Abstract
The virgin acrylonitrile butadiene styrene (ABS), polycarbonate (PC) and polyoxy methylene (POM) available in a plastic mix were separated from each other by a flotation technique with the aid of several depressants. Also, a Design-Expert® statistical software was used to predict plastics flotation using input data including conditioning time, flotation tank temperature and pH and depressants concentration. It revealed that the flotation technique was effective for separation of studied plastics by selected depressants. Understanding the adsorption–desorption phenomena and effective parameters on this process was crucial to explain the activity and selectivity of a depressant on a plastic surface. The increasing conditioning time up to 15 min had adverse effect on the floatability of the all studied plastics conditioned with tannic acid (TA). TA and methyl isobutyl carbinol (MIBC) had not any effect on floatability of PC and POM in all studied depressant concentrations. The flotation of ABS reached to 85% for flotation tank pH of 6.5 with 15 min depressant conditioning at 35 °C as flotation tank temperature. A complicate phenomenon involved and predominated in flotation of studied plastics. Proposed equations by Design-Expert® predicted close plastic flotation values when compared with corresponding experimental values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production and accumulation of the waste polymers including plastics and rubbers is a serious concern for modern civilization. They threaten the human–environment and ecosystem. This is due to long degradation period of the most polymers [1]. Besides, from economic viewpoint, substitution virgin polymers with recycled polymers saves the resources [2]. Normally, in waste streams, the plastics are available as mixed. Because each polymer has own individual properties and to re-using each polymer, they should be separated from each other [3].
The most important separation techniques are electrostatic, density difference, optical, selective dissolution, and new developed flotation. The electrostatic techniques [4,5,6] separates the polymers based on their different electrical characteristics. The density separation is a low cost and simple technique [7,8,9]. However, using aforementioned techniques have been limited because available polymers in waste streams have very close surface charging characteristics and densities [3]. The selective polymer dissolution [10,11,12] is an expensive process. It is a discontinuous technique and toxic organic solvents used in this technique are harmful for human body.The optical techniques rely on indirect sorting and need expensive equipment [13].The flotation separation technique initially was used for mineral ore purification [14, 15] and then extended for plastic separation [16]. In this technique, the hydrophilicity of the plastic surface alters selectivity by various methods [3]. This alteration changes the wettability of the selected polymer in the used liquid media resulting in sinking or flotation of the selected polymer. The polymer surface hydrophilicity can be changed by three main methods, adsorption of wetting agent (depressant) on polymer surface, the physical changing of the media environmentand or physical amendment of the polymer surface and chemical bonds between a chemical agent and the polymer surface.Burat et al. [17] separated a mix of PVC–PET with 96 and 99.7% using DIB and ELO as depressant, respectively.In another attempt, Thongchai and co-workers [18] recovered polyoxymethylene from a mix of PVC–PET–POM by CaLS completely. They also recovered PET with 98% purity from a mix of PET–PVC using the same depressant. Kangal and colleagues [19] studied ona mix of PET–HDPE. They found 100% recovery for HDPE (floated) with the assistance of DIB as a depressant adsorption.Basarova et al. [20] studied on a mix of PC–PS–POM using various depressants including CaLS, TA, Terpineol and PDGE. Their outcomes showed POM floatedby CaLS while all plastics floated by Terpineol. Other researchers [21,22,23,24,25] also studied on separation of ABS, HIPS, PC, PVC and PET using several depressants NaCMC, Quebracho, SDS, SCMC, NP-7, DOP, DBS, DIB, LA and TX-100. The most results showed proper separation of the selected plastics in their mix’s.Also, a numerous researchers tried to separate a mix of plastics by physical changing of the media environment. Beckman et al. [26] separated a mix of LDPE, HDPE, PP, PS and EPS by aid of CO2, a mix of PVC,PET and EPS by SF6. Pascoe and colleagues [27] used flame and oven thermos treatment on a PET–PVC system. Wang and co-workers [28] recommended boiling treatment for separation of ABS and PS. This strategy was observed in other reports [29,30,31,32,33] using various media environment physical conditions and or physical amendment of the polymer surface.
