Abstract
The influential separation of an individual plastic from a mixture of waste plastics is a crucial factor for the quality evaluation of the recycled product. The selected engineering plastics available in municipal and industrial wastes including, acrylonitrile butadiene styrene, polycarbonate, and poly oxy methylene microwave irradiated for different microwave powers. The irradiated plastics conditioned with the selected dual depressants with different concentrations and after that, they introduced into the flotation tank with tap water as liquid media. The effects of microwave power and depressant concentration on the hydrophilic-hydrophobic (sink-float) properties of the plastic surface evaluated. It revealed, the microwave irradiation changed the capacity (the adsorbed depressant mono or multilayer molecules), numbers, and concentration of the plastic surface active sites. It resulted in different sink-float properties of the studied plastics. It was beneficial for the flotation of some plastics and depressants. The results evidenced by different techniques including, contact angles (θ), ATR-FTIR spectra, and AFM and SEM images. The driven equations by a design of experiment software (Design-Expert \(\circledR\)) showed suitable conformity between the predicted and actual plastic flotation values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The annual average growth rate of global plastics consumption was estimated as 6% [1] and, it already reached 390 million MT by 2020. This massive growth refers to replacing plastics with other materials, i.e., metals, woods, and ceramics, in daily applications. The plastics owe their eligibility to some of the distinguished specifications compared with other consumable materials including, hygienic, lightweight, and cost-effective. Hence, the increase of plastics in waste streams is the logical consequence of the consumption growth rate [2]. The availability of waste plastics may cause environmental severe issues because most of them need hundreds of years to degrade at ambient conditions. Recycling plastics not only reduces their environmental impacts but also saves our resources, namely, oil, coal, and natural gas [3]. The most plastics are thermoplastic. They have the capability to re-melt several times without major deterioration in their chemical structures to produce new articles. This is the first strategy in plastics recycling known as re-using. However, several difficulties usually available in front. The plastics in the waste stream typically are not pure, and we face a mixture of them in the environment. As any plastic has its individual physical properties, i.e., melting temperature, thermal stability, the presence of any impurity may alter the properties of the finished (recycled) product [4]. The influential separation of an individual plastic from a mixture of waste plastics is a crucial factor for the quality evaluation of the recycled product. Presently, regardless of manual separation, several techniques are used for plastics separation including, triboelectric separation [5,6,7], selective dissolution [8,9,10] and, gravity or density separation [11,12,13,14]. However, the techniques mentioned above suffer from different drawbacks, namely: labor-intensive and cost, low efficiency, high operative expenses, and toxic operations [1]. The recently developed float-sink (selective flotation) technique is an efficient, safe, simple, and cost-effective and straight forward method for separating individual plastic from a mixture of plastics [15, 16]. In this technique, a depressant (chemical agent) adsorbs on the plastic’s surface selectively. The adsorption of the depressant on the plastic surface alters the surface energy of the polymer, and make it more hydrophilic or hydrophobic, depending on the chemical nature of the depressant. If liquid media will be water, for the former case, the plastic sinks at the bottom of the flotation tank, and for the latter, it floats on the top of the liquid media (water). During the flotation process of a plastic particle, some air bubbles (air bubbles provide by a series of nozzles located at the bottom of the flotation tank) attach to the surface of the plastic make the density of the bubble-plastic complex (\({\rho }_{a}\)) less than the density of the liquid media. The consequence is the flotation of the plastic particle on the top of the liquid media surface. The following equation was proposed [17]:
where \({\rho }_{p}\),\({V}_{p}\), \({V}_{b}\) and \(n\) are the density and volume of the plastic particle, the volume of the bubble, and the number of attached bubbles, respectively.
The researchers already studied on float-sink separation of PVC, PS and PET [18], POM,PC and ABS [19], PVC and PET [20], PVC, PET and POM [21], PET and HDPE [22], PC,PS and POM [23] and ABS, HIPS, PC, PVC and PET using different depressants [24,25,26,27,28].
