Abstract
In the present work, we report on the role of the methanol medium and ball-milling time in the substitution of carbon in carbon nanotube CNT-MgB2 superconductors. In our samples, we find that the CNTs are intact and well dispersed. However, the liquid medium (methanol) used for dispersion of the constituent materials is also acting as a source of C for substitution. However, the substitution of C from methanol is not direct; rather, B2O3, which had been considered as just an impurity, plays a positive role in binding the methanol molecules to the surface of B. The detailed mechanism of methanol absorption and the role of B2O3 and ball-milling time are presented here. In addition, we present the J C(H, T) data, which show that approximately 3% of C substitution provides the best critical current density at 20 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Superconductivity in MgB2 at 39 K [1] has attracted worldwide interest for both fundamental studies and applications since its discovery. The relatively high critical temperature, the low cost, and the weak-link-free grain boundaries make MgB2 a potential candidate for the replacement of commercial low-temperature superconductors such as Nb-Ti [2]. However, the critical current density (J C) of pure MgB2 drops rapidly with increasing magnetic field because of the lack of a strong pinning force, lower H C2, and poor grain connections [3]. In recent years, significant progress has been made in improving the superconducting properties by nanocarbon doping, especially in enhancing the critical current density (J C) in a magnetic field [3,4,5,6,7]. So far, various dopants have been investigated; among them, nanocarbon such as nano-SiC and metal carbide [2, 7,8,9,10,11], carbon nanotubes (CNTs) [12], and nanodiamond [13] have been proven to be the most effective way for increasing J C and H C2. The special geometry of CNTs (i.e. high aspect ratio) [14, 15] and their physical properties, such as high electrical and thermal conductivity [16], make them an interesting source of carbon dopant. These properties of CNTs will enhance the thermal stability of the MgB2 conductor if the CNTs are well dispersed in the MgB2 [17]. Moreover, the robust mechanical axial strength of carbon nanotubes makes them a suitable dopant for practical applications of superconducting wire [18]. However, the strong structure of CNTs makes it difficult to break off the C atoms for substitution in the B-plane of the MgB2 lattice at the sintering temperature [19]. Most CNTs remain in their original form, and they function as an additive in the MgB2 matrix and form CNT-MgB2 composites [19]. These CNTs provide excellent mechanical strength, as well as good thermal and electrical conductivity in the normal state [20]. Various studies have been reported on the improvement of superconducting properties by the addition of CNTs only [14,15,16,17]. Only a very small amount of C is substituted as a result of CNT addition at the lower sintering temperatures. CNTs may provide C substitution during high-temperature sintering at around 1000 °C; however, at that temperature, the crystallinity of MgB2 deteriorates, and the formation of other phases also occurs; hence, the overall J C(H,T) performance decreases [21]. This small amount of substitution might originate from the edge C of CNTs or from the organic liquid used for mixing the CNTs. An optimum level of C substitution is imperative for high-field performance of MgB2 superconductors. In order to substitute adequate C into CNT-MgB2 composites, another source of C is needed in addition to the CNTs. In the present study, we have used a novel method for the additional C substitution in CNT-MgB2 composites: We utilized the organic liquid methanol that is used for mixing the constituent materials as a source of C. The presence of unwanted B2O3 in the commercial boron source helps in binding the methanol molecule at the surface of the boron grains. The mechanism of binding at the surface of the B will be discussed in detail in this paper.
The planetary ball milling (BM) technique is extensively used for mixing the constituent materials uniformly in synthesizing the bulk MgB2 samples because of its flexibility of operation (dry and wet), faster mixing, and easy scale-up for commercial production [22, 23]. We have utilized the BM technique for the uniform dispersion of CNTs. The effects of ball-milling time (BMT) in the wet medium (i.e. in methanol) were studied, and we found that BMT is also crucial in controlling the substitution of C. We discuss the mechanism whereby ball milling reduces the B2O3 and optimizes the binding of methanol molecules at the surface of the B grains and thereby optimizes the coating of C onto the B surface.