A several researchers focused on polymer surface chemical modification for different plastics mixes [34,35,36,37,38,39,40]. In this strategy, because of chemical bonds between the wetting agent and polymer surface, the flotation is more stable. However, changing the composition of the polymer surface is a drawback. The engineering polymers (plastics) have better mechanical, dynamical and or thermal characteristics when compared with traditional plastics [41]. Because they are relatively expensive, the recycling of them has enough profit for saving the resources. In recent years, among of engineering plastics, the production of acrylonitrile butadiene styrene (ABS), polycarbonate (PC) and Polyoxymethylene (POM) in waste streams increased remarkably.This waste accumulation needs modern, cheap, safe and easy technique for separation of mentioned plastics for returning them in production cycle and re-using. The selected plastics, ABS, PC and POM were categorized as engineering plastics. Because their densities were remarkably different with traditional available plastics in waste streams, i.e., PE, PP, PVC and PS, they may be separated from the aforementioned plastics by a density difference technique. However, further separation by the latter technique is not possible because selected engineering plastics have close densities. The flotation technique was chosen to separate the engineering plastics from each other. The authors already studied [3] on the separation of a mixture of traditional plastics, polystyrene (PS) polyethylene terephthalate (PET) and polyvinylchloride (PVC) using flotation technique with depressant adsorption strategy. The used depressants were polyethylene glycol (PEG), methyl cellulose (MC), polyvinyl alcohol (PVA), tannic acid (TA) and methyl isobutyl carbinol (MIBC), individually or in combination with each other. In this study, the same strategy with the same depressants were used for the separation of a mixed selected engineering plastics, PC, ABS and POM by flotation technique. Subsequently, the results were reported and discussed. The industrial and commercial application of this study was our main goal. Hence, flotation agents with three crucial properties, i.e., cheap, safe and accessible in large scale in market were chosen.
Experimental
Materials
Acrylonitrile butadiene styrene (ABS), polycarbonate (PC) and polyoxymethylene (POM), commercial granule gradesprocured from local market. The used depressants characteristics were mentioned elsewhere [3]. The used plastics shape was sphere with average 4 mm diameter. However, for better distinguishing, they had different colors. They carefully treated with de-ionized water and subsequently dried at ambient temperature before preparing for flotation process.
Methods
Equipment and Testing Procedure
The used flotation equipment and procedure already explained [3]. As mentioned, a glass made flotation tank with dimensions of 20 cm (diameter) by 80 cm (height) was employed for flotation tests. Each test was performed three times and the median value was monitored. Figure 1 [3] represents the flotation equipment. A circular air blowing system with a 13 cm air distributor diameter and a flow rate of 4 L/min was used to facilitate the flotation process. The flotation time was about 2 min in all experiments depending on the condition of flotation tank. The experiments were repeated three times and the median values were reported.
The flotation equipment and parts [3]
Design of Experiment (DOE)
The design of experiments (DOE) is a suitable technique for predicting a desired property as an output, with changing selected input parameters [42]. A Design-Expert® statistical software was used to process the input data (conditioning time, flotation tank temperature and pH and depressants concentration) and the outcome was the flotation % of the selected plastics. The response surface methodology (RSM) with D-Optimal method with aforementioned factors was employed to process the data.
Results and Discussion
The selected depressants are environment friendly materials and because of their chemical structures, they may adsorb on plastic surface and change the hydrophilicity of the plastic surface temporary [3]. The chemical composition of polymer surface is one of the major parameters affecting polymer surface wettability and also the capacity for depressant adsorption on the surface and consequent float or sink of the plastic [43]. However, the reader should also consider other effective parameters including, particle shape, equivalent diameter and density, air or gas bubble coverage on the particle surface and bubble diameter and flow rate [44]. The surface energies of the POM, PC and ABS were 44.6, 42.9 and 42.7 mN/m, respectively. If the reader compares the above mentioned values with the surface tension (energy) of the water with the value of 72.0 mN/m, then it was expected a weak wettability or large contact angles for aforementioned plastics when they immerse in this liquid media. However, because the densities of all plastics ranged between 1.05 to 1.41 g/cm3 (density of pure water 1 g/cm3), all of them were sunk at the bottom of flotation tank before treatment with depressants.
