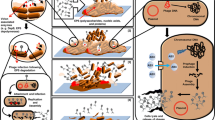
Abstract
Bacterial biofilms are widespread in the environment, and bacteria in the biofilm are highly resistant to antibiotics and possess host immune defense mechanisms, which can lead to serious clinical and environmental health problems. The increasing problem of bacterial resistance caused by the irrational use of traditional antimicrobial drugs has prompted the search for better and novel antimicrobial substances. In this paper, we review the effects of phage endolysins, modified phage endolysins, and their combination with other substances on bacterial biofilms and provide an outlook on their practical applications. Phage endolysins can specifically and efficiently hydrolyze the cell walls of bacteria, causing bacterial lysis and death. Phage endolysins have shown superior bactericidal effects in vitro and in vivo, and no direct toxicity in humans has been reported to date. The properties of phage endolysins make them promising for the prevention and treatment of bacterial infections. Meanwhile, endolysins have been genetically engineered to exert a stronger scavenging effect on biological membranes when used in combination with antibiotics and drugs. Phage endolysins are powerful weapons for controlling bacterial biofilms.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bacterial biofilms are structural communities of bacteria wrapped in an extracellular polysaccharide matrix composed of lipoproteins, and fibronectin, produced by the organisms [1]. Biofilm formation is part of a survival strategy for an organism to resist suboptimal environmental conditions such as limited nutrient availability or lethal antibiotic concentrations. In nature, in some industrial settings (e.g., fermentation and wastewater treatment), and humans and animals, most bacteria grow as biofilms attached to the surface of living or non-living objects, rather than in a planktonic manner [2]. Bacteria within biofilms have a strong affinity for nutrients and are highly resistant to exogenous substances, making them independent and resilient in micro-ecosystems. Once pathogenic microorganisms form biofilms, they become more resistant to antimicrobial drugs and the host’s immune system, causing persistent infection in the body [1, 2]. Biofilms can contain up to 97% water. In addition to water and bacteria, biofilms may contain secreted macromolecules, adsorbed nutrients and metabolites, and lysed bacterial products. Therefore, biofilms contain various major biomolecules, such as proteins, polysaccharides, DNA, RNA, peptidoglycan, lipids, and phospholipids. The formation of a biofilm multicellular structure is a dynamic process that includes the stages of initial bacterial adhesion, biofilm development, and maturation; bacteria in biofilms exhibit different physiological and biochemical properties at each stage (Fig. 1).
The effect of antimicrobial agents is usually significantly reduced in biofilms, presumably owing to their inherent properties [5]. Studies have shown that bacteria enclosed in biofilms are 10–1000 times more resistant to antibiotics than those in the planktonic form [6]. While it is difficult to remove biofilms using antibiotics or common disinfectants, numerous studies have shown that phages and their endolysins have good lytic effects on biofilms.
Bacteriophage endolysins (endolysins) are enzymes encoded by double-stranded DNA phages that depend on host bacteria for their synthesis, and they are produced late in the lytic cycle [7]. Most phages rely on endolysins to hydrolyze the bacterial cell wall [8]. The hydrolyzing of the cell wall is essential for releasing the phage progeny. Endolysins can kill many types of bacteria including the pathogenic and antibiotic-resistant. Endolysin therapy has been proposed based on the continuous emergence and spread of drug-resistant bacteria. The antimicrobial effect of therapeutically applied endolysins applies only to Gram-positive bacteria and Gram-negative are resistant due to the presence of a protective outer membrane unless the endolysins are genetically modified to overcome it. In this review, we will provide an outlook on the practical application of endolysins in the context of their bacteriolytic mechanism and removal of biofilms.
2 Structure and Bacteriolytic Mechanism of Phage Endolysins
Phage lysins of Gram-positive bacteria usually have a C-terminal cell wall-binding domain (CBD) that determines the specificity of cell wall-binding and an N-terminal catalytic domain (CD) that determines the catalytic activity of the enzyme (Fig. 2). However, the CD of Gram-negative bacteriophage lysins (e.g., Pseudomonas aeruginosa phage lysin) is generally located at the C-terminus, whereas the CBD is located at the N-terminal end [9, 10]. Some endolysins possess two or even three catalytic domains and a binding domain [11], such as the lytic enzyme HydH5 encoded by a Staphylococcus aureus phage Φ11, which possesses two catalytic domains and no conjugation domain [12]. Yang et al. [13] found that some endolysins have a separate spore-binding domain in their structure, which can recognize specific species of bacterial spores.
The structural domain of endolysins. a Structural domain of Gram-positive endolysin: CD is an N-terminal catalytic domain, CBD is a C-terminal cell wall-binding domain, b Structural domain of Gram-negative endolysin: CD is generally located at the C-terminus, ‘+ + + +’ represents 2–3 possible catalytic structural domains
Endolysins mainly act on peptidoglycan peptides and glycosidic bonds (Fig. 3). Depending on the site of action of endolysins, they can be classified as endolysins/muramidase, which acts on the β-1,4-glycosidic bond of the glycan backbone in the cell wall; endopeptidases, which act on the polypeptide chain; or amidases, which hydrolyze the amide bond between the glycan backbone and polypeptide chain [14]. Because the amide linkage of peptidoglycan and the β-1,4-glycosidic linkage between aminosaccharides tend to be conserved among bacterial species [15], amidases have a broader cleavage spectrum. Moreover, peptidoglycans in bacterial cell walls are more conserved and less likely to be resistant to phage endolysins. Therefore, phage endolysins have great potential as antimicrobial agents.
