Abstract
Novel macroporous copolymers of glycidyl methacrylate and ethylene glycol dimethacrylate with mean pore size diameters ranging from 150 to 310 nm were synthesized by dispersion polymerization and modified with ethylenediamine. The glutaraldehyde and periodate method were employed to immobilize horseradish peroxidase (HRP) onto these carriers. The activity of the immobilized enzyme was greatly affected by the pore size of the carrier. The highest specific activities of 9.65 and 8.94 U/g of dry weight were obtained for HRP immobilized by the periodate-route onto poly(GMA‐co‐EGDMA) carriers with pore size diameters of 234 and 297 nm, respectively. Stability studies showed an improved operational stability of immobilized peroxidase at 65 °C and in an organic solvent. HRP immobilized on a copolymer with a pore size of 234 nm, showing the highest specific activity and good stability, had higher activities at almost all pH values than the native enzyme and the increased Km value for pyrogallol oxidation. Immobilized HRP retained 80% of its original activity after five consecutive cycles of the pyrogallol oxidation and 98% of its initial activity in a storage stability study. Enzyme immobilized onto the macroporous copolymer with the pore size diameter of 234 nm showed a substantial degree of phenol removal achieved by immobilized peroxidase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An enzyme immobilization on various solid carriers provides an increased enzyme stability and allows for a good recovery and reuse of biocatalysts in different bioreactors for an appropriate amount of time [1]. Among all immobilization techniques (the adsorption, covalent binding, entrapment within a solid matrix and cross-linking), the most common method is the covalent binding of an enzyme to a carrier. This method provides a strong linkage between the carrier and enzyme, thus preventing the enzyme leakage. Moreover, it increases the enzyme stability and improves its stereospecificity [2].
Different materials can be used as carriers for the enzyme immobilization. Among all materials, synthetic polymers are preferable since their properties can easily be tailored during the synthesis by a careful choice of reaction parameters. Furthermore, they are uniform materials with well-defined chemical structure. Appropriate functional groups, evenly distributed along a polymer chain, can be achieved by a judicious selection of monomers. These functional groups allow for an enzyme binding to a polymer. Different polymers employed as carriers for the enzyme immobilization have been reported in literature [3,4,5].
Macroporous copolymers have been widely used as adsorbents in chromatography, but their potential application in the enzyme immobilization is also well known. Copolymers based on styrene and divinylbenzene are among the most commonly used carriers for the enzyme immobilization, mainly due to their high surface area [3]. Epoxy‐activated carriers, composed of glycidyl methacrylate (GMA) and ethylene glycol dimethacrylate (EGDMA), poly(GMA‐co‐EGDMA), are gaining an increasing attention in the immobilization of various enzymes [4]. A wide application of these carriers has been enabled by the presence of allyl glycidyl (epoxide) groups that can be easily transformed into amino, keto, carboxyl or hydroxyl groups. This facilitates strong binding of an enzyme to the carrier and prevents a leakage of the immobilized enzyme. Commercially available macroporous copolymers are known by the name of Sepabeads® and Eupergit® [5, 6]. These copolymers have been frequently used for the enzyme immobilization due to their wide commercial availability and resistance to chemical and mechanical stresses [7].
Several authors have confirmed that bonds formed between the enzyme and synthetic macroporous carriers are more stable than those formed between the enzyme and other carriers, such as natural polysaccharides, silica gel, glass beads etc. [8]. It has been shown that one of the most important parameters that directly affects the activity and stability of the immobilized enzyme is a porosity of the carrier [8]. Properties of macroporous copolymers obtained by the suspension copolymerization have been controlled by a careful choice of the type and amounts of inert compounds and a cross-linking agent during the synthesis procedure [9, 10].
Horseradish peroxidase (HRP, E.C.1.11.1.7), which belongs to the ferroprotoporphyrin group of peroxidases, is the most commonly used peroxidase from plant. During its long‐term use, a higher activity and operational stability can be achieved by immobilizing the enzyme on different carriers. This also increases the potential for repeated use of the enzyme. Horseradish peroxidase can be immobilized on various support materials, from natural polymers such as alginate or chitosan [11,12,13] to magnetic-beads [14]. Immobilized horseradish peroxidase can be widely used for different purposes, such as the phenol and p‐chlorophenol removal from wastewaters [13, 14], decolorization of industrial textile effluents [15] and manufacture of biosensors [16, 17].
The removal of phenol by HRP immobilized on different carriers has been examined by many research groups. Jing et al. have used HRP immobilized onto hydrous-titanium to remove phenol from water. The phenol removal of over 90% has been achieved after 15 min at 37 ± 3 °C [18]. HRP immobilized on polyacrylonitrile beads, modified with ethane diamine and chitosan, has removed 90% of 2,4-dichlorophenol with a lower removal efficiency than that of the free enzyme [19]. Lu and coworkers have applied HRP immobilized on glutaraldehyde activated carbon nanospheres to remove chlorophenols, 4-methoxyphenol and bisphenol A from an aqueous solution [20]. Pradeep et al. have compared the efficiency of the phenol removal of HRP both free and immobilized onto an alginate gel. The lower efficiency of the immobilized enzyme has been ascribed to the less number of active sites available in immobilized HRP [21]. Horseradish peroxidase covalently immobilized on polycarbonate supports using a photolabile linker has been applied to remove phenol from wastewater. The phenol content was reduced to 93% in a 3 L reactor filled with spiked wastewater after the treatment [22]. Chang et al. have removed over 95% of 2,4-dichlorophenol and 65% of phenol by HRP immobilized onto Graphene oxide/Fe3O4 nanoparticles [23]. The phenol removal by HRP immobilized on chitosan–halloysite hybrid-nanotubes was studied by Zhang et al. After four cycles, the enzyme maintained more than 60% of its activity [24]. Kim and coworkers have reported the phenol removal activity decrease to 73% of the initial activity when HRP immobilized onto montmorillonite activated with fulvic acid has been used to remove phenol [25]. Alemzadeh et al. have achieved five cycles with the removal efficiency lowered to half of its initial value. They used HRP immobilized on calcium alginate [13]. HRP immobilized on magnetic poly(glycidylmethacrylate-co-methylmethacrilate) has been applied for the treatment of phenolic wastewaters in continuous systems. The phenol and p-chlorophenol degradation rates were affected by the residence time. It was determined that the enzyme reactor lost 8 and 21% of its initial activity after 48 h of continuous operation with phenol and p-chlorophenol-containing wastewaters, respectively [14]. Vasileva et al. have immobilized HRP on modified acrylonitrile polymer membranes and used to remove phenol from water. They have determined that the degree of phenol oxidation was affected by the initial phenol concentration. A high degree of phenol oxidation of 95.4% was accomplished with the immobilized enzyme and hydrogen peroxide in a 100 mg/L phenol solution [26]. Horseradish peroxidase and soybean peroxidase, immobilized onto an aldehyde glass have been used to remove 4-chlorophenol. The effect of the enzyme type, concentrations of enzyme, hydrogen peroxide and 4-chlorophenol on the removal efficiency has been examined. Also, it was shown that soybean peroxidase was less susceptible to inactivation than HRP and was more efficient in the 4-chlorophenol removal [27]. Gómez et al. have immobilized soybean and horseradish peroxidase on glutaraldehyde-activated aminopropyl glass beads and used in the phenol removal. At lower enzymes concentrations (< 0.028 mg/mL) soybean peroxidase removed more phenol than HRP. With increasing the enzyme dose above the critical concentration, the opposite was found. They also showed the lower phenol removal capacity of HRP due to its susceptibility to inhibition [28].