The Effect of Depressant Concentration on the Sink-Float Behavior of the Used Plastics
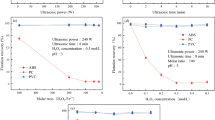
As observed from Figs. 2, 3, 4 the floatability of studied plastics, ABS, POM, and PC was effected by used depressants, TA, MC,PEG,PVA and MIBC. However, there were some exceptions, i.e., TA and MIBC had not significant effect on floatability of PC and POM in all studied depressant concentrations, respectively (Figs. 2, 4). In general, the increasing of TA, PEG and MIBC concentration was beneficial for studied plasticsflotation where for MC and PVA, it was improved or deteriorated depending on the depressant concentration. Understanding the adsorption–desorption phenomena and effective parameters on this process is crucial to explain the activity and selectivity of a depressant on a plastic surface.For depressant adsorption–desorption process following aspects may be considered [45,46,47]. (a) The plastic surface (adsorbent) must have effective and suitable sites for adsorption of the depressant (adsorbate). However, the number of sites are limit and depend on the characteristics of the adsorbent surface. (b) Each site may adsorb only one adsorbate molecule but the adsorbed molecules are not necessarily monolayer. (c) The enthalpy of adsorption for sites may not be equivalent (d) The enthalpy of adsorption depends on the degree of depressant molecules coverage on the plastic surface. (e) There are two competitive phenomenon, adsorption and desorption of the adsorbate. However,at equilibrium, the rates of adsorption and desorption are equal. (f) The rate of adsorption is small because the activation energy is small, i.e., 1 kcal/mol but it is reversible just unlike chemisorption. (g) Thermodynamically and as a spontaneous phenomenon, the Gibb’s free energy of the process should be negative. The sign and value of Gibb’s free energy depends on the sign and values of enthalpy and entropy variation of the system during adsorption and or desorption. (h) The adsorbed molecules have direct and indirect interaction on adjacent molecules and also on other molecules which still have not been adsorbed.
The main structure of TA includes hydroxyl and carboxyl groups and also benzene ring. The availability of numerous hydroxyl group in TA structure makes it capable for rapid hydrolysis with water molecules (the TA solubility in water, 2850 g/L [3]). Also, existence of carbonate ester group in PCshows the tendency of this plastic for water absorption. It seemed,the interaction between the water molecules and TA molecules were stronger when compared with the interaction between the TA and PC molecules because PC was not floated by TA in none of studied concentrations (Fig. 2). POM showed 5% flotationfor TA concentration beyond of 1600 mg/L. However, ABS was floated up to 10% when TA concentration reached to 1600 mg/L and then reduced to 5% because desorption process was speeded up. The reason refers to aqueous boundary layer diffusion (ABLD) mechanism and tendency of joining adsorbed TA molecules to TA cluster in liquid media [48]. The floatability behavior of studied plastics by MC and PVA had not any regular pattern (Figs. 2, 3). When for some MC and PVA concentrations, the floatability increased, for the rest MC and PVA concentrations, it was reduced. As an illustration, the floatability of ABS for MC concentrations of 400 and 1600 mg/L were 65 and 70% while for 800, 1200 and 2000 mg/L, it was 55, 60 and 35%, respectively. As an another example, the flotation %’s of POM for PVA concentrations of 400, 800, 1200, 1600 and 2000 mg/L were 80, 85, 70, 65 and 75%, respectively. Here, at least three mechanisms involve in adsorption–desorption process. Tendency of depressant adsorption on the plastic surface due to chemical structure similarity (MC has oxygen and methyl groups in structure), competition between water molecules and depressant molecules for adsorption on the solid surface and aqueous boundary layer diffusion. The outcome was different flotationbehaviors for used plastics for various depressant concentrations. Interestingly, the PEG was remarkably effective on flotation of all studied plastics (Fig. 3). With increasing PEG concentration to 400 mg/L, the flotation of POM, ABS and PC reached to the values of 80, 60 and 55%, respectively. Further increasing of the PEG concentration had not sensitive benefit for plastics flotation and the flotation % increased only 5 to 10%. Apparently, the chemical structure similarity (PEG has ethyl group and oxygen atom in structure) was the predominant phenomenon for adsorption of PEG molecules on the plastics surface.
MIBC is well known as a frother agent rather than depressant and normally it uses in combination with other agents. However, as observed from Fig. 4, it was ineffective for POM flotation but floated the ABS and PC, 65–70% and 20–25%, respectively. MIBC is a small molecule with hydroxyl group. It seemed the high hydrolysis capability of MIBC along with faster adsorption of water molecules in competition with MIBC molecules on the POM surface, was the reason for negligible effect of this depressant on POM flotation.