Gram-positive and Gram-negative bacteria have different cell envelope structures (Fig. 4). The cell envelope in Gram-negative bacteria has a thinner peptidoglycan layer, an outer membrane and bacterial capsule or mucus layer covering the outer membrane. This makes it more difficult for endolysins to lyse Gram-negative bacteria from outside. Therefore, there are usually differences in the structure of endolysins that target Gram-positive and Gram-negative bacteria. Gram-positive endolysins have evolved to utilize a modular design in which catalytic activity and substrate recognition are performed by two different types of functional structural domains called CBDs and enzymatically active domains (EADs), respectively [10, 18]. EAD confers the catalytic mechanism of the enzyme (i.e., cleaves specific bonds within the bacterial peptidoglycan). However, endolysins with CBDs target proteins to their substrates and keep CBDs tightly bound to cell wall fragments after cell lysis, thereby preventing diffusion and disrupting the surrounding intact cells that have not yet been infected by phages [19]. In contrast, the outer membrane of Gram-negative bacteria can prevent such collateral damage by restricting endolysins from entering the peptidoglycan layer from the outside, which may explain why endolysins from phages infecting Gram-negative host bacteria are predominantly small single-domain globular proteins (molecular weight between 15 and 20 kDa) that usually do not have a specific CBD module [9]. Such endolysins may perform better as enzymes (aiding multiple catalytic reactions during cell lysis) than endolysins of Gram-positive bacteria, which bind to a site with a very low release rate [20]. Nonetheless, there are exceptions, such as the endolysins of P. aeruginosa (Gram-negative bacteria) phages, KZ144 and EL188, with modular structures of N-terminal CBD and C-terminal EAD. Both KZ144 and EL188 have a modular structure consisting of an N-terminal substrate-binding domain and a predicted C-terminal catalytic module, a property previously only demonstrated in endolysins originating from phages infecting Gram-positives and only in an inverse arrangement. Both binding domains contain conserved repeat sequences, consistent with those of some peptidoglycan hydrolases of Gram-positive bacteria [9].
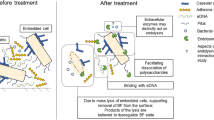
Shen et al. [21] suggested that there are two main mechanisms by endolysin PlyC act: (1) direct lysis of host bacteria and (2) degradation of extracellular matrix components of the biofilm, which exposes the bacteria. Based on the available results, the following speculation was made regarding the mechanism of biofilm removal by phages or their endolysins: the polysaccharide on the outer surface of the bacteria is known to be the main component of the extracellular matrix of the biofilm, and these “smart” phages recognize and degrade the polysaccharide as the main component by producing polysaccharide lysins. The biofilm structure is then destroyed and the intrinsic bacteria are exposed. Once inside the biofilm, the phages invade and lyse the host bacteria, releasing more progeny phages and accelerating the lysis of the bacteria while also preventing the formation and maintenance of the new biofilm. Notably, bacteria within biofilms often exhibit multidrug resistance. The ineffectiveness of antibiotics against biofilms and their large-scale use is contributing to the growing problem of antimicrobial resistance. Phages and their endolysins can potentially be applied to remove biofilms.
3 Native Phage Endolysins Clear Biofilm
Sharma et al. [22] demonstrated that phages can play a role in scavenging biofilms by degrading the extracellular polysaccharides of P. aeruginosa. Indiani et al. [23] noticed, in in vitro experiments, that lysin CF-301 has an extremely strong ability to lyse biofilms and drug-resistant S. aureus biofilms that are formed in human synovial fluid. After adding CF-301, all biofilms in the catheter were removed, and the number of bacteria was reduced by five orders of magnitude. Poonacha et al. [24] found that lysin P128 could degrade host biofilms and kill bacteria within biofilms. Electron micrographs showed that 8.0 μg/mL of lysin P128 significantly degraded Staphylococcus epidermidis, Staphylococcus Lugdunensis, and Staphylococcus haemolyticus biofilms; 15.0–31.0 μg/mL of lysin P128 killed 99% of S. epidermidis and S. haemolyticus and 62.5 μg/mL killed 99% of S. haemolyticus. Singh et al. [25] determined the Staphylococcus aureus biofilm-disrupting ability of chimeric P1y187AN-KSH3b lysin and gentamicin via fluorescence imaging and demonstrated that the lysin exhibited an extremely strong Staphylococcus aureus biofilm-disrupting ability, whereas gentamicin alone failed to disintegrate Staphylococcus aureus biofilms. Lood et al. [26] observed the degradation effect of lysin P1yF307 on the biofilm of Acinetobacter baumannii; the A. baumannii biofilm growing on the catheter was significantly reduced after treatment with lysin P1yF307, both in vivo and in vitro. In addition, phage lytic enzyme LySMP was more than 80% effective in clearing biofilms formed by Streptococcus suis SS2-4 and SS2-H strains [27]; the removal rate of biofilms by phage or antibiotics alone was usually less than 20%, compared to that of the phage lytic enzyme LySMP. Furthermore, different cell wall endolysins encoded by Streptococcus pneumoniae or its phage could effectively eliminate the organism in vitro and in animal models [28]. Among them, LytA is a major S. pneumoniae autolysis enzyme, which is an N-acetyl cytosolic acyl-l-alanine amidase that removes biofilms formed by S. pneumoniae. In addition to LytA, other cell wall endolysins include LytC, Pal, Cpl-1, Cpl-7, and Ejl; furthermore, Lyt-A and Cpl-1 can act synergistically to effectively remove S. pneumoniae biofilms. These studies showed that phage-encoded endolysins exhibited better scavenging and killing effects on biofilms and the bacteria protected within them. This finding can be further exploited for future clinical management of infectious diseases associated with biofilms.
4 Improving the Anti-biofilm Activity of Phage Endolysins
The inhibitory effect of endolysin on biofilms has been previously studied, and the rate of endolysin inhibition in biofilms has been improved. Leitch et al. [29] investigated the ability of lactoferrin to enhance the activity of vancomycin and endolysin against the biofilms of a clinical S. epidermidis isolate. Lactoferrin treatment significantly (p < 0.05) reduced the number of viable biofilms and biofilm-released cells at an endolysin concentration of 16 mg/mL. The in vitro effect of endolysin (0–1000 µg/mL) on eukaryotic Candida albicans biofilm development was also investigated [30]. The action of lactoferrin is likened to that of cationic substances [31] such as protamine sulphate, which potentiates the action of vancomycin against S. epidermidis biofilms in vitro [32] and in vivo [33] and platelet microbicidal protein, which increases the vancomycin susceptibility of suspended S. aureus isolates [34]. In 2017, Hukić et al. [30] investigated two basic questions regarding endolysin activities on the selected microorganisms were investigated: (1) Whether endolysin inhibits biofilm formation and (2) Which concentration of the enzyme is required to change the natural biofilm-producing capacity of different strains of S. aureus (methicillin-sensitive and resistant), S. pyogenes, P. aeruginosa, and Gardnerella vaginalis. The effect of endolysin on the biofilm-forming capacity of 16 selected microbial strains was investigated in vitro using a test tube method including four replicates; it was concluded that the potential of endolysin to alter biofilm-forming capacity depends on its concentration, the bacterial species, and the microbial strain used [30]. Some of the studies involving phage lysin treatment in bacterial biofilms and their characteristics are listed in Table 1.