In the present work, we synthesized few micrometers large micro-beads of macroporous poly(GMA‐co‐EGDMA) copolymers with different mean pore size diameters and surface characteristics. These carriers were prepared by the dispersion polymerization in the presence of inert compounds. Fine particles of the macroporous copolymers obtained in this way were a lot smaller than those obtained by the suspension polymerization, used in our previous research [29]. These copolymers were used as carriers for the horseradish peroxidase immobilization. The activity, stability and operational stability were tested. The immobilized enzyme was used for the removal of phenol in a batch reactor using continual internal delivery of hydrogen peroxide.
Experimental
Materials
Polyvinylpirrolidone (PVP, Mw = 24 000 g/mol), glycidyl methacrylate (GMA), ethylene glycol dimethacrylate (EGDMA), 1-dodecanol and cyclohexanol were purchased from Sigma Aldrich (St. Louise, Mo, USA). Ethylenediamine was obtained from Merck (Kenilworth, New Jersey, USA). Toluene and 96% ethanol were purchased from Zorka (Šabac, Serbia). The initiator 2,2′-azobis(2-methylpropionitrile) (AIBN) (98%, Akzo Nobel, Amsterdam, Netherlands) was recrystallized twice from ethanol. Horseradish peroxidase (150–250 U/mg), glucose oxidase (160 U/mg), pyrogallol used as a substrate for the peroxidase reaction, glutaraldehyde (25% solution in H2O, grade II) and sodium periodate were purchased from Sigma Aldrich (USA). Hydrogen peroxide and 1,4‐dioxane were obtained from AppliChem GmbH (Darmstadt, Germany) and Merck (USA), respectively. Phenol and sodium dihydrogen phosphate anhydrous were purchased from Centrohem (Stara Pazova, Serbia). 4‑aminoantipyrene and sodium acetate were obtained from Fluka (Buchs, Switzerland), whereas potassium ferricyanide and glycerol (from plant, for laboratory use) were purchased from MP Hemija (Belgrade, Serbia) and Serva (Heidelberg, Germany), respectively.
Copolymer Preparation and Amination
A continuous phase consisting of 45 mL of 2.78 wt% PVP (Mw = 24 000 g/mol) in ethanol was heated to 70 °C in a 250 mL reactor equipped with an anchor stirrer. A monomer phase (5.0 g of both the monomer GMA and cross-linking agent EGDMA (GMA/EGDMA = 90/10; 80/20; 60/40; 40/60)), initiator (0.05 g of AIBN) and inert phase (2.25 g of 1-dodecanol and 2.25 g of cyclohexanol) was added to the continuous phase under stirring (the stirring rate was 100 rpm). The reaction was stopped after 5 h. The obtained copolymer was washed 5 times with ethanol and dried at room temperature. Yields were in a range of 70 to 90%.
Polymer samples, poly(GMA-co-EGDMA), with various contents of the monomer and cross‑linking agent, were washed 3 times with both ethanol and water. Each polymer (0.7 g) was suspended in 18 mL of water. Ten-fold excess of ethylenediamine in relation to epoxide groups was added and the reaction was left to stir over night at 25 °C. Furthermore, the reaction mixture was heated to 80 °C for 7 h and subsequently, left to stir over night at 25 °C. The polymer particles were removed and washed first with ethanol and subsequently, water by filtration until the pH value of the filtrate was 6. The samples were dried in the oven at 50 °C for couple of hours. Conversions were ≈ 45%.
Copolymer Characterization
The pore size distributions of the synthetized copolymers poly(GMA-co-EGDMA) were determined by a mercury porosimetry (Carlo Erba 2000, software Milestone 200). Mercury intrusion porosimetry measurements of the synthetized copolymers poly(GMA-co-EGDMA) were performed on a high-pressure unit PASCAL 440 (Thermo Scientific) in a CD3-P type dilatometer in the pressure range 0.1–200 MPa. Although two consecutive intrusion-extrusion cycles were performed, only the second cycle was used to calculate the porosimetric parameters, since the second cycle provides only information on the intraparticle porosity of the particles and excludes the contribution of the interparticle voids. A SOLID Software System PC interface was used for automatic data acquisition and all textural parameter calculations. Before analysis, the powder sample was dried at 50 °C for 24 h in an oven and additionally evacuated for 2 h in a sample holder at the analytical position of a Macropore Unit 120 (Carlo Erba Strumentazione) used for mercury filing of the dilatometer. Scanning electron microscope (Tescan FE-SEM Mira 3 XMU) operated at 20 keV was employed to characterize the morphology of poly(GMA-co-EGDMA). All samples were coated with a tin gold layer using a sputter coater (Polaron SC503, Fisons Instruments) prior to the SEM analysis. The efficiency of transformation of epoxy groups into amino groups was confirmed by the elemental analysis. The copolymer samples were analyzed for their carbon, hydrogen and nitrogen content at Microanalysis Laboratory, Department of Chemistry, University of Belgrade.
Immobilization of Horseradish Peroxidase Using Glutaraldehyde Method
An appropriate amount of aminated copolymers was deaerated for 10 min in sodium phosphate buffer pH 8 (0.1 mol/L) and rinsed twice with the same buffer. Prepared copolymers were incubated in a glutaraldehyde solution in sodium phosphate buffer pH 8 (0.1 mol/L) for 2 h. Subsequently, the copolymers were rinsed 3 times and incubated with different amounts of horseradish peroxidase (1, 5, 15 and 25 mg/g) per gram of the carrier for 48 h at room temperature. After the incubation, copolymers were rinsed twice with sodium phosphate buffer pH 7 (0.1 mol/L). Washings were collected, the copolymers with the immobilized enzyme were resuspended in the same buffer and stored at 4 °C until further use.