It was concluded with the exception of TA for PC and MIBC for POM, the rest used depressants showed remarkably high flotation % values for all studied plastics.
The Effect of Temperature on the Sink-Float Behavior of the Used Plastics
Table 1 depicts the effect of various flotation temperatures, 15, 25, 35, 45, and 55 °C on floatability of the POM, ABS and PC for studied depressants, TA, MC, PEG, PVA, and MIBC. The depressant concentrations in these experiments were 400 and 1600 mg/L (for MIBC, 1 and 3 ml/L) with 15 min conditioning time at a pH of 6.5.
The results showed for 400 mg/L TA concentration, increasing temperature from 15 to 25 °C had not any effect on flotation of PC and POM. However, beyond this temperatures, 20% and 30–65% flotation were observed for POM and PC, respectively. Increasing temperature had not any regular effect on flotation of ABS by this depressant. It reached to 85% when temperature increased to 35 °C and subsequently reduced to 45% at 55 °C. The effect of temperature for 1600 mg/L TA concentration was remarkably different. As observed, the flotation of all studied plastics reached to a maximum value at 35 °C and gradually reduced when temperature increased to 55 °C. For MC at 400 mg/L concentration and for PVA for both 400 and 1600 mg/L concentrations, increasing temperature had adverse effect on all plastics flotation. For example, the flotation values for MC ranged 65–20%, 50–0% and 35–0% for ABS, PC and POM, respectively when temperature increased from 15 to 55 °C. These values for PVA at 400 mg/L were 80–10%, 60–0% and 60–0% and for 1600 mg/L, they ranged 65–10%, 60–0% and 55–0% for the same plastics. However, for 1600 mg/L MC concentration, no regular trend was observed. For the left depressants, i.e., PEG and MIBC, no regular patterns were observed for plastics flotation for studied depressant s concentrations. Interestingly, 90% recovery was seen for ABS and PC using 1600 mg/L PEG concentration at 15 °C.
However, the other studies [49,50,51] showed increasing the temperature in some cases was beneficial while in other cases, it deteriorated the adsorption process. For spontaneous adsorption process, the sign of Gibbs free energy change in the following equation must be negative [3]:
where \(\Delta G_{ad}\), \(\Delta H_{ad}\), \(\Delta S_{ad}\) and \(T\) were Gibbs free energy, enthalpy and entropy changes of the system during adsorption and absolute temperature, respectively. The enthalpy change is a temperature dependent thermodynamic property. The sign of this property is negative for exothermic processes while it is positive for endothermic ones. With increasing the temperature, the values of this property change and it’s sign may also change. In addition, the sign of Eq. 1 also depends on the absolute temperature and entropy change during the adsorption process. Normally, the entropy change during adsorption is negative. Hence, the enthalpy change sign is a crucial factor. If the sign of \(\Delta G_{ad}\) becomes positive, then the adsorption process stops and the desorption process will be predominant phenomena. As a conclusion and according with observed results, temperature had different effects on flotation of studied plastics. Increasing the temperature in some temperatures and for some depressants and plastics improved adsorption process while for other cases, it increased the desorption process.Our founds were in conformity of other reports [49,50,51].
The Effect of pH on the Sink-Float Behavior of the Used Plastics
Figures 5, 6, 7 represent the ABS, POM and PC flotation versus liquid conditioning pH, 4.5, 6.5, 8.5 and 10.5 for several studied depressants for 800 mg/L (for MIBC, 2 mL/L) depressant concentration with 15 min conditioning time at 25 °C. The selected conditions were favorable from economic view point in related flotationindustry. As observed, for TA and MIBC (Figs. 5, 7), up to pH of 6.5, the flotation of all plastics reduced with different rates. Increasing pH beyond this value improved the flotation of studied plastics to the values of 55, 50 and 45% (for TA) and 55, 75 and 60% (for MIBC) for POM,ABS and PC, respectively. It was obvious that in acidic environment, the \(H^{ + }\) ion reacts with OH group of the TA and MIBC and makes them inactive for adsorption on plastic surface. However, for MC, increasing the pH up to 8.5 was beneficial for flotation of studied plastics. The flotation values ranged between 5–30%, 25–60% and 40–75% for POM, PC and POM, respectively. For the PEG, it was not observed any regular trend for flotation of plastics by increasing the pH values. The utmost flotation values for POM, ABS and PC were 50, 85 and 55%. Here, it seemed several mechanisms were simultaneously involved in adsorption of depressant on the plastic surface. Unlike of TA and MIBC, the flotation of conditioned plastics with PVA increased up to pH of 6.5 and beyond this value, it was reduced remarkably. The maximum flotation values were 85, 55 and 50% for ABS, PC and POM at studied pH values, respectively.