The microwave frequencies and wavelengths lie down between 300 MHz to 300 GHz and 0.001 to 1 m, respectively [29]. Among various industrial applications, i.e., in rubber recycling, microwave irradiation uses for breaking the sulfur bridges between macromolecules in vulcanized rubber [30,31,32]. The microwaves modify the plastic surface by changing the roughness as well as type and concentration of the attached functional groups on the plastic surface [33]. Only limited previous research activities reported in the literature for the flotation of the plastics with the aid of the microwave irradiation [34,35,36,37,38]. The studies focused on the few plastics and depressants and no systematic study observed in related field.
Since 1940, the acrylonitrile butadiene styrene (ABS) Plastic was known as a common thermoplastic and it is the most popular engineering polymer. It is a copolymer of three different monomers acrylonitrile, butadiene, and styrene. It has excellent physical properties including, excellence resistance to chemicals, dimensional stability, and creep resistance. The production (consumption) of the ABS increases continuously and it reached to the value of 10.8 million MT in 2016 [19]. Poly oxy methylene (POM) is an engineering thermoplastic and it was discovered by a German scientist named H. Staudinger in 1953 [19]. Owing to unique stiffness, dimensional stability, and low friction, it widely uses in the precision parts. Polycarbonate (PC) as an engineering thermoplastic [19] was used in electronic applications widely. It shows perfect electrical insulation, flame retardant, and heat resistance properties.
The above-mentioned engineering plastics usually available in waste streams in mix and they should separate from each other for efficient further re-using.
In this study, the surface of several engineering plastics including, Poly oxy methylene (POM), polycarbonate (PC), and acrylonitrile butadiene styrene (ABS) pre-irradiated by microwaves at different microwave level (%). In the next stage, their surfaces were conditioned with selected dual depressants, methylcellulose (MC), tannic acid (TA), polyvinyl alcohol (PVA), and polyethylene glycol (PEG) at different operative conditions before introducing them into the flotation tank. Subsequently, their float-sink behaviors recorded and the results discussed. The outcomes of the study may use in plastics waste management in commercial scale.
Experimental
Materials
The specifications of the used plastics, poly oxy methylene (POM), acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) shown in Table 1. As observed, the shape of all granules were sphere with the same diameter (4 mm). The polyvinyl alcohol (PVA, depressant, BP 20, LIWEI CHEMICAL CO. LTD, China), methylcellulose (MC, depressant, 274429 Sigma, average molecular weight, 40000, SIGMA ALDRICH, USA), polyethylene glycol (PEG, depressant, PEG-400, Shree CHEM, India) and tannic acid (TA, depressant, 100773, Merck, Germany) were prepared and used. All plastics were washed with de-ionized water and subsequently well dried before microwave pre-irradiation.
Equipment and Testing Procedure
A 30 L laboratory microwave (GMO-530, GOSONIC) with a maximum (100%) output power of 900 W, frequency of 2000 MHz used to irradiate the studied plastics surfaces. In total, 60 granules of the mixed plastics (20 from each plastic) were introduced into the microwave apparatus at different output powers for 20 s. The selected irradiation time guarantees the prevention of un-controlled temperature rise and subsequent deformation of the plastics granule. For convenience, the flotation tank media pH kept as 6.5 at ambient temperature. The residence time of granules in the flotation tank was 1 min. The employed flotation tank and test procedure explained already [18, 19]. The dimensions of the glass made flotation tank were 20 cm (diameter) by 80 cm (height). Each test repeated three times, and the median value reported. The flotation tank was equipped with an air blowing system (13 cm circular air distributor diameter) with 4 L/minute as air bubble flow rate.
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR, AVATAR 370FT-IR, TERMO Nicolet, USA) analysis was used to study the adsorption status of the depressant on a plastic surface. The morphology of the plastics surfaces was examined by an MES OEL-PV 1450 field emission gun scanning electron microscope (SEM) with a resolution of 2 nm and 20 kV with a 12 mm working distance. The contact angle (\(\theta\)) between a water droplet (50µL) and the plastic surface measured by a stereomicroscope (SZH10) equipped with a camera (Olympus DVP1) with 25 × magnitude. The surface specifications of the plastics evaluated by atomic force microscopy (AFM, full plus series 0101, ARA, Iran). The samples glued on a glassy surface, and the mean difference between the highest peaks and lowest valleys (\({R}_{z})\) was determined and reported.