4.1 Genetic Engineering Modification
4.1.1 Genetically Engineered Phage Endolysin Clears Biofilms
Many in vivo and ex vivo experiments have revealed the great potential of endolysins as an antibacterial agent [10, 45]. However, the lytic activity of endolysins against Gram-positive bacteria is higher than that of their Gram-negative counterparts. In addition, the host specificity and tendency to form inclusion bodies when expressed in prokaryotes limit the activity and application of endolysins for multiple bacterial infections (e.g., some mucosal infections). In addition, the C-terminal binding domain of endolysins has a strong hydrophobic and repetitive transmembrane region, which makes endolysins poorly water-soluble and reduces their application. To overcome these drawbacks and improve the lytic activity and host spectrum of endolysins, scientists have utilized molecular biology to design and modify natural phage endolysins to produce improved antibacterial reagents. Researchers have targeted the specificity of the C-terminal binding structural domain of phage endolysins to develop rapid detection reagents for bacteria. Bacterial biofilms exhibit resistance to antimicrobial therapy and clearance by the host immune system, making eradication very difficult. To address this issue, Lu et al. [47] engineered bacteriophage to express a biofilm-degrading enzyme during infection to simultaneously attack the bacterial cells in the biofilm and the biofilm matrix. The authors show that the efficacy of biofilm removal by this two-pronged enzymatic bacteriophage strategy is significantly greater than that of nonenzymatic bacteriophage treatment and cleared up 99.997% of the E. coli population in biofilms [46]. Therefore, the use of endolysins for treating bacterial biofilms has been supported and advocated by many researchers internationally.
4.1.2 Chimeric Enzymes
Some endolysins can alter their specificity and catalytic activity by substituting their structural domains. The peptide chain endonuclease structural domain of streptococcal phage lysin λSA2 (the λSA2 cleavage site is present on the peptidoglycan of Streptococcus and Staphylococcus [47]) was combined with the SH3b-binding structural domains of staphylococcal phage lysin LysK and staphylococcal endolysin to form a chimeric enzyme that not only produced higher lytic activity against S. aureus (including penicillin-resistant strains) but also maintained its original activity [48, 49]. Furthermore, the three fusion proteins HydH5SH3b (HydH5 + SH3b structural domain), CHAPSH3b (cysteine and histidine-dependent aminohydrolase/peptidase (CHAP) structural domain + SH3b structural domain), and HydH5Lyso (HydH5 + Lysostaphin) of the S. aureus phagocytic lysin HydH5 and staphylococcal endolysin lysostaphin both exhibited higher cleavage capacity than HydH5 [50]. Jagielska et al. [51] combined LytM, an autolysis enzyme of S. aureus, with the CBD of the S. aureus lytic enzyme to create a chimeric enzyme with a lytic capacity that was 540-fold higher than that of the original lytic enzyme. Yang et al. [52] combined the CD of the lytic enzyme Ply187 from S. aureus and the binding domain of the phage lytic enzyme phiNM3 to form the chimeric enzyme ClyH. The latter not only increased the lytic capacity but also expanded the host spectrum. Dong et al. [53] combined the CD Ply187 N (1–157 aa) of the lytic enzyme Ply187 from S. aureus and the binding domain (146–314 aa, V12C) of the phage lytic enzyme PlyV12 to form the chimeric enzyme Ply187 N-V12C. This lysed not only S. aureus but also streptococci (S. aureus, S. lactis, S. pyogenes) and Enterococcus (Enterococcus faecalis), increasing the host spectrum of the lytic enzyme. The bactericidal function of endolysins was enhanced by combining different lytic enzyme structural domains. Yang et al. [54] constructed the lytic enzyme ClyH by fusing Ply187 (Pc) with the non-SH3b-like cell wall-binding structural domain of phiNM3, which exhibited good bactericidal activity in vitro and in vivo and disrupted the biological periplasm formed at different times [55]. Fernandes et al. [56] combined the CD of E. faecalis phage lysin F168/08 and the binding domain of phage87 lysin Lys87b to form a chimeric enzyme. The latter enzyme not only expanded the lysis spectrum but also increased its water solubility. Other investigators designed ClyS as a chimeric lysin by splicing the CD of phage Twort lysin plyTW and CBD of phage phNM3 lysin [57]. The designed C1yS lysin enhanced its water solubility and lytic activity, laying the foundation for clinical applications [58]. SINGH et al. [59] determined the biofilm-disrupting ability of chimeric lysins Ply187AN-KSH3b and gentamicin by fluorescence imaging method, respectively. The results demonstrated that the chimeric lysin Ply187AN-KSH3b had a strong ability to disrupt biofilms, while gentamicin could not lyse biofilms. The fusion protein SMAP-29-KZ144 was formed by fusing the antimicrobial peptide SMAP-29, which can penetrate the outer membrane of bacteria with the N-terminal end of lytic enzyme KZ144. The in vivo killing effect of a series of antimicrobial peptide endolysins designed along these lines against P. aeruginosa and its biofilms has been demonstrated in a nematode infection model [60].