2 Experimental Work
The conventional solid-state reaction method was used to synthesize the samples. Mg powder (45 µm, 99.6% purity; CERAC), amorphous B powder (1 µm, 99.9% purity; Sigma-Aldrich), and multi-walled carbon nanotube (MWCNT) powder (8–15 nm in outer diameter, 10–50 µm in length, and 95% purity from Cheap Tubes, Inc.) were mixed in the stoichiometric ratio corresponding to the chemical formula (MgB0.96C0.04). Here, the amount of MWCNTs was taken as fixed according to the above formula, which is a 4% atomic ratio. Although the boron is described as amorphous by Sigma-Aldrich, it is, in fact, not amorphous when examined by X-ray diffraction (XRD). Initially, B and CNTs were sonicated together in the methanol for 10 min using an ultrasonicator to remove their agglomeration; subsequently, Mg was added to the mixture and mixed using the planetary ball milling machine for different BMTs (1, 4, 8, and 12 h) in the methanol. In all cases, the rotation speed per minute (rpm) and the amount of methanol used were the same. The bowl and balls were made of agate. Finally, the mixed powder was dried in an oven and pressed into the form of square pellets at 10 tons pressure. One sample of pure MgB2 (without CNTs) was fabricated under the same conditions for use as a reference. Throughout this paper, we will refer to these samples as the pure, 1-h, 4-h, 8-h, and 12-h samples. These pellets were wrapped in Ta foil, placed inside a soft Fe tube, and heated up to 650 °C for 2.5 h in a pure argon gas environment. The heating rate was 5 °C per min.
The crystal structure was characterized from the XRD (PANalytical, X’Pert PRO) patterns of the samples using Cu K α radiation within the 2θ range of 20° – 80°. The microstructure was studied by SEM (JEOL JSM-7600F). A PPMS®; four-probe station from Quantum Design, Inc., was used to investigate the temperature dependence of the normal-state resistivity (ρ(T)) from room temperature to 10 K. The magnetizations of these samples were measured using a PAR-4500 vibrating sample magnetometer (VSM) at 20 K in magnetic fields up to 10 T. The J C values were calculated from the M-H loops using the Bean critical-state model.
3 Results and Discussion
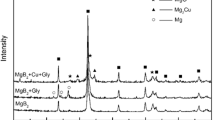
Figure 1 presents the XRD patterns of the samples. It is clear that all the samples include the hexagonal phase of pure MgB2 with traces of MgO impurity peaks at 2θ angles of 36.9° and 62.4° as shown by the asterisk symbol. This figure does not show the XRD peak of CNTs generally observable at 25° [24] because of the low percentage (4%) of CNTs in the composite.
The lattice parameters of all samples in the present study were calculated from the XRD graph using the PowderX analytical program [25] and also by the MAUD program [26, 27]. The results for lattice parameters a and c are shown in Figs. 2 and 3, respectively. The c parameter is almost constant as a function of BMT; on the other hand, the variation in the a parameter is very pronounced as a function of BMT. The decrease in the lattice parameter a in comparison to that of the pure sample is an indication of the substitution of C atoms in the B-plane of the MgB2 lattice. However, the a parameter increases as a function of BMT and becomes saturated after 8 h of BMT. The amount of C substituted in all samples was calculated from the c/a ratio by using the formula X = 7.5 × Δ(c/ a) [28]. In fact, this method gives better results for the calculation of the carbon concentration (X) than does the method using only the a value. This is because the c/a ratio is independent of diffractometer calibration errors [28]. The value of X as a function of BMT is shown in Table 1. The result shows that the value of C substituted decreases with the increase of BMT and becomes approximately constant after 8 h. The value of X for the 1-h sample is 5.6% which is more than the amount of CNTs added (4%).
This result initially seemed very strange to us, and we thought there might be some experimental mistake in the sample synthesis and/or the XRD characterization. However, we repeated the experiments from the initial synthesis of the sample to its characterization and found exactly the same results. This made us confident that the experimental results are correct. This means that there is some extra source of C in the samples, and one possible source might be the mixing medium (methanol) itself [29]. We started looking for the mechanism by studying the phase analysis and microstructure. From the phase analysis, we found that the percentage of MgO increases as a function of BMT. This gives an indication that there is some source of oxygen present in the starting composition that oxidizes the Mg during the ball milling. As we can see from Table 1, the percentage of MgO increases with BMT and almost saturates after 8 h. This means that the Mg exhausts the source of oxygen in the starting materials after 8 h of BMT. The ball milling was done in methanol; therefore, absorption of oxygen from the air is negligible, and the source of O2 must come from the commercial boron itself, which contains B2O3. It has been reported that boron generally absorbs oxygen at room temperature and forms B2O3 on the surface of the boron grains [30]. This B2O3 reacts with Mg according to the following chemical reaction:
The purpose of increasing the BMT is to enhance the homogeneity and increase the contact between Mg and B2O3 and hence increase the formation of MgO until it exhausts most of the B2O3.