Immobilization of Horseradish Peroxidase Using Periodate Method
Horseradish peroxidase was oxidized with the 50 mmol/L solution of sodium periodate in sodium acetate buffer pH 5 (50 mmol/L) for 6 h in the dark at 4 °C. The oxidation reaction was stopped by adding glycerol to a final concentration of 0.2% (v/v). Oxidized HRP was dialyzed overnight against sodium acetate buffer pH 5. The aminated copolymers were first deaerated for 10 min in sodium phosphate buffer pH 7 (0.1 mol/L), rinsed with the same buffer and subsequently, incubated for 48 h with different amounts of oxidized HRP (1, 5, 15 and 25 mg/g) per gram of copolymer. The copolymers with the immobilized enzyme were rinsed twice with sodium phosphate buffer pH 7 (0.1 mol/L) and thereafter, stored in the same buffer at 4 °C until further use.
Washings were collected and used for determination of the activity of the unbound enzyme.
Enzyme Activity Studies
Pyrogallol and hydrogen peroxide (H2O2) were used as substrates in the assay employed to determine the peroxidase activity. In the most common test, 10 µL of the enzyme dilution from the washings and 10 µL of H2O2 (9.7 mmol/L) were introduced into 1 mL of the pyrogallol solution (13 mmol/L) in sodium phosphate buffer pH 7. Absorbance was measured for 3 min at 420 nm using UV–VIS spectrophotometer (Shimadzu Corporation UV-2501PC, Japan). The value of the enzyme activity was calculated from the absorbance coefficient of purpurogallin (12 m/g cm2). The activity of the immobilized enzyme was determined by introducing 9.0 mg of the copolymer with immobilized HRP and 30 µL of H2O2 into 3 mL of pyrogallol. Every 60 s aliquots were taken out from the mixture, filtrated and the absorbance at 420 nm was measured. One unit of enzyme activity was defined as the amount of enzyme that produces 1 mg of purpurogallin in 20 s at 20 °C. The specific activity of the enzyme was calculated per gram of dry weight of the copolymer.
Stability Studies
In order to examine the temperature stability of immobilized horseradish peroxidase, a specific amount of the enzyme immobilized on the copolymer was introduced into sodium phosphate buffer pH 7 (0.1 mol/L) and incubated at 65 °C. Subsequently, the immobilized enzyme was cooled down to room temperature. Residual specific activity of the immobilized enzyme was determined as described previously.
The enzyme stability in organic solvents, such as 1,4‐dioxane, was also examined. Both the soluble and immobilized enzyme were incubated at 20 °C in the 80% 1,4-dioxane solution (v/v) in sodium phosphate buffer pH 7 (0.1 mol/L). After certain time intervals specific activities of the immobilized and soluble enzyme were determined by employing the above-described assay.
Activity Measurement at Different pH Values
Series of 0.1 mol/L phosphate-citrate buffer with pH values from 2 to 8 was used to determine the enzyme activity of free and immobilized horseradish peroxidase. The enzyme stability at pH 9 was examined in sodium glycinate buffer (0.1 mol/L). The relative activity of immobilized horseradish peroxidase at different pH values was determined according to the above-described procedure. Obtained results were then normalized to the maximum activity at optimum pH, i.e. relative activity, expressed as a percentage, was presented.
Determination of Kinetic Parameters
The effect of a pyrogallol concentration on the initial activity of both free and immobilized horseradish peroxidase was studied by varying the pyrogallol concentration. Kinetic parameters (Km and Vmax) were straightforwardly determined from the Michaelis–Menten model by nonlinear regression analysis using the OriginLab software and used for calculation of catalytic constant, Kcat.
Reusability Studies and Storage Stability
In order to determine the operational stability of immobilized peroxidase, several consecutive cycles of the pyrogallol oxidation in a batch reactor were performed. At the end of each cycle, the carrier with the immobilized enzyme was rinsed twice with 0.1 mol/L sodium phosphate buffer pH 7. This procedure was repeated several times with fresh aliquots of substrates (pyrogallol and H2O2).
The storage stability of immobilized HRP was also investigated. HRP immobilized on the poly(GMA-co-EGDMA) copolymer was stored in 0.1 mol/L sodium phosphate buffer at 4 °C for the specific period of time. Specific activity of the immobilized enzyme was determined at the initial point and 14 days after storage in a buffer by employing the above-described method for determination of the enzyme activity.
Phenol Removal in a Batch Reactor
Horseradish peroxidase immobilized on the macroporous copolymer with the optimal pore size diameter (in terms of the enzyme activity and stability), was used to eliminate phenol from a solution. A phenol removal was performed in a batch reactor by adding HRP immobilized on the copolymer (80 mg) into the phenol solution (2 mmol/L) in Tris HCl buffer pH 7 (50 mmol/L). A system composed of glucose oxidase and glucose was used for the internal delivery of hydrogen peroxide. The concentrations of 0.187 U/mL of glucose oxidase and 3 mmol/L of glucose were used for this purpose. The immobilized enzyme, glucose oxidase and glucose were introduced into the phenol solution (3 mL). The reaction mixture was stirred for an appropriate period of time. Aliquots of 40 µL were taken every 60 min, transferred into 760 µL of Tris HCl buffer pH 7 and subsequently, 100 µL of 83.4 mmol/L potassium hexacyanoferrate (III) solution (K3Fe(CN)6) and 100 µL of 20.8 mmol/L 4‐aminoantipyrine were added to the solution. Absorbance was measured at 510 nm by UV–VIS spectrophotometry after 10 min of color development. The concentration of removed phenol was determined using the calibration curve for phenol.
Results and Discussion
Synthesis and Characterization of Macroporous Copolymers
The enzyme immobilization on different carriers provides several benefits such as the improved enzyme activity at elevated temperatures and in organic solvents, long-term stability and reusability of the enzyme [30]. Synthetic polymers are preferable carriers for the enzyme immobilization since their structure and thus, properties can easily be tailored during the synthesis by a careful choice of monomer units. Macroporous poly(GMA-co-EGDMA), obtained by the suspension polymerization, has already been proven as an outstanding carrier for the enzyme immobilization [8, 29, 31,32,33]. In this paper macroporous poly(GMA-co-EGDMA) is synthetized by the dispersion polymerization. Unlike suspension polymerization, the dispersion polymerization allows for the formation of uniform beads, 0.1–10 µm in diameter. Smaller beads have lower diffusional limitations due to minor influence of internal diffusion, resulting from decreased internal diameter of beads and therefore, offer higher specific activities of the immobilized enzyme.
Dispersion polymerization is performed in ethanol as a solvent since the stabilizer (PVP), monomer mixture (the monomer and cross-linking agent), inert mixture (1-dodecanol and cyclohexanol) and initiator are soluble in it, whereas the obtained copolymer precipitates in ethanol. Copolymer particles are mainly spherical aggregates with diameter of around 1.5 µm as presented in Fig. 1.
