Abstract
This work aimed to study the mechanism of toughness improvement of polylactic acid (PLA) by using a biobased plasticizer. Ozonized soybean oil (OSBO), acting as a biobased plasticizer, was prepared by the ozonolysis reaction. The functional groups and chemical structure of OSBO were characterized by FTIR and NMR techniques, respectively. Plasticized PLA specimens were prepared by compounding using a corotating twin-screw extruder and sheet forming using a compression molding machine. The influences of OSBO contents, varying from 0 to 15 wt%, on PLA were evaluated via mechanical and thermal testing and morphological analysis. After the ozonolysis reaction, there was an increase in ester groups and the formation of hydroxyl groups in OSBO. With increasing OSBO content, it was found that elongation at break and impact strength tended to increase, whereas tensile strength decreased. The glass transition, crystallization and melting temperatures of PLA continuously decreased as a function of OSBO content. The presence of OSBO enhanced crystallization of PLA. OSBO at low content acted as a plasticizer for PLA, whereas OSBO at 15 wt% formed fine oil droplets acting as an impact absorber by energy dissipation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polylactic acid (PLA) is an aliphatic polyester that is classified as a biodegradable biobased plastic. With its good strength and high stiffness, PLA is one of the potential choices to be used for the replacement of conventional petroleum-based plastics, especially for packaging [1, 2]. The plastic waste problem, especially from plastic packaging, is currently one of the violent environmental concerns. It has become an important driving force to introduce the application of biodegradable plastic in the packaging industry. However, the low flexibility and toughness of PLA [3] limit PLA in extensive packaging applications. Improvements in the toughness of PLA in the literature have been accomplished by various methods, such as blending PLA with ductile polymers [4,5,6,7,8,9], adding a toughening modifier [10,11,12], and increasing the chain mobility of PLA with a plasticizer [13,14,15].
Improvement of the toughness of PLA with a plasticizer is a favored choice for commercial applications because the method is not complicated and inexpensive. Generally, plasticizers can reduce the glass transition temperature (Tg) and polymer stiffness. These factors lead to increases in both flexibility and toughness. The miscibility of plasticizers with polymers is an important factor in plasticization. Plasticizers tend to migrate toward the polymer surface, leaving less plasticizer content in the polymer bulk with time. As a result, the abovementioned properties of the plasticized polymer would change over time. The miscibility of a plasticizer with a polymer depends on the polarity, solubility, chemical structure, and molecular weight of the plasticizer compared with that of the polymer. In terms of the effect of the molecular weight of the plasticizer, Tanrattanakul and Bunkaew [14] found that the lowest molecular weight of the plasticizer studied led to the greatest decreases in strength and strain at break in PLA because it induced the highest reduction in the intermolecular attraction of PLA. Avolio et al. [15] indicated that plasticizers with low molecular weights induced plasticizer aggregation and phase separation between the polymer and plasticizer and then plasticizer migration toward the polymer surface. This phenomenon results in undesired changes in properties. An increase in the molecular weight of plasticizers can slow plasticizer diffusion and lead to lower migration. Nevertheless, a higher molecular weight of the plasticizer also results in decreases in the miscibility and efficiency of the plasticizer in the plasticizing polymer [16, 17]. Burgos et al. [17] studied the effect of different molecular weights of the oligomer of lactic acid (OLA) on the efficiency as a function of plasticizer in PLA. They found that a higher molecular weight of OLA led to a smaller decrease in Tg. From previous research work, suitable plasticizers for PLA should contain ester or hydroxyl functional groups, e.g., glycerol [18], poly(ethylene glycol) [18,19,20], ester compounds [14, 21, 22], and hydroxyl end-capped OLA [23]. The work of Avolio et al. [23] revealed that the interaction of hydroxyl end-capped OLA with PLA was stronger than that of carboxyl end-capped OLA. Biobased plasticizers are interesting choices because of their many advantages, such as nontoxicity, renewability, wide molecular weight distribution, low cost and biodegradability. However, biobased plasticizers must be modified such that they contain PLA-compatible functional groups. The ozonolysis reaction for oil is a reaction between unsaturated fatty acids, ozone and ethylene glycol. The triglycerides of soybean oil are composed of five major fatty acids (palmitic, stearic, linoleic, oleic and linolenic). The high content of unsaturated fatty acids in soybean oil includes linoleic acid (50.8%), monounsaturated oleic acid (22.8%) and polyunsaturated linolenic acid (6.8%) [24]. Scheme 1 shows the ozonolysis reactions of unsaturated triglycerides in soybean oil with ethylene glycol by an alkaline catalyst [25]. During the ozonolysis reaction, ozone reacts with the double bonds of unsaturated fatty acids in soybean oil to form unstable ozonide rings. The presence of the alkaline catalyst and ethylene glycol (Diols) can induce the reaction of ethylene glycol with unstable ozonide rings to form polyols with ester linkages [25, 26]. The cleavage of double bonds by ozone also leads to a reduction in the molecular weight of ozonized soybean oil (OSBO). The increases in compatible functional groups (hydroxyl and ester groups) and shorter chains of OSBO were expected to help increase the miscibility between oil and PLA. Therefore, this work selected the ozonolysis reaction as a modification method of soybean oil.