4.1.3 Endolysin Truncation
Notably, some endolysins, such as Mur encoded by Lactobacillus deuterium phage LL-H, still induced lytic activity when the C-terminus was removed, whereas other enzymes had increased activity with the deletion or partial deletion of the C-terminus [61]. Loessner et al. [62] noticed that the whole enzyme activity of S. aureus lytic enzyme P1y187 was low, but its N-terminal amino acid (1–157 aa) had high activity, whereas 158–227 aa and 158–628 aa were inactive. Mutant forms of group B streptococcal lysin P1yGBS with multiple fragment losses exhibited increased activity, retaining only the N-terminal 1–141 aa and C-terminal 13 amino acids, with a 28-fold higher lytic activity than the full enzyme [62]. Meng Wu et al. [63] found that when the whole enzyme of Ply187 was cut off and only its CHAP structural domain was expressed, it showed strong antibacterial activity against both S. aureus and its biofilms. When some skin chains of the staphylococcal lytic enzyme LysK were truncated, leaving only the CHAP structural domain, it maintained its staphylococci (including MRSA) lysing activity [64, 65]. The Clostridium difficile phage lysin CD27L was truncated, leaving the N-terminal domain CD27L1–179, which not only increased the lytic activity against C. difficile but also expanded its lytic spectrum, whereas the other half of the lysin, CD27L180–270, had no lytic activity [66]. Thus, this is another way to modulate the specificity of the lytic enzyme. A truncated lytic enzyme, even a single structural domain protein with a greatly reduced relative molecular mass, might reduce the mounting of an immune response. Fenton et al. [67] also demonstrated that the peptidase CHAPk, produced by the truncated structural domain (cysteine, histidine-dependent amidohydrolase/peptidase) CHAPK from phage K lysin LysK of S. aureus, can act as a biocide to rapidly degrade the biofilm formed by S. aureus and prevent and treat biofilm-associated staphylococcal infections. Pure CHAPk can eliminate biofilms of S. aureus DPC5246 within 4 h. In addition, CHAPk prevented the formation of S. aureus DPC5246 biofilms and reduced the number of S. aureus colonies on the skin surface.
By modifying the endolysins, we can increase their lytic activity and make them more target specific for different pathogenic bacteria to optimize the endolysins. In conclusion, combining different structural protein domains allows the design of endolysins with high activity against bacterial biofilms, laying the foundation for future clinical applications.
4.2 Combining Enzymes with Antibiotics or Membrane Permeation Agents
Several endolysins combined with antibiotics can result in synergistic effects and improve bactericidal effects. Mixing the endolysins HydH5 and LysH5 of S. aureus phage phiIPLA88 with different sites of action produced better in vitro anti-staphylococcal effects [48]. Notably, the combined use of the lytic enzyme Cpl-1 (2.5 µg) and Pal was more bactericidal than when 5 µg of Cpl-1 or Pal was used alone [68]. Other studies also confirmed that the combination of the lytic enzyme LysK and staphylococcal lysins exhibited synergistic effects [69]. In addition, the combination of S. pneumoniae lytic enzyme Cpl-1 with antibiotics has similar synergistic effects. Cpl-1 combined with gentamicin at less than the minimal inhibitory concentration can improve the killing of S. pneumoniae. Cpl-1 can also synergize penicillin to lyse penicillin-resistant bacteria. Therefore, a rational combination of enzymes and antibiotics can potentially control specific antibiotic-resistant bacteria [70]. McCarthy noted that Exebacase (Lysin CF-301) is an attractive antimicrobial agent because it demonstrates rapid bacteriolytic activity against staphylococcal species, including Staphylococcus aureus, has a low resistance profile, eradicates biofilms, and acts synergistically with other antibiotics [71].
In recent years, phage endolysins have also been studied in combination with membrane permeabilizers [e.g., polymyxin B and ethylene diamine tetraacetic acid (EDTA)] to overcome the outer membrane barrier of Gram-negative bacteria. Briers et al. [72] combined P. aeruginosa phage lysin OBPgp279 and Salmonella phage PVP-SE1 gp146 with various outer membrane permeating agents (e.g., polycationic peptide, hydrophobic pentapeptide, parasin l, and lycotoxin l) to form fusion proteins. “Artilysins” (outer membrane-penetrating endolysins) exhibit superior lytic activities in vitro. The phage lytic enzyme SPN1S [73], which has an endolysin-like superfamily domain, can kill most Gram-negative strains and maintain stable antibacterial activity at different pH (pH 7.0–10.5) and temperature (25–45 °C) ranges. When combined with the chelating agent EDTA, the ability of SPNlS to pass through the bacterial outer membrane and its lytic activity was significantly enhanced. Another lytic phage Ts2631 reduced all Enterobacteriaceae pathogens, including multi-drug-resistant Citrobacter, below the detection limit [by 6(log (CFU/mL))] [74] when combined with EDTA. Liu et al. [75] identified and prepared two phage endolysins, LysWL59 and LysWL60, from phage LPST10. The lytic activity of both enzymes was extensive against Gram-negative bacteria after chloroform treatment. LysWL59 showed more stability than LysWL60 and maintained good lytic activity at pH 6.0–10.0 and a temperature of 4–90 °C. When LysWL59 was combined with an outer membrane-permeant, live Salmonella typhimurium cells suspended in Tris–HCl buffer were lysed. LysWL59 (2.50 mmol/L) in combination with EDTA (0.50 mmol/L) removed 93.03% of S. typhimurium biofilms on lettuce within 1 h [75].