Another issue is how the BMT affects the substitution of C in MgB2 especially in the methanol medium. This can be explained as follows: Generally, B2O3 is present on the surface of boron grains because of partial oxidation of B in contact with the ambient environment. B2O3 is unavoidable in commercially available boron [31]. It is known that the surfaces containing B2O3 adsorb methanol by chemisorption or by hydrogen bonding [32]. Thus, the oxidized surface of boron may be utilized for the absorption of methanol, which—upon decomposition—provides the C-coated boron that is an efficient source of C for substitution in MgB2 at the sintering temperature. The schematic diagram is shown in Fig. 4.
The effect of increasing the BMT is to reduce the amount of B2O3 present in the boron by forming MgO. At low BMTs, more B2O3 is still present and can absorb the methanol and form the C-coated boron at the decomposition temperature. As we increase the BMT, the amount of B2O3 is reduced; hence, the amount of coated C on boron is also reduced. This C-coated boron is substituted in the MgB2 during its phase formation at the sintering temperature. Thus, we expect that the doping percentage of C will decrease as a function of BMT, which is clear from the C percentage calculated from the XRD plotted in Fig. 1. This is also clear from the SEM pictures shown in Fig. 5. The 1-h SEM image shows considerable inhomogeneity; there are large, hexagonal-shaped crystals as well as very small crystals. The large crystals are the pure MgB2 crystals, whereas the smaller ones are the C-substituted MgB2 crystals. The C substitution hinders the growth of the crystals, and higher C substitution lowers the crystal size. Thus, we see that the 1-h BMT sample has the smallest crystal size because of the larger amount of C supplied from the methanol as we discussed earlier. Since 1 h of ball milling is not sufficient for homogeneity, we find larger pure MgB2 hexagonal crystals in the 1-h BMT samples. As the BMT increases, the samples become more and more homogeneous, and there is a reduction in the amount of B2O3 as described above that reduces the substitution of C. The crystals with less C substitution are larger than the ones with more C substitution; this can be clearly seen by comparing the SEM images of the samples with different BMTs in Fig. 5.
The next question concerns the role of CNTs in the C substitution. Generally, CNTs possess a strong structure, and it is difficult to provide the C atoms at the 650 °C sintering temperature. Only a small amount of C atoms broken from the edge of CNTs can be substituted in the MgB2 lattice. We attempted to look for the CNTs in the samples by using SEM; they are present as such and are well distributed until 12 h of BMT. The distribution of CNTs for the 8- and 12-h samples is shown in Fig. 6. However, at 12 h of BMT, we found that the CNTs were present in lower quantities; this might be due to breaking of CNTs at higher BMTs. The smaller size of the CNTs may provide extra C in the MgB2, and this is expected to cause a slight increase in the amount of C substitutions. It is clear from Fig. 7 that C substitution in the 12-h samples is slightly greater than that in the 8-h ones. The substitution of C in the samples was further verified by measuring the resistivity of all the samples as a function of temperature.
The temperature dependence of resistivity of samples with different BMTs is shown in Fig. 8. It is clear from the figure that the normal state resistivity of the samples increases with the increasing percentage of C doping in the entire range of temperature. This further proves that the C is substituted in the MgB2 lattice. An increase in BMT decreases the substitution. Residual resistivity (ρ 0) was calculated using the Rowell technique described in detail by Rowell [33] for all the samples. The results for the resistivity parameters are tabulated and shown in Table 2. The plot of ρ 0 and T C0 as functions of C doping is presented in Fig. 9, which shows linear relationships. This is a clear indication of C substitution in the lattice of MgB2 [34].