Ozonolysis reaction of triglyceride containing a oleic acid b linoleic acid and c linolenic acid (adapted with permission from Wiley [25])
The objective of this work was to study the efficiency of OSBO as a biobased plasticizer on the improvement of the toughness of PLA and to explain the OSBO plasticizing mechanism, estimated from the mechanical and thermal properties and morphological analysis of PLA. This work was divided into two sections. The first section was the preparation and characterization of OSBO, and the second section involved the effect of OSBO content on the property changes in PLA. OSBO was prepared by the ozonolysis reaction, and then, the changes in functional groups and chemical structure were investigated with FTIR and NMR techniques, respectively. The effect of OSBO content on the properties of PLA was investigated by tensile and impact tests, scanning electron microscopy, differential scanning calorimetry, and dynamic mechanical thermal and thermogravimetric analysis.
Experimental
Raw Materials
PLA was purchased from Nature Works LLC (USA) under the trade name Ingeo 4043D. Degummed soybean oil was obtained from Thanakorn Vegetable Oil Products Co., Ltd. (Thailand). Ethylene glycol and calcium carbonate (CaCO3) were purchased from Thermo Fisher Scientific Australia Pty Ltd. and used for the ozonolysis reaction of degummed soybean oil. Ozone was produced by passing oxygen gas through an ozone generator built by the Department of Physics, King Mongkut’s University of Technology Thonburi (KMUTT).
Modification of Soybean Oil by Ozonolysis Reaction
The ozonolysis reaction of soybean oil proceeded according to the work of Tran et al. [25]. Soybean oil (200 g) and ethylene glycol (150 g) were reacted with ozone in a 500-mL gas wash bottle. CaCO3 (10 g) was used as a catalyst for the ozonolysis reaction. The reaction temperature was maintained at 0 °C by placing the gas wash bottle in an ice water bath. For ozone production, oxygen gas was flown through an ozone generator with a flow rate of 12.0 L/min. Under this condition, the ozone generator can produce ozone at 13.1 g/h. Ozone bubbles were passed through the reaction mixture for 2 h. After the ozonolysis reaction, excess ethylene glycol and CaCO3 were removed from OSBO. Ethylene glycol was washed out from OSBO with 500 mL of distilled water 5 times. CaCO3 was filtered out through a fine filter paper. After that, moisture was removed from OSBO by molecular sieving for 48 h. FTIR and NMR techniques were used to characterize the functional groups and chemical structure of soybean oil before and after ozonolysis. The functional groups of oil were identified with an FTIR spectrometer (Spectrum One, PerkinElmer, Inc., USA) in transmission mode. A small quantity (5 µl) of oil sample was deposited between two transparent KBR disks to create a thin film. All spectra were recorded from wavenumbers of 4000 to 400 cm−1 with a resolution of 4 cm−1 at 20 scans. NMR spectra were obtained from an FT-NMR spectrometer (Inova500 MHz, Varian, Inc., USA). OSBO was dissolved in deuterated chloroform (CDCl3) before the measurement to obtain the 1H and 13C spectra at room temperature.