Properties of the immobilized enzyme are determined by the porous characteristics of the carrier. Too small pores can cause inactivation of the enzyme due to the diffusion limitation and structural rearrangement of the enzyme. Large pores allow for enzymes to cluster together and thus, lose their activity. In order to determine the optimal pore size which provides the highest enzyme activity and stability, various copolymers with different porous characteristics are synthetized. To this purpose, the weight ratio of the monomer to the cross-linking agent is varied and an effect of this ratio on properties of synthetized carriers is examined. Analogous to the suspension polymerization, the inert compounds (1-dodecanol and cyclohexanol) are added to the reaction mixture to contribute formation of the macroporous structure. The experimental conditions investigated in this study are presented in Table 1.
Previous studies have shown that the porous properties of poly(GMA-co-EGDMA) obtained by the suspension polymerization have been affected by the amount of the cross-linking agent [9, 10]. In the dispersion polymerization as well as in the suspension polymerization, an increase in the amount of the cross-linking agent in the reaction mixture and therefore, in the synthetized copolymer, increases the specific pore volume (Table 2). This has been attributed to the decrease in the size of submicroscopic particles which form the copolymer bead, with increasing the EGDMA content [34]. Moreover, with increasing the amount of the cross-linking agent in the reaction mixture, the porosity of poly(GMA-co-EGDMA) increases, as shown in Table 2. The sample with the lowest EGDMA content (G9E1) has the smallest average pore diameter, whereas further increase in the amount of the cross-linking agent results in larger pores (Table 2). Horak et al. have reported comparable findings for the series of samples obtained by the suspension polymerization with the lower degree of the cross-linking agent (the EGDMA content was less than 75 vol%). Lower degrees of cross-linking result in less rigid and “soft” particles, more compromised by interfacial tension [10]. Jovanovic et al. have reported minor changes in the pore diameter and an increase in the specific surface area when increasing the amount of EGDMA in the reaction mixture from 20 to 40 mas% [9]. They have related these findings to the globular structure of copolymers which determines the pore size and porosity. The increase in the amount of the cross-linking agent slightly affects the size of globule and therefore, the size of the pore diameter [9].
Immobilization of Horseradish Peroxidase on Poly(GMA-co-EGDMA) Macroporous Copolymers
In order to allow for immobilization of the enzyme onto macroporous poly(GMA-co-EGDMA), amino groups are introduced into the copolymer chains. This is achieved by treating the epoxide groups with ethylenediamine. Transformation of the epoxide into amino groups is confirmed by the elemental analysis (Table 3).
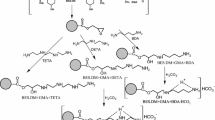
Two different methods are used to immobilize HRP on the aminated copolymers, glutaraldehyde and periodate. The periodate method has been previously optimized for hydrolases [6, 35, 36] and involves covalent binding to a copolymer’s surface through an enzyme carbohydrate moiety previously subjected to oxidation with sodium periodate. On the other hand, the glutaraldehyde method is one of the most commonly used methods for the enzyme immobilization. Binding of an enzyme to a carrier is achieved via amino groups present on the surface of the protein molecule. Differences between binding mechanisms of the enzyme to the copolymer surface using these two methods, are schematically presented in Fig. 2.
In order to determine which method provides better HRP immobilization, different amounts of the enzyme per gram of the copolymer are added and a specific activity of the immobilized enzyme is calculated. Obtained results show that the increase in the amount of the enzyme added per gram of the copolymer results in the increase in the specific activity of the enzyme immobilized by both periodate and glutaraldehyde route (Fig. 3). The enzyme immobilized by the periodate method provides higher specific activities than by the glutaraldehyde method, under the same conditions (the same copolymer and the same amount of immobilized peroxidase). The obtained data corroborates previously reported results for lipase [6]. A higher efficiency of the periodate method is probably a result of oxidation of the carbohydrate moiety which does not distort the structure of the enzyme’s active site. Oxidation also leads to the formation of a large number of aldehyde groups that represent potential binding sites to the macroporous copolymers. Therefore, with increasing the number of reactive groups, the probability of the enzyme multipoint attachment to the carrier also increases.
Based on amounts of the enzyme bound per mg of the carrier, two immobilization methods provide different activities of the immobilized enzyme, as seen in Table 4. Lower amounts of HRP are bound to the carrier when the enzyme is immobilized by the glutaraldehyde method. Knezevic et al. used three different methods to immobilize lipase onto Eupergit C: periodate, direct binding via oxirane groups and binding via spacer made from diamine/glutaraldehyde. They have reported the periodate method as the most effective for the enzyme immobilization [6]. Similar data have also been obtained by Pramparo et al. They have compared the periodate method for the immobilization of HRP on Eupergit C with direct binding by oxirane groups and binding of HRP onto adipic dihydrazide treated Eupergit C [4]. The presence of a spacer in the above‐mentioned methods leads to additional distancing of the enzyme from the carrier, thus placing the enzyme in a better position for the catalytic activity. On the other hand, enzyme binding to carbohydrate groups proved to be more effective, probably, due to more favorable interactions between the enzyme and carrier.
The highest specific activities (9.65 and 8.94 U/g of dry weight) are obtained when HRP is immobilized by the periodate method on aminated macroporous copolymers labeled as G6E4am and G8E2am with mean pore diameters of 234 and 297 nm, respectively. Previous reports of our group have shown that larger pore sizes allow for the highest specific activities [29, 32]. Similar results for various carriers with different porous characteristics are reported in literature [37, 38]. It has been shown that silicate-based carriers with larger mean pore diameters provide higher specific activities of immobilized enzymes [39].
As shown in Table 4, the specific activity of the enzyme immobilized by the periodate route on the copolymer G4E6am with a pore size diameter of 308 nm is similar to that of G8E2am and approximately 1.1 times lower than the specific activity of HRP immobilized on the copolymer G6E4am by the same method. This result can be a consequence of a possibility of oligomer HRP formation when using the periodate method and a higher specific surface area of G6E4 am. Additionally, Chouyyok et al. have found an increased HRP leaching from the silica surface when increasing the pore size of the carrier [38].
Horseradish peroxidase immobilized by the periodate route on the copolymer with the smallest pore diameter (G9E1am, 150 nm) shows the lowest specific activity of 6.05 U/g of dry weight. A similar trend is also observed for specific activities of horseradish peroxidase immobilized by the glutaraldehyde method.
Since the periodate method provides substantially better outcomes in terms of the amount of the enzyme immobilized on the copolymer, further research is proceeded with HRP immobilized by this method. The enzyme immobilized by the periodate route is observed by SEM. However, the presence of HRP is not visible due to its small radius of gyration, which is below the limit of detection (data not shown).
Stability Studies of Immobilized Horseradish Peroxidase
Stability of immobilized enzymes predominantly depends on surface characteristics of the carrier used for the immobilization. In order to determine an effect of immobilization on thermostability of HRP, the immobilized enzyme is incubated at 65 °C for an appropriate period of time (Fig. 4).