Results and Discussion
The Effect of Microwave Irradiation on the Plastic Surface Characteristics
The selection of the studied depressants as sink-float agents refers to their low hazardous, reasonable cost, and availability in commercial quantity. The physical adsorption of a depressant on the plastic surface may change the hydrophilic property of the surface resulting in float or sink of the plastic particle. However, the float or sink of a plastic particle also depends on surface roughness, type and concentration of the functionalized group(s) on the surface, particle shape, density and size, air bubble coverage on the particle surface and air bubble rate and size [39].
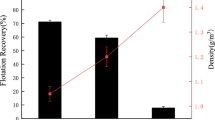
Microwave irradiation is an economical and environment-friendly technique for surface modification of the polymers [40,41,42,43]. It changes the roughness and morphology of the plastic surface, facilitates or de-facilitates the depressant adsorption on the plastic surface depending on the processing condition. It also alters the concentration and type of the probable attached functional groups on the polymer surface [42]. As an illustration and to assess the effect of microwave irradiation on the surface hydrophilic property of the plastic, the contact angles (θ) of a water droplet on the POM surface measured. The θ values for the plastic mentioned above before and after 100% power microwave irradiation were 122 and 118°, respectively. As observed, the microwave irradiation reduced θ value of the POM surface which means, the microwave irradiation increased the hydrophilic property of the plastics surface. In the other word, microwave irradiation caused and accelerated the migration of some hydrophilic groups to the POM surface. Also, conditioning the microwave irradiated of POM with 100% power with TA-PVA (dual depressant containing equal weight ratios of TA and PVA with total concentration of 800 \(\mathrm{mg}/{\mathrm{cm}}^{3})\) was more effective on hydrophilic property enhancement of the POM with the θ value of 106. Increasing the TA-PVA concentration to 2000 \(\mathrm{mg}/{\mathrm{cm}}^{3}\) reduced the θ value to 105. The above-mentioned results conformed with flotation % reduction of non-irradiated POM from 85% to the values of 75, 15, and 0% for irradiated without depressant and with 800 and 2000 \(\mathrm{mg}/{\mathrm{cm}}^{3}\) TA-PVA, respectively.
Figure 1 shows the SEM (Scanning Electron Microscope) images for the same samples. As observed, microwave irradiation was utterly practical on the roughness of the plastic (POM) surface. The microwave irradiation smoothened the plastic surface and reduced the roughness. However, the modified surface may facilitate the depressant’s adsorption (TA-PVA) on the POM surface. The same observations seen from AFM (Atomic Force Microscope) images (Fig. 2). As observed, the \({R}_{z}\) value for the non-irradiated POM surface was 365.8 nm, which was reduced to 97 nm after microwave irradiation. However, after conditioning the POM surface with TA-PVA, the \({R}_{z}\) values again increased to 178 and 165 nm for TA-PVA concentrations of 800 and 2000 mg/L, respectively.
Figure 3 represents the ATR-FTIR (Attenuated total reflectance-Fourier transform infrared spectroscopy) spectra of the mentioned samples. As observed, the microwave irradiation changed the type and concentration of some functional groups. As an illustration, the observed peak around 3400 \({\mathrm{cm}}^{-1}\) dedicated to the O–H group in contributed materials, i.e., water, TA, and or PVA. The intensity of the mentioned peaks was changed before and after irradiation and also after adsorption of TA-PVA on the POM surface with two concentrations of 800 and 2000 mg/L. As another example, the infrared absorption bands for C-O appear at wavelengths 900–1400 [44]. As observed, the intensities of the appeared peaks in mentioned wavelengths were different for studied samples.