Preparation of OSBO Plasticized PLA Specimen
Before mixing PLA and OSBO, PLA pellets were dried in an oven at 80 °C for 4 h. The dried PLA pellets were dry-blended with OSBO by a high-speed mixer for 2 min. OSBO contents were varied from 0 to 15 wt%. A corotating screw twin screw extruder (Enmach, Thailand) was used to prepare neat PLA and OSBO-plasticized PLA with a screw rotating speed of 85 rpm. The extrusion temperature profiles were 80, 120, 140, 150, 150 and 160 °C from the hopper to die zones. The extrudate was solidified by passing into a water bath and granulated by a pelletizer. In the sample preparation process, neat PLA and OSBO-plasticized PLA were again dried in an oven at 80 °C for 4 h. After the drying step, PLA specimens were fabricated by a compression molding machine (QC-601 T, Cometech Testing Machines Co., Ltd, Taiwan). Neat PLA and OSBO-plasticized PLA were made by compression at 170 °C for 6 min using a pressure of 2000 psi.
Characterization
Mechanical Testing
The mechanical properties of neat PLA and OSBO-plasticized PLA were obtained through tensile and impact testing. A universal testing machine (QC-506M1, Cometech Testing Machines Co., Ltd, Taiwan) was used for the measurement of tensile properties according to ASTM D638 at a crosshead speed of 5 mm/min. Dumbbell-shaped specimens (Type I) were used for tensile testing with a gauge length of 50 mm. Izod impact tests were carried out using a 6545 CEAST® Resil Impactor, according to ASTM D256. The specimen dimensions were 12.7 × 63.5 × 3.3 mm3. The reported data for the mechanical test were obtained from the average values of seven independent specimens of each formulation.
Thermal Analysis
The glass transition temperatures of neat PLA and OSBO-plasticized PLA were measured using both differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA). DSC characterization was performed using a calorimeter (DSC-204F1, Netzsch, Germany) under a nitrogen atmosphere. The PLA samples (5–6 mg) were heated at a temperature range of 30–200 °C with a heating rate of 10 °C/min. The glass transition temperature (Tg), cold crystallization temperature (Tc), melting temperature (Tm) and crystallinity contents of neat PLA and OSBO-plasticized PLA after molding were investigated by DSC technique. The percentage crystallinity (Xc) was determined from Eq. (1).
where Hm is the melting enthalpy of the sample, Hc is the cold crystallization enthalpy, Hmo is the melting enthalpy at 100% crystallinity of PLA, which is 97.2 J/g [27]. WPLA is the weight fraction of PLA.
A dynamic mechanical analyzer (DMA 242E, Netzsch, Germany) was used to obtain the Tg values of neat PLA and OSBO-plasticized PLA. The specimen dimensions were 12.0 × 32.0 × 3.3 mm3, and the specimen was heated from a temperature of -50 to 130 °C with a heating rate of 3 °C/min. The specimen was tested under dual cantilever mode with a frequency of 1 Hz and amplitude of 30 μm.
Microstructure Analysis
The fracture surfaces of neat PLA and OSBO-plasticized PLA were examined using a scanning electron microscope (JSM-7610F, Jeol, USA) at an accelerating voltage of 5 kV. Before SEM characterization, the fracture surfaces of impacted specimens were coated with gold to prevent charging.
Results and Discussion
Modification of Soybean Oil by the Ozonolysis Reaction
Figure 1 shows the FTIR spectra of soybean oil before and after the ozonolysis reaction. In the spectrum of OSBO (dashed line), compared to nonozonized soybean oil (solid line), the peak of C=C stretching at 1620 cm−1 cannot be observed to decrease after ozonolysis reaction, while the peaks of =C–H stretching at 3010 cm−1 almost disappear. A new broad peak appears at 3462 cm−1 representing O–H stretching of alcohol. Moreover, there is an increase in the peak intensity of C–O stretching at 1103 cm−1 and a broadening of the C=O peak at 1740 cm−1. These changes indicated an increase in ester groups on soybean oil chains after 2 h of reaction time. The ozonolysis reaction led to cleavage of the double bonds of unsaturated fatty acids in soybean oil by reacting with ozone to form ozonide rings. Ethylene glycol can then react with soybean oil at this molecular position. The ozonolysis reaction results in the formation of hydroxyl groups and an increase in ester groups on OSBO molecular chains.