The enzyme immobilized on the copolymer G6E4am (the pore diameter of 234 nm) shows the highest stability at elevated temperatures, whereas HRP immobilized on other carriers (G8E2am, G4E6am and G9E1am) is slightly less stable. These findings indicate that the pore size of the carrier used for the enzyme immobilization affects the thermostability of the immobilized enzyme. A residual activity of HRP immobilized on the G6E4am copolymer after 30 min of incubation at 65 °C (19.3%) is approximately 2 times higher than the residual activities of the enzyme immobilized on other copolymers. The increased thermostability of the immobilized enzyme is plausibly a consequence of formation of more stable 3D structure of the enzyme, resulting from an enzyme multipoint attachment to the carrier.
The stability of the immobilized enzyme decreases with increasing incubation time at 65 °C. A similar trend has also been observed when horseradish peroxidase was immobilized on macroporous copper with a pore size of 100–200 nm [37].
The enzyme stability in organic solvent solutions is of great importance especially for the enzymatically catalyzed synthetic reactions. The improved stability of immobilized horseradish peroxidase in the presence of organic solvents, such as methanol, acetone or acetonitrile, has already been reported in literature [40].
In order to examine the enzyme stability in 80% dioxane, both immobilized and free HRP are incubated in this organic solution for 120 h at room temperature as shown in Fig. 5.
After 5 days of incubation in 80% dioxane, free horseradish peroxidase is practically inactivated, whereas the immobilized enzyme preserves approximately 10% of its original activity. Diagrams presented in Fig. 5 show a rapid decrease in the enzyme activity within first 72 h of incubation. Subsequently, further increase in incubation time above 72 h does not substantially affect the activity. Similar data have been obtained when the stability of horseradish peroxidase in 20% acetonitrile was examined [41]. The immobilized enzyme retains a large percentage of its original activity, whereas free HRP almost completely loses its activity. The improved activity of the immobilized enzyme in organic solvents is probably caused by reduced diffusional limitations resulting from the adsorption of the enzyme to the carrier. The increased catalytic activity of the immobilized enzyme may also be a consequence of favorable microenvironment for the catalysis provided by the carrier.
Figure 5 also shows that HRP immobilized on the G6E4am copolymer has slightly better residual activity than HRP immobilized on other copolymers. These findings corroborate reports of other researchers who have found that the pore size does not greatly affect the stability of enzyme in organic solvents solutions [42].
Horseradish peroxidase immobilized on the copolymer with the pore size diameter of 234 nm (G6E4am), shows the most promising results regarding the specific activity, thermostability and stability in the organic solvent, and therefore, is further characterized in terms of the pH optimum, Km and Vmax and operational stability.
Kinetic Studies of Immobilized Horseradish Peroxidase
The effect of pH on activities of both the free and immobilized enzyme was studied in the pH range from 2 to 9 as shown in Fig. 6.
With increasing the pH value from acidic to basic, both peroxidases show the same trend regarding the relative activity. Specifically, the increase in pH from 2 to 8 results in the increase of the enzymes’ relative activities. The maximum activity of both immobilized and free peroxidase is achieved at pH 8. Further increase of pH causes the decrease in the enzyme activity (Fig. 6). Immobilized horseradish peroxidase shows higher activities than soluble HRP at almost all pH values. The optimum pH value for both peroxidases is the same (pH 8). Horseradish peroxidase, immobilized onto the macroporous copolymer, maintains the high and stable activity at different pH values, which is of great importance for its practical application.
Similar behavior of immobilized enzymes has been reported in literature [43,44,45]. Horseradish peroxidase immobilized on various carriers has shown the increased stability over a wide range of pH values.
In order to determine kinetic constants of soluble and immobilized horseradish peroxidase, Km and Vmax, pyrogallol and H2O2 are used as substrates. Apparent Km values of free and immobilized HRP are 1.62 and 2.06 mmol/L, respectively. The Km value of immobilized horseradish peroxidase is slightly higher than that of the free enzyme. This indicates that the soluble enzyme has a greater affinity towards the substrate than the immobilized enzyme. This may be caused by the steric hindrance of the inner pore structure formed during the immobilization. The immobilization of enzyme prevents access of the substrate to the enzyme’s active site, thereby reducing the affinity of the enzyme towards the substrate [46]. The apparent Vmax values of free and immobilized HRP are 3.16 and 5.18 U/mL, respectively. Calculated values of Kcat for soluble and immobilized HRP are 556.16 and 182.34 min−1, respectively.
Operational Stability and Storage Stability
Operational stability of immobilized horseradish peroxidase is examined by employing the same batch of the immobilized enzyme in several consecutive cycles for pyrogallol oxidation. The residual activity of the immobilized enzyme has been monitored for 180 min. Subsequently, the copolymers with immobilized HRP are rinsed several times with buffer and used for another round of oxidation. The enzyme activity obtained in the first cycle is considered as 100%.
After five cycles of repeated use, immobilized horseradish peroxidase retains 80% of its original activity. Results presented in Fig. 7 show that the enzyme immobilized on the copolymer G6E4am with the pore diameter of 234 nm has an outstanding operational stability under examined conditions. During five consecutive cycles, the immobilized enzyme loses only 20% of its original activity, which is of great importance for numerous applications.
The operational stability of peroxidase immobilized on different carriers has been reported in literature [29, 37, 44]. Qiu et al. have found that the retention activity of HRP immobilized on macroporous copper after five cycles was around 60% [37]. After four cycles of repeated use peroxidase immobilized onto glycidyl methacrylate based copolymers, obtained by the suspension polymerization, with pore sizes between 120 and 200 nm retained only 45% of the initial activity [29]. When compared to the literature data, HRP immobilized on the novel macroporous copolymers, used in this study, has shown the substantially improved stability within the first five cycles of repeated use, as shown in Fig. 7. Thus, the described enzyme immobilization provides considerably better outcomes regarding the stability and reusability of the enzyme.
In order to examine the time interval in which the immobilized enzyme shows satisfactory results for the pyrogallol oxidation, the copolymer G6E4am with immobilized HRP was stored in sodium phosphate buffer pH 7 at 4 °C for 2 weeks. Residual activity of the immobilized enzyme was measured at the initial moment, as well as 2 weeks after storage at 4 °C. Specific activities of HRP at the initial moment and after 2 weeks of storage are 9.65 and 9.55 U/g, respectively. The obtained results show that HRP immobilized onto this copolymer retained more than 98% of its initial activity. Chang et al. reported that HRP immobilized on magnetic Fe3O4 nanoparticles retained 84% of initial activity after 15 days of storage [47].
When compared with the literature data for free horseradish peroxidase, the obtained results show that the immobilization further stabilizes the enzyme and prolongs its half-life as well as the possibility of its repeated use for an extended period of time. Soluble HRP retains only 30% of its initial activity after 15 days of storage [47, 48].