4.3 Binding to Drug Molecules
Numerous recent studies have combined endolysins with materials such as drug molecules to improve the stability and antimicrobial properties of endolysins. The potential of using nanoparticles (NPs) for biofilm control and eradication has attracted increasing scientific interest [76, 77]. In 2017, Liu et al. immobilized endolysin proteins on a layered zeolite imidazole acid framework (ZIF-8) and analyzed the interaction between AgTiO2 nanoparticles and endolysin, providing important biological applications [78]. Wang et al. [79] obtained endolysin-immobilized chitosan nanoparticles (Lys-CS-NPs) by integrating endolysin into chitosan nanoparticles (CS-NPs) by using an ionic gelation technique, which significantly improved the thermal stability and reusability of endolysin. Furthermore, Lys-CS-NPs exhibited excellent bacterial inhibition based on in vitro killing kinetics and the minimum inhibitory concentration of CS-NPs and Lys-CS-NPs against P. aeruginosa [79]. Liu et al. [80] showed that coating endolysin with polyγ-glutamic acid and chitosan could broaden the antibacterial spectrum and improve the antibacterial activity of composite endolysin nano-reagents. Chhibber et al. used divalent cobalt ions on plates to limit iron content and combined with phage KP01K2, NDP, and endolysin and found that the formation of biofilm of Klebsiella pneumoniae B5055 was prevented. From this result, it is clear that the combination of iron antagonists such as CoSO4 and phage endolysin can be used as an adjuvant therapy to prevent bacterial biofilm formation [81]. Zhang et al. [82] showed by laser confocal microscopy supplemented with electron microscopy that combined treatment with phage, endolysin, and chlorine was an effective method for controlling and eliminating bacterial biofilms on various surfaces. The combined use of 3 × 107 PFU/mL of phage and 210 mg/L of chlorine was able to reduce the growth of 94% of the biofilm and remove 88% of the formed P. aeruginosa biofilm.
5 Conclusion
Bacterial phage endolysins can break the cell wall of bacteria rapidly and efficiently, and no direct adverse effects on humans have been reported. Its specificity is between that of antibiotics and phages, and bacteria are less likely to develop tolerance to it. Currently, it is possible to transform bacteria using recombinant DNA and plasmids, thus expressing the target lytic enzyme in large quantities, making it easily available. Compared with conventional antibiotics, endolysins also possess the following unique properties. (1) An evolutionary advantage: endolysins are derived from phages that have co-evolved with their host bacteria. This phenomenon has been preserved by natural selection. (2) High specificity: the functional endolysin domain that binds to the bacterial cell wall recognizes only specific species of bacteria, making endolysins highly specific. (3) High bactericidal activity: endolysins are “weapons” for releasing daughter phages and are thus naturally efficient. (4) Very low potential for drug resistance development: owing to the pressure of natural selection, phage endolysins only act on the essential and conserved parts of the host bacterium, and bacteria are rarely able to develop resistance to escape this recognition. Therefore, it has some advantages as a novel antibacterial drug. However, phage endolysins have also some problems: (i) some natural endolysins expressed in E. coli are toxic to the expressing strain, and proteins are often expressed in inclusion bodies [83], (ii) endolysins are vulnerable to protease attack after entering the organism and have a short half-life [84], and (iii) it is difficult to determine the optimal time and optimal dose of endolysins in the treatment process [84]. Although endolysins have some drawbacks, theoretical and experimental studies support the use of genetic engineering and protein engineering to mutate prophage genes, replace lytic enzyme genes, modify structural domains, synthesize lytic enzyme chimeras with different lytic activities, and modify and optimize endolysins to achieve the goals of high yield, high efficiency, broad spectrum, and stability, making them ideal antimicrobial substances that clinicians seek. Endolysin has a large developmental value in antibacterial activity and is expected to be an effective candidate for solving the problem of drug-resistant bacteria through continuous research. Thus, phage endolysins may be indispensable weapons against pathogenic biofilms.
Abbreviations
- GlcNAc:
-
N-acetylglucosamine
- MurNAc:
-
N-acetylmuramic acid
- CBD:
-
Cell wall-binding domain
- CD:
-
Catalytic domain
- EADs:
-
Enzymatically active domains
- kDa:
-
KDalton
- L-peptide:
-
Linking-peptide
- Biofilms:
-
Bacterial biofilms
References
Percival SL, Malic S, Cruz H, Williams DW (2011) Introduction to biofilms. Springer, Berlin
Habash M, Reid G (1999) Microbial biofilms: their development and significance for medical device-related infections. J Clin Pharmacol 39:887–898. https://doi.org/10.1177/00912709922008506
Maunders E, Welch M (2017) Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnx120
Simes M, Borges A, Simes L (2020) Recent trends in biofilm science and technology. Academic Press, London
Sass P, Bierbaum G (2007) Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol 73:347–352. https://doi.org/10.1128/AEM.01616-06
Monroe D (2007) Looking for chinks in the armor of bacterial biofilms. PLOS Biol 5(11):e307. https://doi.org/10.1371/journal.pbio.0050307
Loessner MJ (2005) Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol 8:480–487. https://doi.org/10.1016/j.mib.2005.06.002
Young I, Wang I-N, Roof WD (2000) Phages will out: strategies of host cell lysis. Trends Microbiol 8:120–128. https://doi.org/10.1016/s0966-842x(00)01705-4