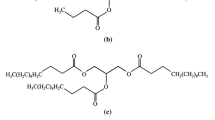
The structural change of soybean oil by the ozonolysis reaction can be confirmed with NMR analysis. The 13C NMR and 1H NMR spectra are shown in Figs. 2 and 3, respectively. Double peaks at 130 ppm were observed in the 13C NMR spectrum of soybean oil (Fig. 2a), whereas they were absent in the spectrum of OSBO, as shown in Fig. 2b. The double peaks at 130 ppm were the signal of double bonds in the unsaturated fatty acid of the soybean oil. The disappearance of double peaks was attributed to the ozonolysis reaction at the double bond in soybean oil. The reaction of ozone with unsaturated fatty acids of oil led to the formation of an unstable ozonide ring (1,2,3 trioxolane). In the relatively anhydrous environment, the primary ozonide ring (1,2,3 trioxolane) rearranges to form the secondary ozonide ring (1,2,4 trioxolane) [28]. Ethylene glycol can react with unstable ozonide rings to form polyols and ester linkages [25, 26]. The new peaks at 62–68 ppm were expected as the signals of a carbon atom attached to the oxygen atom from the reaction of oil and ethylene glycol. The new peak at 62 ppm was the peak of C–OH whereas the peak at 68 ppm was the peak of C–O at the linkage of oil with ethylene glycol [25, 29]. In addition, the appearance of a new peak at 104.5 ppm was the ring carbon of secondary ozonide (1,2,4 trioxolane) assigned by Wu et al. [30]. The new peak at 104.5 ppm was also found in the works of Tran et al. [25] and Soriano Jr et al. [31]. The signal at 104.5 ppm is an evidence of the remained secondary ozonide from its reaction with ethylene glycol. The carbonyl peak of ester (173 ppm) and methylene peaks (25–35 ppm) remained unchanged. Figure 3a and b show 1H NMR spectra of OSBO at reaction times of 0 and 2 h, respectively. After the ozonolysis reaction, the peaks at 2 and 5.3 ppm, which represented the peak of hydrogen atoms attached to carbon atoms at double bonds, were found to decrease. This result corresponded to the previous explanation. In addition, a peak at 3.7 ppm, not present in normal soybean oil, was observed in the 1H NMR spectra of modified oil with a 2 h reaction time. This peak represented a hydrogen atom attached to the oxygen atom in the hydroxyl group. From structural characterizations, the results of OSBO by the ozonolysis reaction were consistent with the work of Tran et al. [25]. The chemical structure of the modified oil changed from unsaturated fatty acids to polyol mixtures with an increased number of ester groups. The hydroxyl and ester groups were expected to improve the oil compatibility with PLA.
Effect of OSBO Content on the Properties of PLA
OSBO with an ozonolysis time of 2 h was used to study the toughness improvement of PLA. Figure 4 shows changes in tensile modulus and elongation at break of plasticized PLA at OSBO contents of 0–15 wt%. The tensile modulus of PLA remained unchanged with the addition of OSBO, whereas elongation at break tended to increase with increasing OSBO contents of more than 5 wt%. The maximum elongation of 10 wt% OSBO was 89% higher than that of neat PLA. The addition of plasticizers usually led to the reduction in tensile modulus whereas elongation at break was increased. For this work, the unchanged values of tensile modulus might be because of the slightly decreased Tg values of plasticized PLA by OSBO, as shown in Table 1. Tg values of plasticized PLA were still as far from room temperature. Therefore, the behavior of plasticized PLA remained in a glassy state. Figure 5 shows the tensile curves of plasticized PLA with different OSBO contents. It was observed that the initial linear stress–strain curves of neat PLA and plasticized PLA yielded similar slopes. However, the fracture behaviors of PLA changed from brittle to ductile upon the addition of OSBO. The plastic deformations of plasticized PLA were more extensive than those of neat PLA. The increase in elongation at break by OSBO was attributed to the increases in free volume and chain mobility of PLA due to the penetration of OSBO into PLA chains.