Phenol Removal in a Batch Reactor
Phenol and phenolic compounds are the most common pollutants present in industrial effluents. These compounds are toxic and mutagenic and therefore, their removal from wastewaters is of the utmost importance for the entire ecosystem. Since phenol is an intermediate in the oxidation process of higher molecular weight aromatic hydrocarbons, this compound has been taken as a model compound in our study, aiming to determine the efficiency of removal of phenolic pollutants from the aqueous system by HRP immobilized on methacrylate-based carriers obtained by the dispersion polymerization.
Optimization of reaction conditions for the phenol removal is performed with horseradish peroxidase immobilized onto the macroporous copolymer with the pore size diameter of 234 nm (G6E4am). This carrier has shown the most promising results in terms of the amount of the bound enzyme, operational stability and reusability. The data obtained in our previous research, in which the parameters for the removal of phenolic compounds with peroxidase immobilized within tyramine-alginate micro‐beads were optimized, are used as a starting point with an aim to determine the optimal conditions for this new system. The removal of phenol is conducted in a batch reactor with 80 mg of the copolymer with immobilized HRP, 3 mL of the phenol solution (2 mmol/L) at room temperature, whereas a system composed of glucose oxidase and glucose is used for the internal delivery of hydrogen peroxide. Thus, a gradual and controlled release of hydrogen peroxide is provided.
Our previous study, in which the phenol removal was performed in the presence of HRP immobilized on tyramine-alginate micro-beads, has shown that the highest removal efficiency was achieved when using glucose oxidase and glucose for the internal delivery of hydrogen peroxide [49]. Considering these findings, the same concentration of glucose oxidase and 3 mmol/L of glucose are employed to test the removal efficiency of HRP immobilized on the copolymer. The phenol removal is examined in cycles, with each cycle lasting 24 h. Obtained results, presented in Fig. 8, show that immobilized peroxidase removes a substantial amount of phenol in the first cycle, 74.3%. However, already in the second cycle, the removal percentage drops rapidly and only 27% of phenol is converted (Fig. 8). The high phenol conversion in the first cycle is promising, but its rapid drop is undesirable since it disables the successful reusability of the system.
The lower reusability of HRP immobilized on the macroporous copolymer G6E4am can be caused by blocking of the pores with the products formed in the oxidation reaction. To overcome this issue, the polymer is rinsed with ethanol between two cycles and phenol removal time is reduced to 4 h per cycle. Lower reaction time results in reduced conversions of phenol and therefore, lower amounts of the oxidation products, which further facilitates disposal of these products from the copolymer surface by rinsing with ethanol. We were guided by the literature data in the selection of reaction conditions for the removal and desorption of polyphenolic compounds. It has been reported that 3 to 5 h is the optimum reaction time for the removal of phenolic compounds [50]. Thereafter, a significant decrease in the enzyme activity, and thus the efficiency of the phenol removal, has been observed. Some research groups have focused their study on capability of appropriate solvents (chloroform, isobutanol and hexane) to desorb polyphenolic compounds accumulated on the surface of different carriers. It has been shown that polar organic solvents have been able to desorb more than 90% of the adsorbed phenol [51]. Higher regeneration efficiencies have been observed when isobutanol was used for desorption. This solvent can form hydrogen bonds with amine groups, located on the surface of the carrier, thus, allowing for the removal of phenol from the surface and reuse of the immobilized enzyme.
Ethanol is chosen as a solvent for rinsing process since it is polar, highly available, economical and is the most environmentally friendly among all the above-mentioned solvents. The efficiency of polymer rinsing with ethanol is illustrated in Fig. 9a. When the HRP-copolymer system is extensively rinsed in between two cycles, the phenol conversion achieved in the second cycle is 1.5 times higher than that obtained in the absence of the rinsing process. This indicates that the rinsing step is efficient in removal of the solid oxidation product from the carrier and thus, contributes to prolonged use of HRP immobilized on the copolymer in the phenol removal process.
a The effect of the carrier rinsing with ethanol on the phenol conversion (1 cycle lasting for 4 h without (the white block) and with (the grey block) ethanol rinsing in between cycles). b The phenol conversion and reusability of horseradish peroxidase immobilized onto the macroporous copolymer under modified conditions: 1 cycle lasting 4 h with rinsing with ethanol in between cycles
By applying the above-mentioned conditions for the phenol removal (0.187 U/mL of glucose oxidase and 3 mmol/L of glucose) and by rinsing the polymer with ethanol, that does not change the structure of the macroporous copolymer and has mild effect on the HRP activity, to eliminate polyphenolic compounds accumulated on the surface of the carrier, the number of cycles for the repeated use in phenol oxidation is increased to 4. After four consecutive cycles, each lasting 4 h, immobilized HRP retains 26.3% of its initial activity (Fig. 9). Obtained results are corroborated by previously reported data for peroxidase immobilized onto electrospun poly(vinyl‐alcohol)‐polyacrylamide nanofibers [52]. By applying the above-elaborated reaction conditions, we managed to increase the number of repeated cycles for the phenol removal to 4 with the adequate phenol removal efficiencies.
Conclusion
In this work, horseradish peroxidase is covalently immobilized onto the macroporous copolymer, poly(GMA-co-EGDMA), by the periodate and glutaraldehyde method. Different porous properties of the copolymer are obtained by varying the ratio of the amounts of the monomer to cross-linking agent during the dispersion polymerization. The effect of the pore size diameter of the carrier on the specific activity of the immobilized enzyme is examined. It is shown that the highest activities are obtained when HRP is immobilized on the copolymers with the largest pores. The periodate method provides higher specific activity of the immobilized enzyme than the glutaraldehyde method. The highest specific activity of 9.65 U/g of dry weight is obtained when peroxidase is immobilized by the periodate method on the copolymer with the pore size diameter of 234 nm (G6E4am). Unlike free peroxidase, which is inactivated after incubation in 80% dioxane for 5 days, immobilized peroxidase retains approximately 10% of its original activity. Immobilized horseradish peroxidase shows increased activities at almost all pH values (from 2 to 9), and the increased value of the apparent Km. The immobilized enzyme shows the excellent reusability during the pyrogallol oxidation since it retains 80% of its original activity after five cycles of repeated use. The stability study shows that HRP immobilized onto G6E4am copolymer retained 98% of its initial activity after 2 weeks storage. The efficiency of the phenol removal in a batch reactor is around 43% in the first cycle and retains around 30% in the following three cycles. Our study demonstrates that peroxidase immobilized on the new type of macroporous copolymer in the described manner can be successfully applied in the phenol oxidation reactions.
