Abstract
Having been activated with glutaraldehyde, modified poly(ethylene terephthalate) grafted acrylamide fiber was used for the immobilization of horseradish peroxidase (HRP). Both the free HRP and the immobilized HRP were characterized by determining the activity profile as a function of pH, temperature, thermal stability, effect of organic solvent and storage stability. The optimum pH values of the enzyme activity were found as 8 and 7 for the free HRP and the immobilized HRP respectively. The temperature profile of the free HRP and the immobilized HRP revealed a similar behaviour, although the immobilized HRP exhibited higher relative activity in the range from 50 to 60 °C. The immobilized HRP showed higher storage stability than the free HRP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive research efforts have been dedicated to evaluate the possibilities offered by enzymes in biotechnological and environmental applications. An effective use of enzymes may be hampered by some peculiar properties of the enzymatic proteins such as their non-reusability, high sensitivity to several denaturating agents and presence of adverse sensory or toxicological effects. Many of these undesirable constraints may be removed by the use of immobilized enzymes. This approach has proven to be more advantageous for catalysis than the use of free enzymes [1, 2]. Immobilization of enzymes (if it is stable) makes these biocatalysts reusable and thus turns the enzyme-based process into a more economically viable approach. Quite frequently, the immobilized enzyme (if multipoint or multisubunit immobilization is achieved) is more stable towards harsh conditions like high temperatures. This allows the biocatalyst to survive longer during process conditions and allows one to operate the process at higher temperature [3]. There are many immobilization techniques available such as adsorption [4], covalent attachment [5] and entrapment [6]. Non-covalent binding processes are simple but have the disadvantage of weak binding of enzyme. Covalent binding is preferred over physical or ionic binding. However, covalent attachment requires the activation of support surface if active groups on the surface are unavailable [7]. However, every system has both advantages and disadvantages. Support material, which plays an important role in the utility of an immobilized enzyme, should be readily available and non-toxic and also should provide a large surface area suitable for enzyme reaction, and substrate and product transport with least-diffusional restriction [8]. Some organic and inorganic supports like glass beads [9], polyketone [10], chitosan [11] and aluminum oxide/polyethyleneimine composite [12] have been used for the immobilization of peroxidases.
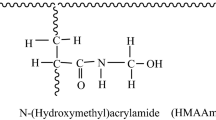
Poly(ethylene terephthalate) (PET) fiber is one of the most important synthetic fibers used in the textile industry. PET fibers have good resistance to most strong acids, oxidizing agents, sunlight and micro organisms [13]. However, it is hydrophobic in nature and do not contain chemically reactive groups. Certain desirable functional groups can be imparted to PET fiber by grafting with different monomers such as 4-vinyl pyridine [14], 4-vinyl pyridine and 2-hydroxyethylmethacrylate [15], N-vinyl–2 pyrrolidone [16]. The modified PET fiber was widely utilized in our previous researches as support materials towards the uptake of heavy metal ions [17–20], acidic and basic dyes [21–23] and recently α-amylase immobilization [24].
Peroxidases (donor: hydrogen peroxide oxido reductase, EC 1.11.1.7) are a group of heme-containing oxidoreductases that act on peroxide as electron donors. They are present in all known organisms and their biological function is related to the removal of the toxic hydrogen peroxide which is a product cell metabolism of plant hormones, lignification and modifications of cell wall. Peroxidases reduce hydrogen peroxide and oxidize a wide number of compounds including phenols, aromatic amines, thioanisoles, halide ions thyocianate ions, fatty acids, and also degrade hydrogen peroxide. The selectivity towards reducing substrates depends on the specific peroxidase [25]. They have been extensively studied and show many attractive properties for biocatalysis such as wide specificity, high stability in solution and easy accessibility from plant materials. They show potentially interesting applications in a number of fields. The most important application so far is in analytical diagnosis, where they are utilized as a key component of biosensors and immunoassays [26].
In the present study, the horseradish peroxidase (HRP) has been covalently immobilized on the PET grafted acrylamide (PET-g-AAm) fiber. For this purpose, amino groups were generated via Hofmann degradation reaction on the surface of the PET-g-AAm fiber. The aminated support material was activated by glutaraldehyde [12, 25, 27]. Then the horseradish peroxidase was immobilized onto the activated fiber. The activities of the immobilized enzyme and the free enzyme were evaluated in point of pH, temperature, substrate concentration, thermal stability and storage stability and the results were compared. The reusability of the immobilized enzyme was also investigated.
