fe-stimulated grain growth through partial melting of MgB2. When the sintering time is very long (8 h — results are not presented), both physical and superconducting properties of bare (SB) and coated (SF) samples deteriorate.
3.4 SEM and EDS Analyses
The surface morphology of the bare and Fe-coated samples annealed for different sintering times (only 15 min, 1 h, 4 h at 900∘C) were examined by SEM and the surface micrographs are displayed in Fig. 4. According to the results of the SEM investigation, it is found that the surface morphology, grain connectivity, and grain size of the Fe-coated samples improve with increasing sintering time up to 4 h. The improvement of crystallinity is associated with better connectivity of the MgB2 grains.
The microstructure of Fe-coated surface is remarkably different from that of bare samples which may be due to prevention of Mg loss from the outer surface during sintering because of Fe layer. Also, the grain structure and morphology seem to be different in Fe-coated samples, the grains have merged into big clusters. Therefore, based on the SEM observations, it seems that the Fe-coated samples are denser and have better connected grains than the bare ones. As the sintering time increases, the grain size increases (Table 1) and surface of the sample becomes smoother.
Figure 5 shows the EDS results of Fe-coated and bare-MgB2 bulk samples of SB 1590 and SF 1590. EDS analysis were done to obtain the chemical composition at the surface of the Fe-coated samples. The total Mg, B, Fe, and O contents in samples were determined by EDS spectrum. For this, analysis of SB 1590 sample was found to have 7.7 wt% oxygen content, and SF 1590 10.9 wt% oxygen content. These results show that Fe coating cause higher oxygen content least at the surface.
3.5 Calculation of Diffusion Coefficient
The active diffusivity may be presented by the diffusion in condensed matter and the pore space of porous media [29, 30]. The most common methods for studying diffusion in superconductors/semiconductors are measurement of lattice parameters from XRD patterns and EDXRF method [31, 32]. In this study, we have employed XRD and room temperature resistivity methods to determine the diffusion coefficient of Fe into MgB2 superconductor.
We studied depth profile for the changes in these physical quantities by successive removal of thin layers of about 25 μ m from Fe diffused surface of the samples. The change of c parameter with thickness of the samples resembles the concentration distribution of diffused Fe impurity. At these considerations, the change in lattice parameter c is studied by XRD measurements. The data about the concentration profile of the diffusing impurity in the Fe-diffused sample were found by measuring the distribution of deformation (Δc/c 0) over a certain thickness of the samples. Δc is the change in the value of lattice parameter c with respect to each step, that is the c parameter difference between Fe-coated sample and cparameter value of uncoated sample with same property (prepared under same conditions with same dimensions), and c 0 is lattice parameter c value of bare MgB2 pellets. The ratio of Δc/c 0 is related with Fe-diffusion concentration profile and its dependence on the samples depth is given in Fig. 6a. Concentration profile of Fe atoms/ions which cause measured parameters to change is likely to follow the curves given in Fig. 6a, b.
Another technique for studying diffusion is to determine the changes in room temperature resistivity (Δρ/ρ 0) over the thickness of the samples, and Fig. 6b illustrates change of ρ 300 K value as Δρ/ρ 0 over the depth of the sample from the coated surface, where Δρ is the resistivity difference between Fe-coated sample and resistivity of uncoated sample and ρ 0 is the ρ 300 K of bare sample with same property. The process for removal of thin layers was repeated eight times in total, achieving a depth of 200 μ m for Fe diffused MgB2 sample.
The full curve represents the calculated profile for diffusion of Fe in MgB2 as impurity diffusion from a constant source into semi-infinite solid [32];
where \(\text {erf}(x/2\sqrt {Dt})\) represents the error function \(y=x/2\sqrt {Dt}\)
where N 0 = N(0,t) is the constant concentration on the surface of the sample, N(x, t) presents the impurity concentration at the distance x from the surface, D refers to the diffusion coefficient and t is the diffusion sintering time.
The experimental data presented in Fig. 6 are in agreement with the theoretical curve calculated according to relation (3). The data in Fig. 6 are fitted to (3) using a nonlinear least-square fit procedure; the resulting concentration profile is plotted with the solid curve in Fig. 6. The same fitting method was used for the samples that are diffusion sintered at five different times between 15 min and 4 h. The values of Fe-diffusion coefficients for each sintering duration were calculated and listed in Table 3.
Based on the results given in Table 3, Fe diffusion in MgB2 superconductor depends strongly on the diffusion-sintering time. This finding supports the idea that diffusion of Fe atoms is through the defects and grain boundaries. The mean values of the diffusion coefficients of Fe in MgB2 obtained from lattice parameter c measurements are presented in Fig. 7a as a function of time and Fig. 7b shows the same information obtained from room temperature resistivity measurements. As proven by these data, both methods gives produces very similar, almost same results for diffusion.
4 Conclusions
We have found that the critical transition temperature and room temperature resistivity values increase with Fe diffusion in MgB2 bulk samples. Improvement of T c indicates the presence of an optimum doping level for Fe. Likewise, changes in unit cell parameter a is insignificant in the case of Fe diffusion regardless of sintering times. The lattice parameter c is bigger in Fe-diffused samples compared to bare samples indicating substitution. Surface morphology and grain connectivity are improved and grain size is enhanced with Fe diffusion and increasing sintering time up to 2 h. Good agreement between diffusion coefficients calculated with two methods show that Fe diffusion into bulk MgB2 can be monitored with changes in room temperature resistivity as well as with changes in lattice parameter c along depth profile. Fe diffusion coefficient spans between 7.3348 × 10−9 cm2/s and 2.3763 × 10−8 cm2/s for different sintering times showing that Fe diffusion in solid MgB2 is very slow and it has a strong time dependence. The diffusion coefficient decreases with increasing sintering time. MgB2 bulk and wire/tape samples with high performances can be produced by sintering at low-temperatures for longer duration provided that other parameters of the process is optimized.