The ultimate tensile strength (UTS) and impact strength of plasticized PLA are shown in Fig. 6. The UTS decreased continuously with increasing OSBO content, whereas the impact strength showed the opposite trend. The decrease in UTS was because OSBO increased the flexibility and chain mobility of PLA chains. The UTS at 15 wt% OSBO, which represents the minimum value, was approximately 36% lower than that of neat PLA. The addition of 15 wt% OSBO improved the impact strength of PLA such that the strength was 25% higher than that of neat PLA. The fracture surfaces of neat PLA and plasticized PLA from impact testing illustrated different fracture behaviors, as shown in Fig. 7. The fracture surfaces of plasticized PLA in Fig. 7c and d were rougher than that of neat PLA in Fig. 7a. The rougher fracture surfaces represent higher energy absorption and more extensive plastic deformation. The more plastic fracture behavior of plasticized PLA was the result of OSBO acting as a plasticizer. The compatibility between OSBO and PLA in Fig. 7b and c was the result of polar-polar interactions between the ester (–COO–) groups in OSBO and PLA and the hydrogen bonding of hydroxyl (–OH) groups in OSBO with both the C=O and C–O groups of PLA. The decreased Tg values with the addition of OSBO in Table 1 could be used to support the plasticization effect of OSBO in PLA. An interesting observation in the changes in the toughness of PLA by adding OSBO was that the value of impact strength at 15 wt% OSBO was higher than that at 10 wt% despite that the Tg values of both by DSC analysis were only slightly different, being 49.4 °C and 50.1 °C, respectively. This indicated that there was another toughening mechanism apart from OSBO acting as a plasticizer. From the micrographs in Fig. 7, phase separation and the formation of fine droplets of OSBO can be observed in plasticized PLA with 15 wt% OSBO only. In Fig. 7d, numerous fine droplets of OSBO dispersed uniformly in the PLA bulk, and the droplet size was less than 2 μm. The fine liquid droplets of OSBO became another part that helped improve the toughness of PLA. A similar behavior was found in the work of Pluta and Piorkowska [6]. The fine droplets of the modifier, which are in the liquid phase at room temperature, enhanced drawability and were responsible for energy dissipation. From the results in this work, toughening of PLA by OSBO might be facilitated by two mechanisms depending on the OSBO content. For a low OSBO content, OSBO, miscible with PLA and penetrating in between PLA chains, acted as a plasticizer for PLA. At a high OSBO content (15 wt% OSBO), OSBO functioned as both a plasticizer and energy dissipator. In addition, the reduced miscibility between OSBO and PLA at 15 wt% OSBO could be used to explain the decrease in elongation at break. A similar result was found in the work of Al-Mulla et al. [32]. They studied the effects of epoxidized palm oil (EPO) as plasticizer on the PLA/PCL blend. They found that the elongation at break tended to decrease at EPO content of higher 10 wt% because of the incomplete miscibility between plasticizer and polymer.
Table 1 shows the changes in thermal transitions of PLA as a function of OSBO content obtained from DSC and DMA techniques. The decrease in the Tg values by DSC analysis was already mentioned in the above paragraph. The decreases were caused by OSBO acting as a plasticizer for PLA. However, the addition of OSBO led to a slight decrease in Tg. The miscibility of OSBO with PLA is not quite well enough for acting as a good plasticizer. It was expected that the excess high molecular weight of OSBO resulted in the decreases in the miscibility and plasticizing efficiency of OSBO with PLA. The near values of Tg between 10 and 15 wt% OSBO content corresponded to the phase separation of OSBO in Fig. 7. In the works using modified vegetable oil as plasticizer [32,33,34,35], Tg values of PLA were similar behaviors with this work. They found no much reduction in Tg values of plasticized PLA by modified vegetable oil. The work of Ali et al.showed that Tg values slightly decreased at epoxidized soybean oil (ESO) content of higher 10 wt% due to phase separation of ESO [35]. The Tc values also decreased with increasing OSBO content. The Tc value of neat PLA was 110.6 °C. The Tc values decreased to 107.7 °C, 99.0 °C and 96.0 °C when plasticized with 5, 10 