Materials and methods
Chemicals
The PET fiber (126 denier, 28 filaments) was kindly provided by SASA Co. (Adana, Turkey). Horseradish peroxidase (EC 1.11.1.7), and glutaraldehyde were obtained from Sigma-Aldrich. Pyrogallol and Folin-Ciocalteu’s phenol reagent were purchased from Fluka. Acrylamide, sodium hydroxide, hydrogen peroxide, benzoyl peroxide, citric acid and acetic acid were purchased from Merck. Ethanol, methanol, acetonitrile, acetone, sodium dihydrogen phosphate and disodium hydrogen phosphate were purchased from Riedel-deHaen. Solutions were prepared with deionized water (Millipore, Elix 3 water purification system).
Apparatus
Pharmacia Biotech Ultrospec 2000 model UV-Visible spectrophotometer was used for the determination of optical density of solutions in the visible region. The infrared spectrums were obtained by Thermo-Nicolet 6700 FT-IR spectrometer attached to an attenuated total reflection (ATR) apparatus, using diamond prism with an incident angle 45°. All pH measurements were performed with HANNA 221 model digital pH meter.
Preparation of support material
After fiber samples (0.30 ± 0.01 g) had been swollen in dichloroethane for 2 h at 90 °C, solvent on the fiber was removed by blotting between a filter paper [14]. As described by Coşkun and Soykan [28], acrylamide monomer was grafted onto the PET fiber by using benzoyl peroxide as an initiator. Swelled PET fiber was placed in 100 ml polymerization tube. In addition, appropriate amount of acrylamide monomer dissolved in 18 ml aqueous and Bz2O2 dissolved in 2 ml acetone were added. The polymerization tube was placed into the water bath at 85 ± 1 °C. The fiber samples taken at the end of the 2 h of polymerization were removed from homopolymer by washing with water at 50 °C for 5 h by changing the washing water five times. The fiber was dried at 50 °C and weighed. The graft yield (GY) was calculated from the weight increase in grafted fiber as follows:
where W i and W g denote the weights of the original and grafted PET fiber, respectively.
Modification of PET-g-AAm fiber
The PET-g-AAm fiber was chemically modified as described in our previous research [24]. The amide groups of poly(acryl amide) were converted to the amine groups by Hofmann degradation reaction. The PET-g-AAm fiber (0.03 g) was immersed in 15 ml of suitable concentration of NaOCl and NaOH aqueous solution at 20 °C for 30 min. After continuous shaking for given period of time, the fiber was removed from the mixture and washed with 10 ml of deionized water four times. The PET fiber was shaken in 5 ml of 2% glutaraldehyde solution at 100 rpm for 18 h [29]. Activated fiber was washed with deionized water four times to remove the unreacted glutaraldehyde.
Immobilization of HRP onto the activated support
The activated PET fiber was shaken in solution of the HRP (0.2 mg ml−1) in 50 mM buffer solution at 100 rpm for 18 h. Then the PET fiber was washed with phosphate buffer solution for 10 min by shaking at 100 rpm four times. Immobilized enzyme was stored in buffer solution at 4 °C.
Protein determination
The enzyme solutions were prepared in the concentration range from 0.06 to 0.2 mg ml−1 protein. The activated fiber (0.42 g) was shaken with enzyme solutions (15 ml) at 100 rpm for 18 h. Then, the fiber was removed from enzyme solution and washed with 10 ml buffer solution for 30 min by shaking at 150 rpm for two times. The enzyme solution and washing solutions were collected and protein contents were determined. The amount of immobilized protein was calculated from the difference between the amount of protein introduced to the coupling reaction medium and the amount of residues protein in the medium. The amount of protein was determined by the Lowry’s method with bovine serum albumin as standard [30].
Activity measurement of free HRP and immobilized HRP
The activities of the free and the immobilized enzymes were determined by using pyrogallol and H2O2 as substrates as described by Halpin et al. [31]. The reaction mixture containing 2 ml of the pyrogallol solution (20 mmol l−1) and 1.5 ml of buffer solution (0.1 mol l−1) was incubated with 0.1 ml the free HRP (0.01 mg ml−1) and the immobilized HRP in shaking water bath at 100 rpm. The reactions were started with the addition of 0.5 ml of 10 mmol l−1 H2O2 solution. The colour densities of product solutions were measured at 420 nm, 1 min after H2O2 addition for the free HRP and 5 min after H2O2 addition for the immobilized HRP. The values obtained in the blank reactions, performed in the presence of H2O2 without enzyme, were discounted from all readings. One unit (U) of enzyme activity was defined as the amount of enzyme which produced 1 μmol purpurogallin per min under assay conditions. The relative activity was calculated as follows:
Thus the highest activity is regarded as 100% for free and immobilized studies separately.