The Effect of Time on the Sink-Float Behavior of the Used Plastics
Figures 8, 9, 10 show the effect of conditioning time, 5, 10, 15 and 30 min on the floatability of POM, PC and ABS for the several studied depressants. The depressant concentration was kept at 800 mg/L (for MIBC, 2 ml/L) with 6.5 pH at 25 °C. Figure 8 shows increasing the conditioning time up to 15 min had adverse effect on the floatability of all studied plastics conditioned with TA. However, extra increasing of the conditioning time improved this parameter. It seemed, at initial stage of conditioning, rapid hydrolysis of TA with water molecules controlled the process and limited the adsorption of TA molecules on the plastics surface. The increasing of the MC conditioning time had not any remarkable effect on the floatability of the POM and PC because, all active sites on aforementioned plastics surface were covered by depressant after 5 and 10 min for PC and POM, respectively.The same trend also observed for PEG (Fig. 9). The studied plastics reached to ultimate flotation after 5 min and increasing conditioning time to 30 min caused only 10% variation on this parameter. No regular trends were observed for conditioning plastics with PVA and MIBC at different conditioning times. Here, different parameters affected on adsorption–desorption process as described already in text.
The Effect of Depressants Ratio on the Sink-Float Behavior of the Used Plastics
Table 2 shows the effect of a dual mixing of the studied depressants on the flotation of the ABS, PC, and POM. The temperature, conditioning time, total depressant concentration and pH were kept as 25 °C, 15 min, 800 mg/L and 6.5 with variable depressant ratios, respectively. The depressant ratios in dual systems were 0.8–0.2, 0.6–0.4 and 0.5–0.5.As observed from Table 2 for TA–PEG dual system, increasing the PEG ratio up to 0.4 increased the ABS flotation to 25%. Further increasing caused flotation reduction to 15%. Surprisingly, for PEG ratio of 0.8, the flotation reached to a maximum value of 30%. For PC, increasing PEG had adverse effect on floatability of this plastic. Although, the flotation was not exceeded than 10%. The same trend was also observed for POM with increasing PEG in TA–PEG dual system. For TA–PVA dual system, increasing PVA with the exception of 0.4–0.6 (TA–PVA) had not any remarkable effect on the floatability of ABS. However, it was completely ineffective on flotation of PC. Increasing PVA up to 0.6 increased the floatability of POM to 30% and beyond this value, it reduced the flotation of this plastic to 10%. The dual system of TA–MC showed efficient effect on floatability of ABS and it reached the floatability of above mentioned plastic to 60% when the MC ratio in dual TA–MC system reached to 0.6. However, this system was less effective on flotation of PC and POM.It seemed, a complicate phenomenon involves and predominates in flotation of studied plastics for aforementioned dual systems.
Actual Versus Predicted Results by Design of Experiment (DOE)
During the past years several number of excellent software products to assist experimenters and researchers in the design and analysis phases have appeared. Design-Expert® was widely available general-purpose statistical software packages that have excellent data analysis capabilities and it handles the analysis of the experiments with both fixed and random factors. Design-Expert® is a package focused exclusively onexperimental design which has many capabilities for construction and evaluation of designs and extensive analysis features [52]. The used response surface methodology (RSM) is a useful tool for analysis of the data [53, 54]. As an illustration and based on input data (conditioning time, flotation tank temperature and pH and MIBC concentration), the software proposed following second order polynomials for predicting the individual plastic flotation for conditioning plastics with MIBC:
In the preceding equations, T and C refer to temperature (°C) and MIBC concentration (mL/L), while t and pH are equal to conditioning time (min) and flotation vessel pH.