Briers Y, Volckaert G, Cornelissen A, Lagaert S, Michiels CW, Hertveldt K, Lavigne R (2007) Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages φKZ and EL. Mol Microbiol 65:1334–1344. https://doi.org/10.1111/j.1365-2958.2007.05870.x
Borysowski J, Weber-Dabrowska B, Górski A (2006) Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood) 231:366–377. https://doi.org/10.1177/153537020623100402
Navarre WW, Ton-That H, Faull KF, Schneewind O (1999) Multiple enzymatic activities of the murein hydrolase from staphylococcal phage φ11. J Biol Chem 274:15847–15856. https://doi.org/10.1074/jbc.274.22.15847
Rodríguez L, Martínez B, Zhou Y, Rodríguez A, Donovan DM, García P (2011) Lytic activity of the virion-associated peptidoglycan hydrolase HydH5 of Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88. BMC Microbiol 11:138. https://doi.org/10.1186/1471-2180-11-138
Yang H, Wang DB, Dong Q, Zhang Z, Cui Z, Deng J, Yu J, Zhang XE, Wei H (2012) Existence of separate domains in lysin PlyG for recognizing Bacillus anthracis spores and vegetative cells. Antimicrob Agents Chemother 56:5031–5039. https://doi.org/10.1128/AAC.00891-12
Young R (1992) Bacteriophage lysis: mechanism and regulation. Microbiol Rev 56:430–481. https://doi.org/10.1128/mr.56.3.430-481.1992
Vollmer W, Blanot D, de Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167. https://doi.org/10.1111/j.1574-6976.2007.00094.x
Shannon R, Radford DR, Balamurugan S (2020) Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Crit Rev Food Sci Nutr 60:1631–1640. https://doi.org/10.1080/10408398.2019.1584874
Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ (2008) Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J Appl Microbiol 104:1–13. https://doi.org/10.1111/j.1365-2672.2007.03498.x
Hermoso JA, García JL, García P (2007) Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol 10:461–472. https://doi.org/10.1016/j.mib.2007.08.002
Loessner MJ, Kramer K, Ebel F, Scherer S (2002) C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol 44:335–349
Schmelcher M, Shabarova T, Eugster MR, Eichenseher F, Tchang VS, Banz M, Loessner MJ (2010) Rapid multiplex detection and differentiation of listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol 76:5745–5756. https://doi.org/10.1128/AEM.00801-10
Shen Y, Köller T, Kreikemeyer B, Nelson DC (2013) Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J Antimicrob Chemother 68:1818–1824. https://doi.org/10.1093/jac/dkt104
Sharma G, Rao S, Bansal A, Dang S, Gupta S, Gabrani R (2014) Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals 42:1–7. https://doi.org/10.1016/j.biologicals.2013.11.001
Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R (2019) The antistaphylococcal Lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob Agents Chemother 63:e02291-e2318. https://doi.org/10.1128/AAC.02291-18
Poonacha N, Nair S, Desai S, Tuppad D, Hiremath D, Mohan T, Vipra A, Sharma U (2017) Efficient killing of planktonic and biofilm-embedded coagulase-negative Staphylococci by bactericidal protein P128. Antimicrob Agents Chemother 61:e00457-e517. https://doi.org/10.1128/AAC.00457-17
Singh PK, Donovan DM, Kumar A (2014) Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother 58:4621–4629. https://doi.org/10.1128/AAC.00126-14
Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, Schuch R, Fischetti VA (2015) Novel phage lysin capable of killing the multidrug-resistant Gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59:1983–1991. https://doi.org/10.1128/AAC.04641-14
Wang Y, Sun JH, Lu CP (2009) Purified recombinant phage lysin LySMP: an extensive spectrum of lytic activity for swine streptococci. Curr Microbiol 58(6):609–615. https://doi.org/10.1007/s00284-009-9379-x
Fang, Y. Z., Wang, Y., & Sun, J. H. (2011) Expression of the phage lysin lysmp in Lactococcus lactis and studies on its antibiotic bioactivities. Journal of Shanghai Jiaotong University (Agricultural Science)
Leitch EC, Willcox MD (1999) Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Curr Eye Res 19:12–19. https://doi.org/10.1076/ceyr.19.1.12.5342
Hukić M, Seljmo D, Ramovic A, Ibrišimović MA, Dogan S, Hukic J, Bojic EF (2018) The effect of lysozyme on reducing biofilms by Staphylococcus aureus, Pseudomonas aeruginosa, and Gardnerella vaginalis: an in vitro examination. Microb Drug Resist 24:353–358. https://doi.org/10.1089/mdr.2016.0303
Ellison R, Giehl T, Laforce FM (1988) Damage of the outer membrane of enteric Gram-negative bacteria by lactoferrin and transferring. Infect Immun 56(11):2774–2781. https://doi.org/10.1128/IAI.56.11.2774-2781.1988
Lee CK, Rubin LG, Moldwin RM (1995) Synergy between protamine and vancomycin in the treatment of Staphylococcus epidermidis biofilms. Urology 45:720–724. https://doi.org/10.1016/S0090-4295(99)80074-0
Teichman JM (1994) Protamine sulphate and vancomycin are synergistic against Staphylococcus epidermidis prosthesis infections in vivo. J Urol 152:213–216. https://doi.org/10.1016/S0022-5347(17)32864-1
Yeaman MR, Norman DC, Bayer AS (1992) Platelet microbicidal protein enhances antibiotic–induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother 36:1665–1670. https://doi.org/10.1128/AAC.36.8.1665
Kuiper Jesse WP, Hogervorst Jolanda MA, Herpers Bjorn L, Bakker Astrid D, Jenneke KN, Nolte Peter A, Krom Bastiaan P (2021) The novel endolysin XZ700 effectively treats MRSA biofilms in two biofilm models without showing toxicity on human bone cells. Biofouling. https://doi.org/10.1080/08927014.2021.1887151
Żebrowska J, Żołnierkiewicz O, Ponikowska M, Puchalski M, Krawczun N, Makowska J, Skowron P (2022) Cloning and characterization of a thermostable endolysin of bacteriophage TP-84 as a potential disinfectant and biofilm-removing biological agent. Int J Mol Sci 23(14):7612. https://doi.org/10.3390/ijms23147612