Results and discussion
The PET-g-AAm fiber was used as support material for immobilization of the HRP. The schematic representation for overall processes was illustrated in Fig. 1.
The infrared spectra of the PET (spectrum a), the PET-g-AAm (40.1% graft yield) (spectrum b), the grafted PET after Hofmann degradation reaction (spectrum c), the glutaraldehyde coupling PET (spectrum d) and the HRP binding PET fiber (spectrum e) are shown in Fig. 2. The examination of these spectra (a and b) revealed that there exists a typical amide I band (~1,652 cm−1), consisting of C=O strech of poly(AAm) and amide II band (~1,606 cm−1), including N–H vibration in spectrum. These data showed that amide groups were attached to the fiber structure. The comparison of these spectra (b and c) revealed that the intensity of carbonyl peak decreased at 1,652 cm−1 of AAm. This result is attributed to the fact that amide groups were converted to amine groups on PET-g-AAm fiber by Hofmann reaction. In the spectrum d, the peak (~1,652 cm−1) broadened and the intensity increased due to the carbonyl group of glutaraldehyde and imine bound (C=N stretching vibration). These data showed that the glutaraldehyde was attached to grafted PET fiber. In addition, the intensity and area of the peak at 1,650 cm−1 was increased by immobilization of HRP (in spectrum e), resulting from the effect of the imine bound.
Optimization of immobilization conditions
The effect of graft yield on the enzyme activity was investigated by performing in the grafting range of 3.7–40.1%. The results are shown in Fig. 3. It is clear from the Figure that the activity of the immobilized HRP increased as the graft yield increases. This increase in activity of the immobilized HRP can be attributed to the increase in the amine groups obtained from amide groups in the fiber structure by graft copolymerization. The activity represents the amount of the immobilized enzyme, which depends on the amount of the functional group on the PET-g-AAm fiber.
The enzyme immobilizations (covalent and adsorption) were carried out at different pH values of immobilization media. The effect of pH on the immobilization was studied in the pH range from 4 to 9 and results were described in Fig. 4. As can be seen in that Figure, the activity of covalent immobilization of HRP is higher than the activity of immobilization of HRP without activation with glutaraldehyde. The formation point of covalent bonds between the surface of the support and enzyme regions probably depends on the pH value of the coupling media. The highest immobilization efficiency was obtained in the pH interval 6–8 of the coupling media. This is presumably because the active site of the HRP was masked in order to prevent covalent bond formation the near active site. In other words, the amino groups in the enzymatic regions far from the active site might have reacted with glutaraldehyde on the PET-g-AAm during immobilization process. Similar results were reported by Fernandes et al. [32] using polyaniline for immobilization of the HRP.
The effect of the protein concentration on ‘the immobilization percentage’ and ‘the activity’ was also investigated, which is shown in Fig. 5 and 6 respectively. As shown in Fig. 5, the maximum bounded percentage of the protein was 34% attained at 0.1 mg ml−1 concentration. It can be seen in Fig. 6 that the activity of the enzyme increased as the concentration of the protein increased. The maximum activity was obtained as 0.035 U at 0.1 mg ml−1 concentration of protein and beyond that point it almost remained unchanged.
Effect of pH on enzyme activity
pH has an important role for enzymatic reactions. The behaviour of an enzyme molecule may be modified by its immediate microenvironment. An enzyme in solution may have a different pH optimum from that of the same enzyme immobilized on a solid matrix, depending on the surface and residual charges on the solid matrix and the pH value in the immediate vicinity of the active site. A change in the optimum pH normally accompanies the immobilization of the enzymes, depending upon the polymer used as matrix. Since the enzyme activity is markedly influenced by environmental conditions, especially pH, the change in behavior caused by enzyme immobilization is useful for understanding the structure-function relationships of the enzyme. Therefore, it is very useful to compare the activity of the soluble and the immobilized enzymes as a function of pH [33]. The activities of the free HRP and the immobilized HRP were assayed at varying pH (3–9) at 20 °C. The pH dependence of the activity of the immobilized HRP has been compared to that of the free HRP and is presented in Fig. 7. The maximum activity of the immobilized enzyme was observed at pH 7 whereas the free enzyme exhibits maximum activity at the pH 8. Similar results involving decrease in the optimum pH value of the HRP after immobilization have been reported in the literature [34, 35]. In contrast to the free HRP, the relative activity of the immobilized HRP is higher for the pH interval from 3 to 7 and at pH 9. There was a general improvement in the stability of the immobilized enzyme to denaturation processes caused by extreme value of pH. In general, the immobilized enzymes have either a broader pH range than the free enzymes or the same pH range as the free ones. The stability of the immobilized enzymes at low pH values can be due to the amine groups because they can bind to proton in the matrix, resulting in a decrease of the local concentration of proton near the enzyme molecules [36].