Figure 11 illustrates and compares the predicted values versus actual values for PC. As observed, the values were close to each other. These observations proved the eligibility of the derived equations for predicting the flotation of the studied plastics at different studied conditions.
To obtain the optimum floatation %, the Eqs. 2–4 should be differentiated with each independent variable (parameter), i.e., T, C, t and pH. In total, twelve equations (four from each equation) were derived and after solving the derived equations for aforementioned four independent variables, the optimum values were reported (Table 3). By applying and inserting the obtained optimum parameters values in Eqs. 2–4, the optimum floatation %’s for ABS, POM and PC were calculated as 96, 78 and 91%, respectively.
Figures 12, 13, 14 illustrates three-dimensional (3D) response surfaces to evaluate the interactive effect of dual independent variables, i.e., conditioning time (t) and floatation vessel temperature (T) on floatability % of the ABS, PC and POM conditioned with MIBC, respectively. As observed, for ABS (Fig. 12), the increasing the conditioning time caused the floatation % to reach to a maximum value. Beyond this value, the floatation % reduced. The reason refers to predominant phenomena, physical adsorption and desorption of MIBC molecules on ABS surface in short and long conditioning times, respectively. However, increasing temperature from 15 to 55 °C had positive effect on floatability % of the ABS. It was predicted, because increasing the temperature improves the adsorption process normally [47]. The same trends were observed for PC and POM when conditioning time increased from 5 to 30 min (Figs. 13, 14). However, increasing the temperature was beneficial for the floatation of the aforementioned plastics up to 40 °C. Apparently, increasing temperature beyond this value speeded up the desorption process of the MIBC from plastic surface.
Concluding Remarks
-
The flotation technique was effective for separation of ABS, PC and POM by selected depressants.
-
Understanding the adsorption–desorption phenomena and effective parameters on this process is crucial to explain the activity and selectivity of a depressant on a plastic surface.
-
TA and MIBC had not any effect on floatability of PC and POM in all studied depressant concentrations.
-
The floatability behavior of studied plastics by MC and PVA had not any regular pattern with depressants concentration variation.
-
For MC at 400 mg/L concentration and for PVA for both 400 and 1600 mg/L concentrations, increasing temperature had adverse effect on all studied plastics flotation.
-
Unlike of TA and MIBC, the flotation of conditioned plastics with PVA increased up to pH of 6.5 and beyond this value, it was reduced remarkably.
-
The increasing conditioning time up to 15 min had adverse effect on the floatability of the all studied plastics conditioned with TA.
-
A complicate phenomenon involves and predominates in flotation of studied plastics for selected dual systems.
-
Proposed equations by Design-Expert® predict close plastic flotation values when compared with corresponding experimental ones.
-
All experiments were performed on virgin plastics. Further investigation will be required for real available plastics in waste streams.
References
Thompson RC, Moore CJ, VomSaal FS, Swan SH (2009) Plastics, the environment and human health: current consensus and future trends. Philos T R Soc B 364:2153–2166
Gu F, Guo J, Zhang W, Summers PA, Hall P (2017) From waste plastics to industrial raw materials: a life cycle assessment of mechanical plastic recycling practice based on a real-world case study. Sci Total Environ 601–602:1192–1207
Negari MS, Ostad Movahed S, Ahmadpour A (2018) Separation of polyvinylchloride (PVC), polystyrene (PS) and polyethylene terephthalate (PET) granules using various chemical agents by flotation technique. Sep Purif Technol 194:368–376
Silveira AVM, Cella M, Tanabe EH, Bertuol DA (2018) Application of triboelectrostatic separation in the recycling of plastic wastes. Process Saf Environ 114:219–228