The Sink-Float Behavior of the Studied Plastics
The microwave power level (%) selected as criteria for the assessing the rate of microwave irradiation on the plastic surface alteration. Figures 4, 5, 6, 7, 8 and 9 show the poly oxy methylene (POM), polycarbonate (PC), and acrylonitrile butadiene styrene (ABS) flotation at different concentrations of MC-PVA, MC-PEG, PVA-PEG, TA-PEG, MC-TA, and TA-PVA for various microwave power levels (%), respectively. The flotation tank media pH kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to the flotation tank. Figures 4, 6 and 7 (only for PC and ABS), 8 and 9 show that the conditioning of the plastic surface with depressants MC-PVA, PVA-PEG, TA-PEG, MC-TA, and TA-PVA, respectively, reduced the flotation of all plastics dramatically. In the mentioned samples, most of the floated plastics sunk at the bottom of the flotation tank after microwave irradiation and subsequent conditioning with a depressant. As shown in Fig. 4, the flotation of POM, PC, and ABS without depressant and all microwave power %’s ranged between 55–85%, 65–90%, and 75–95%, respectively. Using the MC-PVA as a dual depressant at studied concentrations 400, 800, 1200, 1600, and 2000 mg/L reduced the flotation % of the mentioned plastics POM, PC, and ABS to 5–45%, 0–10%, and 5–45%, respectively. The corresponding flotation % values for MC-TA (Fig. 8) and TA-PVA (Fig. 9) were 0–75% and 0–40% for POM, 0–55% and 0–35% for PC and 0–55% and 0–25% for ABS, respectively. The effect of the rest depressants, MC-PEG, PVA-PEG (for POM only), and TA-PEG (for POM only) on the flotation of the studied plastics were lower when compared with MC-PVA, MC-TA and TA-PVA. As an illustration, the flotation of ABS (Fig. 5) without and with 400, 800, 1200, 1600 and 2000 mg/L depressant MC-PEG, were ranged between 75–95%, 60–90%, 55–95%, 50–95%, 45–90% and 75–95%, respectively.
The poly oxy methylene (POM), polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) flotation (%) at different concentrations of MC-PVA for various microwave power levels (%). The flotation tank media pH was kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to flotation tank
The poly oxy methylene (POM), polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) flotation (%) at different concentrations of MC-PEG for various microwave power levels (%). The flotation tank media pH was kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to flotation tank
The poly oxy methylene (POM), polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) flotation (%) at different concentrations of PVA-PEG for various microwave power levels (%). The flotation tank media pH was kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to flotation tank
The poly oxy methylene (POM), polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) flotation (%) at different concentrations of TA-PEG for various microwave power levels (%). The flotation tank media pH was kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to flotation tank
The poly oxy methylene (POM), polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) flotation (%) at different concentrations of MC-TA for various microwave power levels (%). The flotation tank media pH was kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to flotation tank
The poly oxy methylene (POM), polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) flotation (%) at different concentrations of TA-PVA for various microwave power levels (%). The flotation tank media pH was kept as 6.5 at ambient temperature. All plastics were irradiated by microwaves for 20 s before introducing to flotation tank
The initial flotation % of POM, PC and ABS without microwave irradiation and without any depressant were 85, 80, and 90%, respectively (Figs. 4, 5, 6, 7, 8, 9). However, after microwave irradiation, their flotation %’s altered to the values of 55–85% (POM), 65–90% (PC), and 75–95% (ABS) depending on used microwave power. The minimum flotation %’s for POM, PC and ABS were 55 at 60% microwave power, 65% at 80% microwave power and 75% at 60% microwave power, respectively. As observed, microwave irradiation and depressant conditioning of the plastic surface were influential on the flotation ability of all studied plastics. However, the effectiveness was with different values and trends. For some microwave powers and depressants, the plastic flotation improved, while, in some cases, they were not beneficial for the plastic flotation. Instead, they helped the plastics to sink at the bottom of the flotation tank.
To describe the adsorption of a chemical (depressant) on the plastic surface, a deep understanding of the adsorption–desorption mechanisms is required [45,46,47].