Christine L, Vera O, Lenka PT, Timo S, Rocío B, Leen VS, Mario V, Lorenzo C (2022) Preclinical data on the gardnerella-specific endolysin PM-477 indicate its potential to improve the treatment of bacterial vaginosis through enhanced biofilm removal and avoidance of resistance. Antimicrob Agents Chemother. https://doi.org/10.1128/aac.02319-21
William J, Alicia W, Frederique KW, Christopher D, Lee BJ, Suzanne H, David C, Matthew C, Gordon R, Ryan K (2023) In vitro bacterial vaginosis biofilm community manipulation using endolysin therapy. Biofilm. https://doi.org/10.1016/j.bioflm.2022.100101
Fursov MV, Abdrakhmanova RO, Antonova NP, Vasina DV, Kolchanova AD, Bashkina OA, Rubalsky OV, Samotrueva MA, Potapov VD, Makarov VV, Yudin SM, Gintsburg AL, Tkachuk AP, Gushchin VA, Rubalskii EO (2020) Antibiofilm activity of a broad-range recombinant endolysin LysECD7. Vitro In Vivo Study. https://doi.org/10.3390/v12050545
Hou-Qi N, Hong L, Jing-Xue W (2021) Synergistic effects of endolysin Lysqdvp001 and ε-poly-lysine in controlling Vibrio parahaemolyticus and its biofilms. Int J Food Microbiol. https://doi.org/10.1016/j.ijfoodmicro.2021.109112
Baliga P, Goolappa PT, Shekar M, Kallappa GS (2022) Cloning, characterization, and antibacterial properties of endolysin LysE against planktonic cells and biofilms of Aeromonas hydrophila. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09880-7
Oh HK, Hwang YJ, Hong HW, Myung H (2021) Comparison of Enterococcus faecalis biofilm removal efficiency among bacteriophage PBEF129, Its endolysin, and cefotaxime. Viruses 13:426. https://doi.org/10.3390/v13030426
Oliveira H, Thiagarajan V, Walmagh M, Sillankorva S, Azeredo J (2014) A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against Gram-Negative pathogens in presence of weak acids. PLoS ONE 9(10):1–11. https://doi.org/10.1371/journal.pone.0108376
Zhang J, Xu L-L, Gan Dan, Zhang X (2018) In vitro study of bacteriophage AB3 endolysin LysAB3 activity against Acinetobacter baumannii biofilm and biofilm-bound A. baumannii. Clin Lab 64:6. https://doi.org/10.7754/Clin.Lab.2018.180342
Fenton M, Ross P, McAuliffe O, O’Mahony J, Coffey A (2010) Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. https://doi.org/10.4161/bbug.1.1.9818
Lu TK, Collins JJ (2007) Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci USA 104:11197–11202. https://doi.org/10.1073/pnas.0704624104
Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR (2007) LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol 73:7150–7154. https://doi.org/10.1128/AEM.01783-07
Donovan DM, Dong S, Garrett W, Rousseau GM, Moineau S, Pritchard DG (2006) Peptidoglycan hydrolase fusions maintain their parental specificities. Appl Environ Microbiol 72:2988–2996. https://doi.org/10.1128/AEM.72.4.2988-2996.2006
Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM (2009) Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 443:32–41. https://doi.org/10.1016/j.gene.2009.04.023
Rodríguez-Rubio L, Martínez B, Rodríguez A, Donovan DM, García P (2012) Enhanced staphylolytic activity of the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 HydH5 virion-associated peptidoglycan hydrolase: fusions, deletions, and synergy with LysH5. Appl Environ Microbiol 78:2241–2248. https://doi.org/10.1128/AEM.07621-11
Jagielska E, Chojnacka O, Sabała I (2016) LytM fusion with SH3b-like domain expands its activity to physiological conditions. Microb Drug Resist 22:461–469. https://doi.org/10.1089/mdr.2016.0053
Yang H, Zhang Y, Yu J, Huang Y, Zhang XE, Wei H (2014) Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 58:536–542. https://doi.org/10.1128/AAC.01793-13
Dong Q, Wang J, Yang H, Wei C, Yu J, Zhang Y, Huang Y, Zhang XE, Wei H (2015) Construction of a chimeric lysin Ply187N-V12C with extended lytic activity against staphylococci and streptococci. Microb Biotechnol 8:210–220. https://doi.org/10.1111/1751-7915.12166
Yang H, Zhang Y, Yu J, Huang Y, Xian-En H (2014) Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 58:536–542. https://doi.org/10.1128/AAC.01793-13
Yang H, Zhang Y, Huang Y, Yu J, Wei H (2014) Degradation of methicillin-resistant Staphylococcus aureus biofilms using a chimeric lysin. Biofouling 30:667–674. https://doi.org/10.1080/08927014.2014.905927
Fernandes S, Proença D, Cantante C, Silva FA, Leandro C, Lourenço S, Milheiriço C, de Lencastre H, Cavaco-Silva P, Pimentel M, são-José C, (2012) Novel chimerical endolysins with broad antimicrobial activity against methicillin-resistant Staphylococcus aureus. Microb Drug Resist 18:333–343. https://doi.org/10.1089/mdr.2012.0025
Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA (2011) A novel chimeric lysin shows superiority to Mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother 55:738–744. https://doi.org/10.1128/AAC.00890-10
Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA (2010) Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. https://doi.org/10.1128/AAC.01625-09
Signph PK, Donovan DM, Kumar A (2014) Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother 58(8):4621–4629. https://doi.org/10.1128/AAC.00126-14
Schmelcher M, Tchang VS, Loessner MJ (2011) Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb Biotechnol 4:651–662. https://doi.org/10.1111/j.1751-7915.2011.00263.x
Vasala A, Välkkilä M, Caldentey J, Alatossava T (1995) Genetic and biochemical characterization of the Lactobacillus delbrueckii subsp. lactis bacteriophage LL-H lysin. Appl Environ Microbiol 61:4004–4011. https://doi.org/10.1128/aem.61.11.4004-4011.1995
Loessner MJ, Gaeng S, Scherer S (1999) Evidence for a holin-like protein gene fully embedded out of frame in the endolysin gene of Staphylococcus aureus bacteriophage 187. J Bacteriol 181:4452–4460. https://doi.org/10.1128/JB.181.15.4452-4460.1999
Meng WU, Hai-Rong LU, Qingshan H (2016) Expression of CHAP structural domain of Staphylococcus aureus phage lytic enzyme Ply187 and analysis of antibacterial activity. Biotechnol Bull 32(9):232–238. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2016.09.031