Li J, Wu G, Xu Z (2015) Tribo-charging properties of waste plastic granules in process of tribo-electrostatic separation. Waste Manag 35:36–41
Wu G, Li J, Xu Z (2013) Triboelectrostatic separation for granular plastic waste recycling: a review. Waste Manag 33:585–597
Gent M (2009) Recycling of plastic waste by density separation: prospects for optimization. Waste Manag Res 27:175–187
Gent R, Malcolm Menendez M, Toraño J, Torno S (2011) Optimization of the recovery of plastics for recycling by density media separation cyclones. Resour Conserv Recycl 55:472–482
Lee JJS, Mo JPT, Wu DY (2012) Polymer recovery from auto shredder residue by projectile separation method. Sustainability-Basel 4:643–655
Zhao Y, Lv X, Yang W, Ni H (2017) Laboratory simulations of the mixed solvent extraction recovery of dominate polymers in electronic waste. Waste Manag 69:393–399
Pappa G et al (2001) The selective dissolution/precipitation technique for polymer recycling: a pilot unit application. Resour Conserv Recycl 34:33–44
Weeden GS, Soepriatna NH, Wang NL (2015) Method for efficient recovery of high-purity polycarbonates from electronic waste. Environ Sci Technol 49:2425–2433
Singh N, Hui D, Singh R, Ahuja IPS, Feo L, Fraternali F (2017) Recycling of plastic solid waste: a state of art review and future applications. Compos Part B 115:409–422
Deiringer G, Edelmann G, Rauxloh B (1993) U.S.Patent 5248041
Gu GH, Hu YH, Qiu GZ, Wang H, Wang DZ (2002) Potential control flotation of galena in strong alkaline media. J Cent South Univ Technol 9:16–20
Izumi S, Tanaka H (1975) Method for separation of mixture of plastics, US
Burat F, Güney A, OlgaçKangal M (2009) Selective separation of virgin and postconsumer polymers (PET and PVC) by flotation method. Waste Manag 29:1807–1813
Takoungsakdakun T, Pongstabodee S (2007) Separation of mixed post-consumer PET–POM–PVC plastic waste using selective flotation. Sep Purif Technol 54:248–252
Kangal MO (2010) Selective flotation technique for separation of PET and HDPE used in drinking water bottles. Min Proc Ext Met Rev 31:214–223
Basařová P, Bartovská L, Kořínek K, Horn D (2005) The influence of flotation agent concentration on the wettability and flotability of polystyrene. J. Colloid Interf Sci 286:333–338
Yuce AE, Kilic M (2015) separation of PVC/PET mixture from plastic wastes using column flotation technique. J Environ Prot Ecol 16:705–715
Pascoe RD (2005) The use of selective depressants for the separation of ABS and 65 HIPS by froth flotation. Miner Eng 18:233–237
Yenial U, Burat F (2013) Separation of PET and PVC by flotation technique without using alkaline treatment. Min Proc Ext Met Rev 34:412–421
Güney A, Özdilek C, Kangal MO, Burat F (2015) Flotation characterization of PET and PVC in the presence of different plasticizers. Sep Purif Technol 151:47–56
Guo J, Li X, Guo Y, Ruan J, Qiao Q, Zhang J, Bi Y, Li F (2016) Research on flotation technique of separating pet from plastic packaging wastes. Proc Environ Sci 31:178–184
Beckman EJ (1992) Separation of physically co-mingled plastics using a supercritical fluid to facilitate recycling, US
Pascoe RD, Connell BO (2003) Development of a method for separation of PVC and PET using flame treatment and flotation. Miner Eng 16:1205–1212
Wang C, Wang H, Wu B, Liu Q (2014) Boiling treatment of ABS and PS plastics for flotation separation. Waste Manag 34:1206–1210
Pongstabodee S, Kunachitpimol N, Damronglerd S (2008) Combination of threestage sink–float method and selective flotation technique for separation of 67 mixed post-consumer plastic waste. Waste Manag 28:475–483
Zhang X, Zhang C, Hankett JM, Chen Z (2013) Molecular surface structural changes of plasticized PVC materials after plasma treatment. Langmuir 29:4008–4018
Qureshi A, Shah S, Pelagade S, Singh NL, Mukherjee Tripathi SA, Shripathi UPDA (2010) Surface modification of polycarbonate by plasma treatment. J Phys 208:1–6
Bakker EJ, Rem PC, Fraunholcz N (2009) Upgrading mixed polyolefin waste with magnetic density separation. Waste Manag 29:1712–1717
Vesel A, Mozetic M (2012) Surface modification and ageing of PMMA polymer by oxygen plasma treatment. Vacuum 86:634–637