As an illustration, the microwave irradiation together with aqueous alkaline environment provided by the available humidity in atmosphere and also, aqueous depressants solution accelerated the nitrile and butadiene hydrolysis of the ABS [48]. The nitrile and butadiene hydrolysis increases the hydrophilicity of the ABS surface and helps the ABS granules to sink at the bottom of the flotation tank (Fig. 4). Here, the hydroxide group (OH) attaches the nitrile group and generates a carboxyl group with an intermediate alcohol group [48, 49]. The researcher also should consider several more effective factors, including the activation energy of the adsorbed depressant, the number of depressant molecules adsorbed by an individual effective site, the equilibrium constant for adsorption and desorption processes, the difference between the enthalpy of adsorption of different effective sites, the numbers of effective sites on the plastic surface, the Gibb’s free energy of the adsorption process and the interaction between depressant molecules with each other and also with the molecules on the surface of the plastic. On the other hand, from a thermodynamic point of view, for spontaneous adsorption, the sign of Gibbs free energy change (Eq. 1) should not be positive [19]:
where \(T\), \(\Delta {S}_{ad}\), \(\Delta {H}_{ad}\) and \(\Delta {G}_{ad}\) were temperature, entropy and enthalpy and absolute Gibbs free energy changes of the system during adsorption, respectively. Based on the results of this study, the authors believe that microwave irradiation changes acceptance capacity (mono or multilayer), types, and numbers of the plastic surface active- sites. It causes the change of the entropy of the system. The entropy change may be positive or negative, depending on the processing conditions. If microwave irradiation increases the entropy of the system, then the entropy change will be positive and in case of the remaining other two parameters (\(\Delta {H}_{ad}\) and \(T\) ) unchanged, the sign of \(\Delta {G}_{ad}\) may become negative and spontaneous adsorption observes. On the contrary, if microwave irradiation reduces the system’s entropy, then the \(\Delta {G}_{ad}\) may become positive and the depressant desorption process will be the dominant phenomenon. However, more careful studies should done to confirm the above-mentioned conclusions in the future. It was concluded, the microwave irradiation individually, and in combination with depressant concentration was influential on the flotation of the studied plastics with different mechanisms.
Design of Experiment (DOE)
A suitable technique for predicting a desired property as an output, with changing selected input parameters is known as the design of experiments (DOE) [50]. A Design-Expert \(\circledR\) statistical software was used to process the input data, microwave power level (%) and depressant concentration and the output, plastic flotation %. The response surface methodology (RSM) with D-Optimal method with the above mentioned factors were used to process the data. Table 2 depicts the suggested equations by Design-Expert \(\circledR\) statist software for flotation % of the studied plastics versus different microwave power level (P) and depressant concentration (C, mg/L).
Figure 10a and b illustrate the three-dimensional (3D) response surfaces to evaluate the interactive effect of microwave power level (P) and depressant concentration (MC-TA mg/L) on the floatability % of the PC and the predicted values by software versus actual value for the same system, respectively. Figure 10a represents the following equation (Table 2) in 3D:
As an example, Eq. 2 (Fig. 10a) predicts 78 and 2% PC flotation for non-irradiated without MC-TA conditioning, and with 100% microwave with 2000 mg/L MC-TA concentration, respectively. The actual values were 80 and 2% (Fig. 8), respectively. As observed, the predicted values conformed with the experimental values and actual and predicted values were close to each other.
Concluding Remarks
The plastic surface microwave irradiation may change the capacity (the adsorbed depressant mono or multilayer molecules), numbers, and concentration of the plastic surface active sites. Consequently, the irradiated surfaces showed different hydrophilic-hydrophobic (sink-float) properties. It was beneficial for the flotation of some studied plastics and depressants. The results evidenced by different techniques including, contact angles (θ), ATR-FTIR spectra, and AFM and SEM images. The driven equations by a design of experiment software (Design-Expert \(\circledR\)) showed suitable conformity between the predicted and actual plastic flotation values. The PC separated from the plastics mix using MC-PVA for all studied concentrations and microwave power levels effectively.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Wang CQ, Wang H, Fu JG, Liu YN (2015) Flotation separation of waste plastics for recycling: a review. Waste Manag 41:28–38