and 15 wt% OSBO, respectively. The gradual decrease in the Tc of PLA with the addition of OSBO indicated that OSBO penetration in PLA chains led to increases in chain mobility and the free volume of PLA. It induced an easier and faster cold crystallization process. A similar result was found in the works of Silverajah et al. [13] and Ali et al. [35]. The difference in Tc values between 10 and 15 wt% OSBO was lower than that in Tc values between 5 and 10 wt% OSBO. This was attributed to the phase-separated OSBO droplets at 15 wt% OSBO obstructing the orientation of PLA chains during the cold crystallization process. The result of Tc value shows similar trend with the results of Tg value and elongation at break. These results indicated that the imperfect miscibility between OSBO and PLA at 15 wt% OSBO led to the reduction of plasticizing efficiency. The DSC thermograms in Fig. 8 show a single melting peak for neat PLA at a temperature of 151.6 °C and two distinct melting peaks for plasticized PLA. Table 1 lists Tm values of the double peaks. The OSBO enhanced the chain flexibility of PLA resulting in a faster cold crystallization. It was assumed that cold crystallization contributed to the double melting peaks [14]. The lower melting temperature at 145.2–149.0 °C (below 150 °C) was the melting of the α’ form crystals or the crystals from the cold crystallization [14, 36, 37]. But the higher melting temperature at 151.6–153.9 °C was the melting of the α form crystals or the original crystals from the sample preparation [14, 36]. The melting temperatures tended to decrease with increasing OSBO content but remained unchanged at 15 wt% OSBO. The decreases in Tm values at the double peaks were due to the higher OSBO content led to easier chain mobility and faster fusion of crystalline PLA. The unchanged Tm value at 15 wt% OSBO was the result of the partially phase-separated OSBO coalescing into liquid droplets. For the percentage crystallinity, it was calculated as following Eq. (1) to report the PLA crystallinity after molding. The crystallinity level slightly increased with increasing OSBO content from 0 to 15 wt% OSBO. Therefore, it indicated that the changes in tensile and impact properties of plasticized PLA were mainly caused by the miscibility and plasticizing efficiency of OSBO with PLA.
Figure 9 shows the effect of OSBO content on the Tan δ curves obtained from DMA analysis. It was found that the peaks of Tan δ shifted toward the left when the OSBO content was increased. This result indicated that the Tg values at the maximums of Tan δ (Tan δmax) tended to slightly decrease with increasing OSBO content. The Tg values obtained from the DMA technique are shown in Table 1, and the changes in the Tg values with varying OSBO content were the same as those obtained from DSC analysis. Above the glass transition, another transition temperature was found in both neat PLA and plasticized PLA. This transition temperature tended to shift to a lower temperature in the presence of OSBO as a plasticizer. It was expected that this transition involved the cold crystallization process.
Conclusion
The efficiency of OSBO acting as a biobased plasticizer for PLA was estimated by using mechanical and thermal tests and morphological analysis. The mechanisms through which OSBO assists in toughness improvement in PLA were explained based on mechanical, thermal and microstructure properties and their relations. In the modification of soybean oil, the ozonolysis reaction increased ester groups and created hydroxyl groups on OSBO chains. These functional groups enhanced the compatibility between OSBO and PLA via polar-polar interactions between the ester groups of OSBO and PLA and hydrogen bonding between the hydroxyl groups of OSBO and the ester groups of PLA. OSBO improved the flexibility and toughness of PLA but decreased its tensile strength. The Tg and Tm values of PLA decreased with OSBO addition but remained unchanged at 15 wt% OSBO. The presence of OSBO enhanced the crystallinity of PLA, as OSBO induced easier arrangements of PLA chains. Phase separation of OSBO at 15 wt% indicated the insufficiency of OSBO as a good plasticizer. However, the coalescence of OSBO into fine liquid droplets led to better impact resistance by increasing energy dissipation.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Code Availability
Not applicable.