Wang C, Wang H, Liu Q, Fu J, Liu Y (2014) Separation of polycarbonate and acrylonitrile–butadiene–styrene waste plastics by froth flotation combined with ammonia pretreatment. Waste Manag 34:2656–2661
Wang C, Wang H, Fu J, Zhang L, Luo C, Liu Y (2015) Flotation separation of polyvinyl chloride and polyethylene terephthalate plastics combined with 68 surface modification for recycling. Waste Manag 45:112–117
Nagy M, Skvarla J, Sisol M (2011) A possibility of using the flotation process to separate plastics. Ann Fac Eng Hunedoara 9:275
Mallampati SR, HoLee B, Mitoma Y, Simion C (2017) Heterogeneous nano-Fe/Ca/CaO catalytic ozonation for selective surface hydrophilization of plastics containing brominated and chlorinated flame retardants (B/CFRs): separation from automobile shredder residue by froth flotation. Environ Sci Pollut R 24:4469–4479
Mallampati R, Lee C, Thanh Truc NT, Lee B (2015) Hazardous PVC plastics separation from ASR by froth flotation after microwave assisted surface modification. Int Conf Adv Environ Res 87:680–749
Wang H, Wang J, Zou Q, Liu W, Wang C, Huang W (2018) Surface treatment using potassium ferrate for separation of polycarbonate and polystyrene waste plastics by froth flotation. Appl Surf Sci 448:219–229
Wang JC, Wang H (2017) Fenton treatment for flotation separation of polyvinyl chloride from plastic mixtures. Sep Purif Technol 187:415–425
IAPD Education Committee. “Amorphous and Semi-Crystalline Engineering Thermoplastics, Module 4”. Basic Plastics Education tutorials. International Association of Plastics Distributors. Archived from the original on 2 March 2012. Retrieved 13 June 2012
Stat-Ease Handbook for Experimenters, Copyright © (2018) Stat-Ease, Inc. 2021 East Hennepin Ave, Suite 480 Minneapolis, MN 55413
Chau TT, Bruckard WJ, Koh PTL, Nguyen AV (2009) A review of factors that affect contact angle and implications for flotation practice. Adv Colloid Interf 150:106–115
Shen H, Forssberg E, Pugh RJ (2002) Selective flotation separation of plastics by chemical conditioning with methyl cellulose. Resour Conserv Recycl 35:229–241
Zhao Y, Yang S, Wen H, Shen Z, Han F (2019) Adsorption behavior and selectivity mechanism of flotation reagents applied in ternary plastic mixtures. Waste Manag 87:565–576
Gregg GC, Sing KSW (1982) Adsorption, surface area and porosity, 2nd edn. Academic press, London
Masel IR (1996) Principles of adsorption and reaction on solid surfaces. Wiley, New York
Endo S, Yuyama M, Takada H (2013) Desorption kinetics of hydrophobic organic contaminants from marine plastic pellets. Mar Pollut Bull 74:125–131
Marsalek R, Pospisil J, Taraba B (2011) The influence of temperature on the adsorption of CTAB on coals. Colloid Surf A 383:80–85
Hameed BH (2007) Equilibrium and kinetics studies of 2,4,6-trichlorophenol adsorption onto activated clay. Colloid Surf A 307:45–52
Sharma P, Kaur R, Baskar Ch, Chung WJ (2010) Desalination 259:249–257
Montgomery DC (2013) Design and analysis of experiments, 8th edn. Wiley, Danvers
Hu M, Shen H, Ye S, Wang Y, Zhang J, Lv S (2018) Facile preparation of a tetraethylenepentaminefunctionalizednano magnetic composite material and its adsorption mechanism to anions: competition or cooperation. RSC Adv 8:10686–10697
Zhang Z, Zheng H (2009) Optimization for decolorization of azo dye acid green 20 by ultrasound and H2O2 using response surface methodology. J Hazard Mater 172(2–3):1388–1393
Acknowledgements
The authors sincerely thank the staffs of the AKAM company laboratory located in MarkazRoshd, the Ferdowsi University of Mashhad for their sincere cooperation. Approval no. 3/45811.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not any financial/commercial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davari, M.R., Ostad Movahed, S. The Flotation by Selected Depressants as an Efficient Technique for Separation of a Mixed Acrylonitrile Butadiene Styrene, Polycarbonate and Polyoxymethyleneplastics in Waste Streams. J Polym Environ 27, 1709–1720 (2019). https://doi.org/10.1007/s10924-019-01467-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01467-2