Effect of temperature on enzyme activity
The effect of reaction temperature on the enzymatic activity of the free HRP and the immobilized HRP was investigated by varying temperatures from 20 to 60 °C. Figure 8 shows the results obtained in the determination of optimum temperature assay for the free HRP and the immobilized HRP. Maximum activity was observed at 45 °C for the free and the immobilized HRP. For the free HRP, the reaction rate increased at temperatures between 20 and 45 °C, and then decreased at higher temperatures. The temperature profile for the immobilized enzyme was very similar over the first part of the curve. However, the decrease in reaction rate of the immobilized HRP at temperatures above 45 °C was much slower than that for the free HRP; high relative activity (>80%) was maintained over the range from 30 to 50 °C. The thermal stability of the immobilized HRP was apparently higher than that observed for the free enzyme considering the reaction time for the immobilised HRP (5 min) and for the free HRP (1 min).
The improvement in denaturation resistance of the immobilized HRP was probably a consequence of increasing conformational stability. Indeed, the catalytic activity of the enzymes is related to their conformation and, generally, the activity value increases with a rise in temperature, as is usually observed for chemical catalysts. However, the enzymes are unstable under heating because the increase in the environmental temperature results in breaking the interactions that are responsible for its proper globular, catalytically active structural conformation. Since immobilization provides a more rigid external backbone for the enzyme molecules, the effect of higher temperatures becomes less serious, so that optimum temperatures are expected to increase [37].
Thermal stability
The thermal stabilities of the free HRP and the immobilized HRP were assayed by immersing them in buffer solutions (50 mmol l−1, pH 7) for 180 min at 50 °C and periodically determining their activities. Figure 9 shows the thermal stability of the free HRP and the immobilized HRP. It can be seen that the immobilized HRP retained relative activity of about 44% whereas the relative activity retained by the free HRP was only 19%. These results indicate that the thermal stability of the immobilized HRP is much better than that of the free one owing to the covalent bond between the enzyme and support, which prevents the conformation transition of the enzyme at high temperature. It was reported that the thermal stability enhancement was one of the general advantages of the immobilized enzymes [6, 35].
Reusability of immobilized enzyme
For the immobilized enzyme, one of the most important advantages is reuse stability, which can effectively reduce the cost in applications. The operational stability of the immobilized HRP in this study was evaluated in a repeated batch process. Figure 10 shows the effect of the repeated use on the activity of the immobilized HRP. The relative activity of the immobilized system reduced with the increase of the number of reuses. The immobilized HRP retained a relative activity of 54% after 20 reuses. This reduction is due to the inactivation of the enzyme caused by the denaturation of protein. The number of reuse represents a more satisfying performance when compared to previous research [3, 6, 38].
Storage stability
For enzyme immobilization, another important opinion needed to be taken into account when assessing immobilization efficiency, is the storage stability of immobilized enzyme. Immobilized and free enzymes were stored in buffer solutions (pH = 7) at 4 °C. Enzyme activity was tested at different times and obtained results were given as relative activity in Fig. 11. The free HRP loses about 72% activity in 60 days while the immobilized HRP shows good storage stability and retains 61% of its activity for 60 days.
Effect of organic solvents on enzyme activity
Organic solvents have been used most extensively as solvents for the polymerization of phenols by the HRP. Organic solvents are needed for an increase in the solubility of the monomers and for obtaining polyphenols of high molecular weight. For synthetic reactions catalyzed by enzymes, stability of the enzymes in the reaction media is important, especially when organic solvents are used [39]. The activity of the free and the immobilized enzymes was determined in the presence of organic solvents (ethanol, methanol, acetone and acetonitrile) whose concentrations in the activity medium 10% (v/v). The results are illustrated in Fig. 12. The free HRP and the immobilized HRP showed very similar behaviour toward ethanol. The stability of the HRP was increased by immobilization in the presence of methanol, acetone and acetonitrile.
Conclusions
Horseradish peroxidase was immobilized on PET grafted acrylamide fiber by covalent attachment method. The covalent binding process consists of four steps: grafting of AAm on PET backbone, conversion of amide groups to amine groups, glutaraldehyde activation and enzyme coupling. The immobilization of HRP increased its thermal stability, storage stability, organic solvent stability and reusability. The immobilization not only stabilizes the enzyme, but also the PET-g-AAm fiber employed for immobilization adsorbs reaction products. The adsorption of reaction product onto the PET-g-AAm would be of obvious benefit in removing toxic products from the waste water in the event that the peroxidase-catalyzed transformation does not lead to extensive detoxification.