References
Magnin D, Dumitriu S, Chornet E (2003) Immobilization of enzymes into a polyionic hydrogel: ChitoXan D. J Bioact Compat Polym 18:355–373. https://doi.org/10.1177/088391103038375
Fernández-Lorente G, Terreni M, Mateo C et al (2001) Modulation of lipase properties in macro-aqueous systems by controlled enzyme immobilization: enantioselective hydrolysis of a chiral ester by immobilized Pseudomonas lipase. Enzyme Microb Technol 28:389–396. https://doi.org/10.1016/S0141-0229(00)00324-0
Hernandez K, Garcia-Galan C, Fernandez-Lafuente R (2011) Simple and efficient immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads. Enzyme Microb Technol 49:72–78. https://doi.org/10.1016/j.enzmictec.2011.03.002
Pramparo L, Stüber F, Font J et al (2010) Immobilisation of horseradish peroxidase on Eupergit®C for the enzymatic elimination of phenol. J Hazard Mater 177:990–1000. https://doi.org/10.1016/j.jhazmat.2010.01.017
Prlainović NŽ, Knežević-Jugović ZD, Mijin DŽ, Bezbradica DI (2011) Immobilization of lipase from Candida rugosa on Sepabeads Ò : the effect of lipase oxidation by periodates. Bioprocess Biosyst Eng 34:803–810. https://doi.org/10.1007/s00449-011-0530-2
Knezevic Z, Milosavic N, Bezbradica D (2006) Immobilization of lipase from Candida rugosa on Eupergit ® C supports by covalent attachment. 30:269–278. https://doi.org/10.1016/j.bej.2006.05.009
Katchalski-Katzir E, Kraemer DM (2000) Eupergit® C, a carrier for immobilization of enzymes of industrial potential. J Mol Catal - B Enzym 10:157–176. https://doi.org/10.1016/S1381-1177(00)00124-7
Prodanović RM, Milosavić NB, Jovanović SM et al (2006) Stabilization of a -glucosidase in organic solvents by immobilization on macroporous poly ( GMA-co-EGDMA ) with different surface characteristics. J Serb Chem Soc 71:339–347. https://doi.org/10.2298/JSC0604339P
Jovanovic SM, Nastasovic A, Jovanovic NN, Jeremic K (1996) Targeted porous structure of macroporous copolymers based on glycidyl methacrylate. Mater Sci Forum 214:155–162. https://doi.org/10.4028/www.scientific.net/MSF.214.155
Horak D, Svec F, Ilavsky M et al (1981) the effect of polymerization conditions on the porosity and mechanical properties of macroporous suspension copolymers from glycidylmethacrylate-ethylenedimethacrylate. Die Angew Makromol Chemie 95:117–127
Monier M, Ayad DM, Wei Y, Sarhan AA (2010) Immobilization of horseradish peroxidase on modified chitosan beads. Int J Biol Macromol 46:324–330. https://doi.org/10.1016/j.ijbiomac.2009.12.018
Bindhu LV, Abraham ET (2003) Immobilization of horseradish peroxidase on chitosan for use in nonaqueous media. J Appl Polym Sci 88:1456–1464
Alemzadeh I, Nejati S (2009) Phenols removal by immobilized horseradish peroxidase. J Hazard Mater 166:1082–1086. https://doi.org/10.1016/j.jhazmat.2008.12.026
Bayramoglu G, Arica MY (2008) Enzymatic removal of phenol and p -chlorophenol in enzyme reactor: Horseradish peroxidase immobilized on magnetic beads. 156:148–155. https://doi.org/10.1016/j.jhazmat.2007.12.008
Gholami-Borujeni F, Mahvi AH, Naseri S et al (2011) Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Res J Chem Environ 15:217–222
Alonso-Lomillo MA, Domínguez-Renedo O, del Román TL, Arcos-Martínez MJ, (2011) Horseradish peroxidase-screen printed biosensors for determination of Ochratoxin A. Anal Chim Acta J 688:49–53. https://doi.org/10.1016/j.aca.2011.01.003
Yu D, Blankert B, Kauffmann J (2007) Development of amperometric horseradish peroxidase based biosensors for clozapine and for the screening of thiol compounds. 22:2707–2711. https://doi.org/10.1016/j.bios.2006.11.013
Ai J, Zhang W, Liao G et al (2016) Immobilization of horseradish peroxidase enzymes on hydrous-titanium and application for phenol removal. RSC Adv 6:38117–38123. https://doi.org/10.1039/c6ra02397e
Wang S, Fang H, Wen Y et al (2015) Applications of HRP-immobilized catalytic beads to the removal of 2,4-dichlorophenol from wastewater. RSC Adv 5:57286–57292. https://doi.org/10.1039/c5ra08688d
Lu YM, Yang QY, Wang LM (2016) Enhanced activity of immobilized horseradish peroxidase by carbon nanospheres for phenols removal. CLEAN Soil Air Water 45:160007. https://doi.org/10.1002/clen.201600077
Pradeep NV, Hampannavar US (2012) Polymerization of phenol using free and immobilized horseradish peroxidase. J Environ Earth Sci 2:31–37
Rajesh Ahirwar, Jai G. Sharma, Bhanumati Singh, et al (2017) A simple and efficient method for removal of phenolic contaminants in wastewater using covalent immobilized horseradish peroxidase. J Mater Sci Eng B. https://doi.org/10.17265/2161-6221/2017.1-2.004
Chang Q, Huang J, Ding Y, Tang H (2016) Catalytic oxidation of phenol and 2,4-dichlorophenol by using horseradish peroxidase immobilized on graphene oxide/Fe3O4. Molecules. https://doi.org/10.3390/molecules21081044
Zhai R, Zhang B, Wan Y et al (2013) Chitosan-halloysite hybrid-nanotubes: horseradish peroxidase immobilization and applications in phenol removal. Chem Eng J 214:304–309. https://doi.org/10.1016/j.cej.2012.10.073
Kim HJ, Suma Y, Lee SH et al (2012) Immobilization of horseradish peroxidase onto clay minerals using soil organic matter for phenol removal. J Mol Catal B Enzym 83:8–15. https://doi.org/10.1016/j.molcatb.2012.06.012
Vasileva N, Godjevargova T, Ivanova D, Gabrovska K (2009) Application of immobilized horseradish peroxidase onto modified acrylonitrile copolymer membrane in removing of phenol from water. Int J Biol Macromol 44:190–194. https://doi.org/10.1016/j.ijbiomac.2008.12.002
Bódalo A, Bastida J, Máximo MF et al (2008) A comparative study of free and immobilized soybean and horseradish peroxidases for 4-chlorophenol removal: protective effects of immobilization. Bioprocess Biosyst Eng 31:587–593. https://doi.org/10.1007/s00449-008-0207-7
Gómez JL, Bódalo A, Gómez E et al (2006) Immobilization of peroxidases on glass beads: an improved alternative for phenol removal. Enzyme Microb Technol 39:1016–1022. https://doi.org/10.1016/j.enzmictec.2006.02.008
Prodanović O, Prokopijević M, Spasojević D et al (2012) Improved covalent immobilization of horseradish peroxidase on macroporous glycidyl methacrylate-based copolymers. Appl Biochem Biotechnol 168:1288–1301. https://doi.org/10.1007/s12010-012-9857-7