The critical current density (J C(H, T)) was estimated from the M-H loop using the Bean critical-state model [35]. Figure 10 shows the magnetic field dependence of J C at 20 K (the expected operating temperature of MgB2 wire for practical application) for all the samples. From the figure, it is clear that the 12-h sample with 2.9% of C has the highest J C over the entire range of magnetic field. The J C value of 104 A/cm2 is achieved near 3 T in the 12-h sample. Values of C doping that are lower or higher than this value show a deterioration in the J C(H, T) behavior. Thus, the optimum value of C for the best performance of MgB2 at 20 K is around 3%. The possible cause of reduced J C for higher and lower values of C doping is the combined effect of connectivity and pinning forces. The pinning force as a function of field is shown in Fig. 11 for all samples. This also clearly indicates that the 12-h samples with 2.9% C doping have the highest pinning force among all the samples. However, the change in the connectivity (F values) is almost the same (see Table 2).
4 Conclusion
In the present work, we prepared CNT-MgB2 composite superconductors using different BMTs in a methanol medium for mixing of the constituent materials. We found that methanol is also the source of C for substitution in CNT-MgB2 superconductor composites. The mechanism has been discussed here in detail. The oxidized surface of B, which contains B2O3, plays a key role in binding the methanol to the surface of the boron particles. During the heat treatment, before the sintering temperature is reached, the methanol bound on the surface of the B decomposes and provides a coating of C on the surface. During the ball milling, which mixes the constituent materials, the Mg reacts with the B2O3 and reduces it to B while forming MgO. Therefore, as we increase the BMT, the amount of B2O3 decreases, and hence, the chemisorption of methanol decreases. The decrease in the chemisorption of methanol reduces the amount of C coating on the B particles, and finally, we get less C substitution after heat treatment. The shorter BMT (1 h) shows inhomogeneity in particle size of MgB2 as can be clearly seen from the SEM results. This is due to poor dispersion of different constituent materials and hence inhomogeneous substitution of C. The CNTs do not break easily and have been found intact; this is clear from the SEM picture. Thus, mixing of CNTs using ball milling in a methanol medium provides a double benefit: dispersing the CNTs uniformly and providing the C source for substitution. In the present case, we have added only 4% of CNTs. This amount could be increased to 10% to get the maximum benefit of CNTs as the dopant along with the substitution of C from methanol. From J C(H) measurements at 20 K, it is clear that the best J C(H) was obtained for the 3% C substitution in the 12-h sample, which achieved 104 A/cm2 at 2.9 T at 20 K, and it is clearly suitable for practical MRI magnet applications.
References
Nagamatsu, J., et al.: Superconductivity at 39 K in magnesium diboride. Nature 410(6824), 63–64 (2001)
Dou, S.X., et al.: Enhancement of the critical current density and flux pinning of MgB2 superconductor by nanoparticle SIC doping. Appl. Phys. Lett. 81(18), 3419–3421 (2002)
Kim, J.H., et al.: The doping effect of multiwall carbon nanotube on MgB2/Fe superconductor wire. J Appl. Phys., 2006. 100(1)
Wilke, R.H.T., et al.: Systematic effects of carbon doping on the superconducting properties of Mg(B1−xCx)2. Phys. Rev. Lett. 92(21), 217003 (2004)
Ma, Y.W., et al.: Significantly enhanced critical current densities in MgB2 tapes made by a scaleable nanocarbon addition route. Appl. Phys. Lett., 2006. 88(7)
Susner, M.A., et al.: Influence of Mg/B ratio and SiC doping on microstructure and high field transport J(c) in MgB2 strands. Phys. C-Superconductivity Appl. 456(1-2), 180–187 (2007)
Collings, E.W., et al.: Prospects for improving the intrinsic and extrinsic properties of magnesium diboride superconducting strands. Superconductor Sci. Technol., 2008. 21(10)
Ye, S.J., et al.: Comparison of SiC and/or toluene additives to the critical current density of internal Mg diffusion-processed MgB2 wires. Phys. C-Superconductivity Appl. 484, 167–170 (2013)