References
Mateo C, Palomo JM, Lorente GF, Guisan JM, Lafuente RF (2007) Enzyme Microb Technol 40:1451
Duran N, Rosa MA, D’Annibale A, Gianfreda L (2002) Enzyme Microb Technol 31:907
Dalal S, Gupta MN (2007) Chemosphere 67:741
Silva RA, Carmona-Ribeiro MA, Petri DFS (2007) Int J Biol Macromol 41:404
Gomez JL, Bodalo A, Gomez E, Bastida J, Hidalgo MA, Gomez M (2006) Enzyme Microb Technol 39:1016
Shukla SP, Modi K, Ghosh PK, Devi S (2004) J Appl Polym Sci 91:2063
Pujari NS, Vaidya BK, Bagalkote S, Ponrathnam S, Nene S (2006) J Membrane Sci 285:395
Li S, Hu J, Liu B (2004) Biosystems 77:25
Rojas-Melgarejo F, Rodriguez-Lopez JN, Garcia-Canovas F, Garcia-Ruiz PA (2004) J Chem Technol Biotechnol 79:1148
Agostinelli E, Belli F, Tempera G, Mura A, Floris G, Toniolo L, Vavasori A, Fabris S, Momo F, Stevanato R (2007) J Biotechnol 127:670
Bindhu LV, Abraham ET (2003) J Appl Polym Sci 88:1456
Oliveira GB, Filho JLL, Chaves MECC, Azevedo WM, Carvalho LB Jr (2008) React Funct Polym 68:27
Sakurada I, Ikada Y, Kawahara T (1973) J Polym Sci 11:2329
Arslan M, Yiğitoğlu M, Şanlı O, Ünal Hİ (2003) Polym Bull 51:237
Yiğitoğlu M, Arslan M (2007) Polym Bull 58:785
Ünal Hİ, Coşkun R, Şanlı O, Yiğitoğlu M (1997) J Appl Polym Sci 64:1437
Yiğitoğlu M, Arslan M (2005) Polym Bull 55:259
Bağ H, Türker AR, Coşkun R, Saçak M, Yiğitoğlu M (2000) Spectrochim Acta Part B 55:1101
Coşkun R, Yiğitoğlu M, Saçak M (2000) J Appl Polym Sci 75:766
Yiğitoğlu M, Ersöz M, Coşkun R, Şanlı O, Ünal Hİ (1998) J Appl Polym Sci 68:1935
Yiğitoğlu M, Arslan M (2007) E-Polymers 055
Arslan M, Yiğitoğlu M (2008) J Appl Polym Sci 107:2846
Arslan M, Yiğitoğlu M (2008) E-Polymers 016
Temoçin Z, Yiğitoğlu M (2007) E-Polymers 070
Vojinović V, Carvalho RH, Lemos F, Cabral JMS, Fonseca LP, Ferreira BS (2007) Biochem Eng J 35:126
Kamal JKA, Behere DV (2008) Biochem Eng J 38:110
Bryjak J (2003) Biochem Eng J 16:347
Coşkun R, Soykan C (2006) J Polym Res 13:1
Betancor L, Lopez-Gallego F, Hidalgo A, Alonso-Morales N, Mateo C, Fernandez-Lafuente R, Guisan JM (2006) Enzyme Microb Technol 39:877
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265
Halpin B, Pressey R, Jen J, Mony N (1989) J Food Sci 54:644
Fernandes KF, Lima CS, Pinho H, Collins CH (2003) Proc Biochem 38:1379
Atia KS, Ismail SA, Dessouki AM (2003) J Chem Technol Biotechnol 78:891
Shukla SP, Devi S (2005) Proc Biochem 40:147
Fernandes KF, Lima CS, Lopes FM, Collins CH (2004) Proc Biochem 39:957
Chang MY, Juang RS (2005) Enzyme Microb Technol 36:75
Paula AV, Urioste D, Santos JC, Castro HF (2007) J Chem Technol Biotechnol 82:281
Caramori SS, Fernandes KF (2004) Proc Biochem 39:883
Fatima A, Husain Q, Khan RH (2007) J Mol Catal B Enzym 47:66
Acknowledgments
We are grateful to the Kırıkkale University Research Fund for the financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Temoçin, Z., Yiğitoğlu, M. Studies on the activity and stability of immobilized horseradish peroxidase on poly(ethylene terephthalate) grafted acrylamide fiber. Bioprocess Biosyst Eng 32, 467–474 (2009). https://doi.org/10.1007/s00449-008-0266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-008-0266-9