Miletić N, Nastasović A, Loos K (2012) Immobilization of biocatalysts for enzymatic polymerizations: possibilities, advantages, applications. Bioresour Technol 115:126–135. https://doi.org/10.1016/j.biortech.2011.11.054
Miletić N, Rohandi R, Vuković Z et al (2009) Surface modification of macroporous poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) resins for improved Candida antarctica lipase B immobilization. React Funct Polym 69:68–75. https://doi.org/10.1016/j.reactfunctpolym.2008.11.001
Prokopijevic M, Prodanovic O, Spasojevic D et al (2014) Soybean hull peroxidase immobilization on macroporous glycidyl methacrylates with different surface characteristics. Bioprocess Biosyst Eng 37:799–804. https://doi.org/10.1007/s00449-013-1050-z
Knežević-Jugović ZD, Žuža MG, Jakovetić SM et al (2016) An approach for the improved immobilization of penicillin G acylase onto macroporous poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) as a potential industrial biocatalyst. Biotechnol Prog 32:43–53. https://doi.org/10.1002/btpr.2181
Horak D, Svec F, Bleha M, Kalal J (1981) The effect of polymerization conditions on the specific surface area of macroporous copolymers from glycidylmethacrylate-ethylendimethacrylate. Die Angew Makromol Chemie 95:109–115
Prodanović R, Jovanović S, Vujčić Z (2001) Immobilization of invertase on a new type of macroporous glycidyl methacrylate. Biotechnol Lett 23:1171–1174. https://doi.org/10.1023/A:1010560911400
Milosavić N, Prodanović R, Jovanović S, Vujčić Z (2007) Immobilization of glucoamylase via its carbohydrate moiety on macroporous poly(GMA-co-EGDMA). Enzyme Microb Technol 40:1422–1426. https://doi.org/10.1016/j.enzmictec.2006.10.018
Qiu H, Lu L, Huang X et al (2010) Immobilization of horseradish peroxidase on nanoporous copper and its potential applications. Bioresour Technol 101:9415–9420. https://doi.org/10.1016/j.biortech.2010.07.097
Chouyyok W, Panpranot J, Thanachayanant C, Prichanont S (2009) Effects of pH and pore characters of mesoporous silicas on horseradish peroxidase immobilization. J Mol Catal B Enzym 56:246–252. https://doi.org/10.1016/j.molcatb.2008.05.009
Ferreira L, Ramos MA, Dordick JS, Gil MH (2003) Influence of different silica derivatives in the immobilization and stabilization of a Bacillus licheniformis protease (Subtilisin Carlsberg). J Mol Catal B Enzym 21:189–199. https://doi.org/10.1016/S1381-1177(02)00223-0
Temoçin Z, Yiğitoğlu M (2009) Studies on the activity and stability of immobilized horseradish peroxidase on poly(ethylene terephthalate) grafted acrylamide fiber. Bioprocess Biosyst Eng 32:467–474. https://doi.org/10.1007/s00449-008-0266-9
Fishman A, Levy I, Cogan U, Shoseyov O (2002) Stabilization of horseradish peroxidase in aqueous-organic media by immobilization onto cellulose using a cellulose-binding-domain. J Mol Catal B Enzym 18:121–131. https://doi.org/10.1016/S1381-1177(02)00075-9
Takahashi H, Li B, Sasaki T et al (2001) Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater 44–45:755–762. https://doi.org/10.1016/S1387-1811(01)00257-8
Fernandes KF, Lima CS, Lopes FM, Collins CH (2004) Properties of horseradish peroxidase immobilised onto polyaniline. Process Biochem 39:957–962. https://doi.org/10.1016/S0032-9592(03)00211-5
Patel AC, Li S, Yuan JM, Wei Y (2006) In situ encapsulation of horseradish peroxidase in electrospun porous silica fibers for potential biosensor applications. Nano Lett 6:1042–1046. https://doi.org/10.1021/nl0604560
Liu W, Wang WC, Li HS, Zhou X (2011) Immobilization of horseradish peroxidase on silane-modified ceramics and their properties: Potential for oily wastewater treatment. Water Sci Technol 63:1621–1628. https://doi.org/10.2166/wst.2011.228
Alshawafi WM, Aldhahri M, Almulaiky YQ et al (2018) Immobilization of horseradish peroxidase on PMMA nanofibers incorporated with nanodiamond. Artif Cells\ Nanomed Biotechnol 46:S973–S981. https://doi.org/10.1080/21691401.2018.1522321
Chang Q, Jiang G, Tang H et al (2015) Enzymatic removal of chlorophenols using horseradish peroxidase immobilized on superparamagnetic Fe3O4/graphene oxide nanocomposite. Cuihua Xuebao/Chinese J Catal 36:961–968. https://doi.org/10.1016/S1872-2067(15)60856-7
Kumar V, Misra N, Goel NK et al (2016) A horseradish peroxidase immobilized radiation grafted polymer matrix: a biocatalytic system for dye waste water treatment. RSC Adv 6:2974–2981. https://doi.org/10.1039/c5ra20513a
Pantić N, Prodanović R, Đurđić KI et al (2020) Optimization of phenol removal with horseradish peroxidase encapsulated within tyramine-alginate micro-beads. Environ Technol Innov. https://doi.org/10.1016/j.eti.2020.101211
Wu J, Taylor KE, Bewtra JK, Biswas N (1993) Optimization of the reaction conditions for enzymatic removal of phenol from wastewater in the presence of polyethylene glycol. Water Res 27:1701–1706. https://doi.org/10.1016/0043-1354(93)90106-R
Ghafari M, Cui Y, Alali A, Atkinson JD (2019) Phenol adsorption and desorption with physically and chemically tailored porous polymers: Mechanistic variability associated with hyper-cross-linking and amination. J Hazard Mater 361:162–168. https://doi.org/10.1016/j.jhazmat.2018.08.068
Temoçin Z, İnal M, Gökgöz M, Yiğitoğlu M (2018) Immobilization of horseradish peroxidase on electrospun poly(vinyl alcohol)–polyacrylamide blend nanofiber membrane and its use in the conversion of phenol. Polym Bull 75:1843–1865. https://doi.org/10.1007/s00289-017-2129-5
Funding
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia [Grant No. 451-03-9/2021-14/200168, University of Belgrade-Faculty of Chemistry; Grant No. 451-03-9/2021-14/200288, University of Belgrade-Innovation Center of the Faculty of Chemistry and Grant No. 451-03-9/2021-14/200053, University of Belgrade-Institute for Multidisciplinary Research].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pantić, N., Spasojević, M., Stojanović, Ž. et al. Immobilization of Horseradish Peroxidase on Macroporous Glycidyl-Based Copolymers with Different Surface Characteristics for the Removal of Phenol. J Polym Environ 30, 3005–3020 (2022). https://doi.org/10.1007/s10924-021-02364-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02364-3