References
Nagamatsu, J., Nakagawa, N., Muranaka, T., et al.: Nature 410, 63–64 (2001)
Dzhafaro, T.D.: Phys. Status Solidi (a) 158(2), 335 (1996)
Terzioglu, C.: Phys. B Condens. Matter 403.18, 3320–3325 (2008)
Goretta, K., Chen, N.: Diffusion and heat treatment of high temperature superconductors Superconducting Materials: Advances in Technology and Applications, pp 218–228 (2000)
Dzhafarov, T.D., Altunbas, M., Varilci, A., Cevik, U., Kopya, A.I.: Mater. Lett. 26(6), 305–311 (1996)
Ozturk, O., Asikuzun, E., Kaya, S., Coskunyurek, M., Yildirim, G., Yilmazlar, M., Terzioglu, C.: J. Supercond. Nov. Magn. 25(7), 2481–2487 (2012)
Ozturk, O.: J. Mater. Sci. Mater. Electron. 23(6), 1235–1242 (2012)
Altin, S., Aksan, M.A., Yakinci, M.E.: J. Phys. Chem. Solids 72(9), 1070–1076 (2011)
Dogruer, M., Zalaoglu, Y., Yildirim, G., Varilci, A., Terzioglu, C.: J. Mater. Sci. Mater. Electron. 24(6), 2019–2026 (2013)
Samuely, P., Szabo, P., Pribulova, Z., Angst, M., Bud’ko, S.L., Canfield, P.C., Kang, B.W.: Physica C: Superconductivity and Its Applications 460, 84–88 (2007)
Fujii, H., Ishitoya, A., Itoh, S., Ozawa, K., Kitaguchi, H.: J. Alloys Compd. 664, 650–656 (2016)
Akdogan, M., Yetis, H., Gajda, D., Karaboga, F., Ulgen, A.T., Demirtrk, E., Belenli, I.: J. Alloys Compd. 649, 1007–1010 (2015)
Kim, J.H., Dou, S.X., Wang, J.L., Shi, D.Q., Xu, X., et al.: Supercond. Sci. Technol. 20.5, 448 (2007)
Kovac, P., Husek, I., Melisek, T.: Supercond. Sci. Technol. 15.9, 1340 (2002)
Holubek, T., Kovac, P., Husek, I.: Acta Physica Polonica-Series A General Physics 113.1, 367–370 (2008)
Devadas, K.M., Rahul, S., Thomas, S., Varghese, N., et al.: J. Alloys Compd. 509.31, 8038–8041 (2011)
Novosel, N., Pajic, D., Skoko, Z., Mustapic, M., Babic, E., Zadro, K., Horvat, J.: Phys. Procedia 36, 1498–1503 (2012)
Singh, K.P., Awana, V.P., et al.: J. Supercond. Nov. Magn. 21(1), 39–44 (2008)
Dogruer, M., Yildirim, G., Yucel, E., Terzioglu, C.: J. Mater. Sci. Mater. Electron. 23(11), 1965–1970 (2012)
Ulgen, A.T, Belenli, I: J. Supercond. Nov. Magn. 30, 1089 (2017). doi:10.1007/s10948-016-3780-z
Scherrer, P.: Gttinger Nachrichten Gesell. 2, 98 (1918)
Patterson, A.: Phys. Rev. 56(10), 978–982 (1939)
Warren, B.E.: J. App. Phys. 12, 375 (1941)
Joubert, J., Shirk, T.: Mat. Res. Bull. 3, 671 (1968)
Avaeva, I., Kravchenko, V.: Izvestija Akad. Nauk SSSR 8N7, 1330 (1972)
Zhou, S., Dou, S.: Solid State Sci. 12(1), 105–110 (2010)
Kim, J.H, et al.: arXiv:cond-mat/0607540 (2006)
Jiang, C.H., Dou, S.X., Cheng, Z.X., Wang, X.L.: Supercond. Sci. Technol. 21(6), 065017 (2008)
Heitjans, P., Karger, J.: Diffusion in condensed matter, Springer Berlin Heidelberg, New York (2005)
Grathwohl, P.: Diffusion in Natural Porous Media: Contaminant Transport, Sorption/Desorption and Dissolution Kinetics. Kluwer Academic, Dordrecht (1998)
Donovan, R.P., Evans, R.: S.S.Electronics 10, 155 (1967)
Abdullaev, G.B., Dzhafarov, T.D.: Atomic Diffusion in Semiconductor Structures. Harwood, New York (1987)
Acknowledgements
This work is supported by the Scientific and Technological Research Council of Turkey, (Project no: 113F080), AIBU Research Fund grant no: 2016.03.02.1067 and in part by Ministry of Development under Grant 2010K120520.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulgen, A.T., Belenli, I. Time-Dependent Diffusion Coefficient of Fe in MgB2 Superconductors. J Supercond Nov Magn 30, 3367–3375 (2017). https://doi.org/10.1007/s10948-017-4113-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-017-4113-6