Al-Salem SM, Lettieri P, Baeyens J (2009) Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag 29:2625–2643
Gu F, Guo J, Zhang W, Summers PA, Hall P (2017) From waste plastics to industrial raw materials: a life cycle assessment of mechanical plastic recycling practice based on a real-world case study. Sci Total Environ 601–602:1192–1207
Hopewell J, Dvorak R, Kosior E (2009) Plastics recycling: challenges and opportunities. Philos Trans R Soc B: Biolog Sci 364:2115–2126
Li J, Wu G, Xu Z (2015) Tribo-charging properties of waste plastic granules in process of tribo-electrostatic separation. Waste Manag 35:36–41
Wu G, Li J, Xu Z (2013) Triboelectrostatic separation for granular plastic waste recycling: a review. Waste Manag 33:585–597
Silveira AVM, Cella M, Tanabe EH, Bertuol DA (2018) Application of triboelectrostatic separation in the recycling of plastic wastes. Process Saf Environ 114:219–228
Pappa G, Boukouvalas C, Giannaris C, Ntaras N, Zografos V, Magoulas K, Tassios D (2001) The selective dissolution/precipitation technique for polymer recycling: a pilot unit application. Resour Conserv Recycl 34:33–44
Weeden GS, Soepriatna NH, Wang NL (2015) Method for efficient recovery of high-purity polycarbonates from electronic waste. Environ Sci Technol 49:2425–2433
Zhao Y, Lv X, Yang W, Ni H (2017) Laboratory simulations of the mixed solvent extraction recovery of dominate polymers in electronic waste. Waste Manag 69:393–399
Malcolm Richard G, Mario M, Javier T, Susana T (2011) Optimization of the recovery of plastics for recycling by density media separation cyclones. Resour Conserv Recycl 55:472–482
Lee JJS, Mo JPT, Wu DY (2012) Polymer recovery from auto shredder residue by projectile separation method. Sustain-Basel 4:643–655
Gent R, Menendez MM, Toraño J, Torno S (2011) Optimization of the recovery of plastics for recycling by density media separation cyclones. Resour Conserv Recycl 55:472–482
Gent M (2009) Recycling of plastic waste by density separation: prospects for optimization. Waste Manag Res 27:175–187
Fraunholcz N (2004) Separation of waste plastics by froth flotation-a review, part I. Miner Eng 17:261–268
Alter H (2005) The recovery of plastics from waste with reference to froth flotation. Resour Conserv Recycl 43:119–132
Shen H, Forssberg E, Pugh R (2001) Selective flotation separation of plastics by particle control. Resour Conserv Recycl 33:37–50
Negari MS, Movahed SO, Ahmadpour A (2018) Separation of polyvinylchloride (PVC), polystyrene (PS) and polyethylene terephthalate (PET) granules using various chemical agents by flotation technique. Sep Purif Technol 194:368–376
Davari MR, Movahed SO (2019) The flotation by selected depressants as an efficient technique for separation of a mixed acrylonitrile butadiene styrene, polycarbonate and polyoxymethyleneplastics in waste streams. J Polym Environ 27:1709–1720
Burat F, Güney A, Kangal MO (2009) Selective separation of virgin and postconsumer polymers (PET and PVC) by flotation method. Waste Manag 29:1807–1813
Takoungsakdakun T, Pongstabodee S (2007) Separation of mixed post-consumer PET–POM–PVC plastic waste using selective flotation. Sep Purif Technol 54:248–252
Kangal MO (2010) Selective flotation technique for separation of PET and HDPE used in drinking water bottles. Miner Process Extr Metall Rev 31:214–223
Basařová P, Bartovská L, Kořínek K, Horn D (2005) The influence of flotation agent concentration on the wettability and flotability of polystyrene. J Colloid Interface Sci 286:333–338
Yuce AE, Kilic M (2015) separation of PVC/PET mixture from plastic wastes using column flotation technique. J Environ Prot Ecol 16:705–715
Guo J, Li X, Guo Y, Ruan J, Qiao Q, Zhang J, Bi Y, Li F (2016) Research on flotation technique of separating pet from plastic packaging wastes. Procedia Environ Sci 31:178–184
Yenial U, Burat F (2013) Separation of PET and PVC by flotation technique without using alkaline treatment. Miner Process Extr Metall Rev 34:412–421
Pascoe RD (2005) The use of selective depressants for the separation of ABS and 65 HIPS by froth flotation. Miner Eng 18:233–237