Horgan M, O’Flynn G, Garry J, Cooney J, Coffey A, Fitzgerald GF, Ross RP, McAuliffe O (2009) Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl Environ Microbiol 75:872–874. https://doi.org/10.1128/AEM.01831-08
Fenton M, Ross RP, McAuliffe O, O’Mahony J, Coffey A (2011) Characterization of the staphylococcal bacteriophage lysin CHAP(K). J Appl Microbiol 111:1025–1035. https://doi.org/10.1111/j.1365-2672.2011.05119.x
Mayer MJ, Garefalaki V, Spoerl R, Narbad A, Meijers R (2011) Structure-based modification of a Clostridium difficile-targeting endolysin affects activity and host range. J Bacteriol 193:5477–5486. https://doi.org/10.1128/JB.00439-11
Fenton M, Keary R, McAuliffe O, Ross RP, O’Mahony J, Coffey A (2013) Bacteriophage-derived peptidase CHAP(K) eliminates and prevents staphylococcal biofilms. Int J Microbiol 2013:625341. https://doi.org/10.1155/2013/625341CHAP(K)
Violeta RC, Pedro G, Gema DP, Ernesto G, Matilde G, Lorena H et al (2007) In vitro interactions of lyta, the major pneumococcal autolysin, with two bacteriophage lytic enzymes (cpl-1 and pal), cefotaxime and moxifloxacin against antibiotic-susceptible and -resistant Streptococcus pneumoniae strains. J Antimicrob Chemother 5:1159–62
Filatova LY, Donovan DM, Ishnazarova NT, Foster-Frey JA, Becker SC, Pugachev VG, Balabushevich NG, Dmitrieva NF, Klyachko NL (2016) A chimeric LysK-lysostaphin fusion enzyme lysing Staphylococcus aureus cells: a study of both kinetics of inactivation and specifics of interaction with anionic polymers. Appl Biochem Biotechnol 180:544–557. https://doi.org/10.1007/s12010-016-2115-7
Djurkovic S, Loeffler JM, Fischetti VA (2005) Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob Agents Chemother 49:1225–1228. https://doi.org/10.1128/AAC.49.3.1225-1228.2005
McCarthy MW (2022) Exebacase: a novel approach to the treatment of Staphylococcal infections. Drugs R D 22:113–117. https://doi.org/10.1007/s40268-022-00383-6
Briers Y, Walmagh M, Van Puyenbroeck V, Cornelissen A, Cenens W, Aertsen A, Oliveira H, Azeredo J, Verween G, Pirnay JP, Miller S, Volckaert G, Lavigne R (2014) Engineered endolysin-based “artilysins” to combat multidrug-resistant Gram-negative pathogens. mBio 5:e01379–e01314
Lim JA, Shin H, Kang DH, Ryu S (2012) Characterization of endolysin from a Salmonella typhimurium-infecting bacteriophage SPN1S. Res Microbiol 163:233–241. https://doi.org/10.1016/j.resmic.2012.01.002
Plotka M, Kapusta M, Dorawa S, Kaczorowska AK, Kaczorowski TTS (2019) Ts2631 endolysin from the extremophilic Thermus scotoductus bacteriophage vB_Tsc2631 as an antimicrobial agent against Gram-negative multidrug-resistant bacteria. Viruses 11:657. https://doi.org/10.3390/v11070657
Liu A, Wang Y, Cai X, Jiang S, Cai X, Shen L, Liu Y, Han G, Chen S, Wang J, Wu W, Li C, Liu S, Wang X (2019) Characterization of endolysins from bacteriophage LPST10 and evaluation of their potential for controlling Salmonella Typhimurium on lettuce. LWT 114:108372. https://doi.org/10.1016/j.lwt.2019.108372
Berini F, Orlandi V, Gornati R, Bernardini G, Marinelli F (2022) Nanoantibiotics to fight multidrug resistant infections by Gram-positive bacteria: hope or reality? Biotechnol Adv 57:107948. https://doi.org/10.1016/j.biotechadv.2022.107948
Liu Y, Shi L, Su L, van der Mei HC, Jutte PC, Ren Y, Busscher HJ (2019) Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev 48:428–446. https://doi.org/10.1039/c7cs00807d
Liu G, Xu Y, Han Y, Wu J, Xu J, Meng H, Zhang X (2017) Immobilization of lysozyme proteins on a hierarchical zeolitic imidazolate framework (ZIF-8). Dalton Trans 46:2114–2121. https://doi.org/10.1039/c6dt04582k
Wang Y, Li S, Jin M, Han Q, Liu S, Chen X, Han Y (2020) Enhancing the thermo-stability and anti-bacterium activity of lysozyme by immobilization on chitosan nanoparticles. Int J Mol Sci 21:1635. https://doi.org/10.3390/ijms21051635
Liu Y, Sun Y, Xu Y, Feng H, Fu S, Tang J, Liu W, Sun D, Jiang H, Xu S (2013) Preparation and evaluation of lysozyme-loaded nanoparticles coated with poly-γ-glutamic acid and chitosan. Int J Biol Macromol 59:201–207. https://doi.org/10.1016/j.ijbiomac.2013.04.065
Chhibber S, Nag D, Bansal S (2013) Inhibiting biofilm formation by Klebsiella pneumoniae B5055 using an iron antagonizing molecule and abacteriophage. BMC Microbiol 13:174. https://doi.org/10.1186/1471-2180-13-174
Zhang Y, Hu Z (2012) Combined treatment of Pseudomonas aeruginosa biofilms with bacteriophages and chlorine. Biotechnol Bioeng 110(1):286–295. https://doi.org/10.1002/bit.24630
Kovalskaya N, Foster-Frey J, Donovan DM (2016) Antimicrobial activity of bacteriophage endolysin produced in Nicotiana benthamiana plants. J Microbiol Biotechnol 26(1):160–170. https://doi.org/10.4014/jmb.1505.05060
Nelson DC, Schmelcher M, Rodriguez-Rubio L, Klumpp J, Pritchard DG, Dong S, Donovan DM (2012) Chapter 7—endolysins as antimicrobials. Adv Virus Res 83:299–365. https://doi.org/10.1016/B978-0-12-394438-2.00007-4
Acknowledgements
The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities, grant number E1E40506 and Weiqiao-UCAS Special Projects on Low-Carbon Technology Development (No. GYY-DTFZ-2022-008).
Author information
Authors and Affiliations
Contributions
Conceptualization, BL, and XL; methodology, BL; software, ZL; validation, QG; formal analysis, BL; investigation, XL; resources, XL; data curation, XG; writing—original draft preparation, BL; writing—review and editing, QG; visualization, ZL, and BL supervision, XL; project administration, XL; funding acquisition, XL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, B., Guo, Q., Li, Z. et al. Bacteriophage Endolysin: A Powerful Weapon to Control Bacterial Biofilms. Protein J 42, 463–476 (2023). https://doi.org/10.1007/s10930-023-10139-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-023-10139-z