Yamamoto, A., et al.: Reactivity of carbides in synthesis of MgB2 bulks. Phys. C-Superconductivity Appl. 445, 801–805 (2006)
Yamamoto, A., et al.: Effects of B4C doping on critical current properties of MgB2 superconductor. Supercond. Sci. Technol. 18(10), 1323–1328 (2005)
Dou, S.X., et al.: Mechanism of enhancement in electromagnetic properties of MgB2 by nano SiC doping. Phys. Rev. Lett., 2007. 98(9)
Yeoh, W.K., et al.: Effect of carbon substitution on the superconducting properties of MgB2 doped with multi-walled carbon nanotubes and nano carbon. IEEE Trans. Appl. Supercond. 17(2), 2929–2932 (2007)
Cheng, C.H., et al.: Doping effect of nano-diamond on superconductivity and flux pinning in MgB2. Supercond. Sci. Technol. 16(10), 1182–1186 (2003)
Dou, S.X., et al.: Effect of carbon nanotube doping on critical current density of MgB2 superconductor. Appl. Phys. Lett. 83(24), 4996–4998 (2003)
Serquis, A., et al.: Correlated enhancement of H-c2 and J(c) in carbon nanotube doped MgB2. Supercond. Sci. Technol. 20(4), L12–L15 (2007)
Shekhar, C., et al.: Improved critical current density of MgB2-carbon nanotubes composite. J. Nanosci. Nanotechnol. 7(6), 1804–1809 (2007)
Patel, D., et al.: Multiwalled carbon nanotube-derived superior electrical, mechanical and thermal properties in MgB2 wires. Scr. Mater. 88, 13–16 (2014)
Treacy, M.M.J., Ebbesen, T.W., Gibson, J.M.: Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 381(6584), 678–680 (1996)
Yeoh, W.K., et al.: Improving flux pinning of MgB2 by carbon nanotube doping and ultrasonication. Supercond. Sci. Technol. 19(2), L5–L8 (2006)
Lekawa-Raus, A., et al.: Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv. Funct. Mater. 24(24), 3661–3682 (2014)
Singh, K.P., et al.: Phase formation and superconductivity of fe-TUBE encapsulated and vacuum-annealed MgB2. Modern Phys. Lett. B 20(27), 1763–1769 (2006)
Parakkandy, J.M., et al.: Effect of ball milling time on critical current density of glucose-doped MgB2 superconductors. J. Supercond. Nov. Magn. 28(2), 475–479 (2015)
Xu, X., et al.: Improved J(c) of MgB2 superconductor by ball milling using different media. Supercond. Sci. Technol. 19(11), L47–L50 (2006)
Wei, J.Q., et al.: Structure and superconductivity of MgB2-carbon nanotube composites. Mater. Chem. Phys. 78(3), 785–790 (2003)
Dong, C.: Powderx: Windows-95-based program for powder X-ray diffraction data processing. J. Appl. Crystallogr. 32(4), 838 (1999)
Lutterotti, L.: Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res., Sect. B 268(3–4), 334–340 (2010)
Lutterotti, L., et al.: Texture, residual stress and structural analysis of thin films using a combined X-rayx analysis. Thin Solid Films 450(1), 34–41 (2004)
Avdeev, M., et al.: Crystal chemistry of carbon-substituted MgB2. Phys. C-Superconductivity Appl. 387 (3-4), 301–306 (2003)
Jun, B.H., Park, S.D., Kim, C.J.: Refinement and carbon incorporation effects on the superconducting properties of MgB2 through wet milling process of low purity boron powder. J. Alloys Compd. 535, 27–32 (2012)
Low, M.J.D., Harano, Y.: An infrared study of the reaction of methanol with siliceous surfaces. J. Res. Institute for Catalysis, Hokkaido University 16(1), 271–286 (1968)
Low, M.J.D., Harano, Y.: An infrared study of the reaction of methanol with siliceous surfaces. J. Res. Institute for Catalysis, Hokkaido University 16(1), 271–286 (1968)
Low, M.J.D., Harano, Y.: An infrared study of the reaction of methanol with siliceous surfaces. J. Res. Institute for Catalysis, Hokkaido University 16(1), 271–286 (1968)
Rowell, J.: The widely variable resistivity of MgB2 samples. Supercond. Sci. Technol. 16(6), R17–R27 (2003)
Lee, S., et al.: Carbon-substituted MgB2 single crystals. Phys. C-Superconductivity Appl. 397(1-2), 7–13 (2003)
Bean, C.P.: Magnetization of high-field superconductors. Rev. Mod. Phys. 36(1), 31–39 (1964)
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-290.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alghamdi, F.S., Shahabuddin, M., Alzayed, N.S. et al. Mechanism of Enhanced Carbon Substitution in CNT-MgB2 Superconductor Composite Using Ball Milling in a Methanol Medium: Positive Role of Boron Oxide. J Supercond Nov Magn 31, 1119–1126 (2018). https://doi.org/10.1007/s10948-017-4279-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-017-4279-y