Güney A, Özdilek C, Kangal MO, Burat F (2015) Flotation characterization of PET and PVC in the presence of different plasticizers. Sep Purif Technol 151:47–56
Motasemi F, Afzal MT (2013) A review on the microwave-assisted pyrolysis technique. Renew Sustain Energy Rev 28:317–330
Molanorouzi M, Mohaved SO (2016) Reclaiming waste tire rubber by an irradiation technique. Polym Degrad Stab 128:115–125
Khavarnia M, Movahed SO (2016) Butyl rubber reclamation by combined microwave radiation and chemical reagents. J Appl Polym Sci 133:43363–43373
Movahed SO, Ansarifar A, Zohuri G, Ghaneie N, Kermany Y (2016) Devulcanization of ethylene–propylene–diene waste rubber by microwaves and chemical agents. J Elastom Plast 48:122–144
Mallampati SR, Lee CH, Park MH, Lee BK (2018) Processinplastics from ASR/ESR waste: separation of poly vinyl chloride (PVC) by froth flotation aftemicrowave-assisted surface modification. J Mater Cycles Waste Manag 20:91–99
Huang L, Wang H, Wang C, Zhao J, Zhang B (2017) Microwave-assisted surface modification for the separation of polycarbonate from poly methyl methacrylate and polyvinyl chloride waste plastics by flotation. Waste Manag Res 35:294–300
Mallampati SR, Lee CH, Park MH, Lee BK (2018) Processing plastics from ASR/ESR waste: separation of poly vinyl chloride (PVC) by froth flotation after microwave-assisted surface modification. J Mater Cycles Waste Manag 20:91–99
Truc NT, Lee BK (2017) Combining ZnO/microwave treatment for changing wettability of WEEE styrene plastics (ABS and HIPS) and their selective separation by froth flotation. Appl Surf Sci 420:746–752
Irannajad M, Mehdilo A, Nuri OS (2014) Influence of microwave irradiation on ilmenite flotation behavior in the presence of different gangue minerals. Sep Purif Technol 132:401–412
Cheng G, Li Z, Cao Y, Jiang Z (2020) Research progress in lignite flotation intensification. Int J Coal Prep Util 40:59–76
Shen H, Forssberg E, Pugh RJ (2002) Selective flotation separation of plastics by chemical conditioning with methyl cellulose. Resour Conserv Recycl 35:229–241
Aumann T, Theirich D, Engemann J (2001) Rapid surface modification of polyethylene in microwave and r.f.-plasmas: comparative study. Surf Coat Technol 142–144:169
Zhao Q, Gu X, Zhang S, Dong M, Jiang P, Hu Z (2014) Surface modification of polyamide 66 fabric by microwave induced grafting with 2-hydroxyethyl methacrylate. Surf Coat Technol 240:197–203
Saleh NS, Movahed SO, Attarbashi F (2018) Study on the anti-biofouling effects of the grafted polyamide 6 fibers by several vinyl chemicals. J Appl Polym Sci 135:46760–46770
Ginn BT, Steinbock O (2003) Polymer surface modification using microwave-oven-generated plasma. Langmuir 19:8117–8118
Kirwan LJ, van Fawell PD, Bronswijk W (2003) In situ FTIR-ATR examination of poly (acrylic acid) adsorbed onto hematite at low pH. Langmuir 19(14):5802–5807
Masel IR (1996) Principles of adsorption and reaction on solid surfaces. Wiley, New York
Zhao Y, Yang S, Wen H, Shen Z, Han F (2019) Adsorption behavior and selectivity mechanism of flotation reagents applied in ternary plastic mixtures. Waste Manag 87:565–576
Gregg GC, Sing KSW (1982) Adsorption, surface area and porosity, 2nd edn. Academic, London
Jiang H, Zhang Y, Wang H (2020) Surface reactions in selective modification: the prerequisite for plastic flotation. Environ Sci Technol 54:9742–9756
Zhang Y, Jiang H, Wang H, Wang C (2020) Separation of hazardous polyvinyl chloride from waste plastics by flotation assisted with surface modification of ammonium per sulfate: process and mechanism. J Hazard Mater 389:121918
Stat-Ease Handbook for Experimenters, Copyright ©2018 Stat-Ease, Inc. 2021 East Hennepin Ave, Suite 480 Minneapolis, MN 55413
Acknowledgements
The authors sincerely thank the staffs of the polymer chemistry laboratory located at faculty of science, Ferdowsi University of Mashhad for their sincere cooperation. Approval no. 3/53616.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
HH, SOM, and SJ designed the experiments. Dr. SOM prepared the manuscript with contributions from all co-authors. The authors applied the SDC approach for the sequence of authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoseini, H., Ostad Movahed, S. & Jourabchi, S. The Float-Sink Behavior of Selected Pre-microwave Irradiated Plastics by Surface Adsorption of Several Dual Depressants. J Polym Environ 30, 2824–2836 (2022). https://doi.org/10.1007/s10924-022-02394-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02394-5