References
Öz AT, Süfer Ö, ÇelebiSezer Y (2017) Poly(lactic acid) films in food packaging systems. Food Sci Nutr Technol 2:131. https://doi.org/10.23880/FSNT-16000131
Jiménez L, Mena MJ, Prendiz J, Salas L, Vega-Baudrit J (2019) Polylactic acid (PLA) as a bioplastic and its possible applications in the food industry. J Food Sci Nutr 5:100048. https://doi.org/10.24966/FSN-1076/100048
Zhao X, Hu H, Wang X, Yu X, Zhou W, Peng S (2020) Super tough poly(lactic acid) blends: a comprehensive review. RSC Adv 10:13316–13368. https://doi.org/10.1039/D0RA01801E
Han L, Han C, Dong L (2013) Morphology and properties of the biosourced poly(lactic acid)/poly(ethylene oxide-b-amide-12) blends. Polym Compos 34:122–130. https://doi.org/10.1002/pc.22383
Wang X, Zhuang Y, Dong L (2013) Study of biodegradable polylactide/poly(butylene carbonate) blend. J Appl Polym Sci 127:471–477. https://doi.org/10.1002/app.37735
Pluta M, Piorkowska E (2015) Tough crystalline blends of polylactide with block copolymers of ethylene glycol and propylene glycol. Polym Test 46:79–87. https://doi.org/10.1016/j.polymertesting.2015.06.014
Wang Y, Wei Z, Leng X, Shen K, Li Y (2016) Highly toughened polylactide with epoxidized polybutadiene by in-stu reactive compatibilization. Polym 92:74–83. https://doi.org/10.1016/j.polymer.2016.03.081
Rashmi BJ, Prashantha K, Lacrampe M-F, Krawczak P (2015) Toughening of poly(lactic acid) without sacrificing stiffness and strength by melt-blending with polyamide 11 and selective localization of halloysite nanotubes. Express Polym Lett 9:721–735. https://doi.org/10.3144/expresspolymlett.2015.67
Gigante V, Canesi I, Cinelli P, Coltelli MB, Lazzeri A (2019) Rubber toughening of polylactic acid (PLA) with poly(butylene adipate-co-terephthalate (PBAT): mechanical properties, fracture mechanics and analysis of ductile-to-brittle behavior while varying temperature and test speed. Eur Polym J 115:125–137. https://doi.org/10.1016/j.eurpolymj.2019.03.015
Ge XG, George S, Law S, Sain M (2011) Mechanical properties and morphology of polylactide composites with acrylic impact modifier. J Macromol Sci B 50:2070–2083. https://doi.org/10.1080/00222348.2011.557585
Meng B, Tao J, Deng JJ, Wu ZH, Yang MB (2011) Toughening of polylactide with higher loading of nano-titania particles coated by poly(ε-caprolactone). Mater Lett 65:729–732. https://doi.org/10.1016/j.matlet.2010.11.029
Song X, Chen Y, Xu Y, Wang C (2014) Study on tough blends of polylactide and acrylic impact modifier. BioResources 9:1939–1952. https://doi.org/10.15376/biores.9.2.1939-1952
Silverajah VSG, Ibrahim NA, Zainuddin N, Yunus WMZW, Hassan HA (2012) Mechanical, thermal and morphological properties of poly(lactic acid)/epoxidized palm olein blend. Molecules 17:11729–11747. https://doi.org/10.3390/molecules171011729
Tanrattanakul V, Bunkaew P (2014) Effect of different plasticizers on the properties of bio-based thermoplastic elastomer containing poly(lactic acid) and natural rubber. Express Polym Lett 8:387–396. https://doi.org/10.3144/expresspolymlett.2014.43
Avolio R, Castaldo R, Avella M, Cocca M, Gentile G, Fiori S, Errico ME (2018) PLA-based plasticized nanocomposites: effect of polymer/plasticizer/filler interactions on the time evolution of properties. Compos Part B-Eng 152:267–274. https://doi.org/10.1016/j.compositesb.2018.07.011
Imre B, Pukánszky B (2013) Compatibilization in bio-based and biodegradable polymer blends. Eur Polym J 49:1215–1233. https://doi.org/10.1016/j.eurpolymj.2013.01.019
Burgos N, Tolaguera D, Fiori S, Jiménez A (2014) Synthesis and characterization of lactic acid oligomers: evaluation of performance as poly(lactic acid) plasticizers. J Polym Environ 22:227–235. https://doi.org/10.1007/s10924-013-0628-5
Martin O, Avérous L (2001) Poly(lactic acid): plasticization and properties of biodegradable multiphase systems. Polym 42:6209–6219. https://doi.org/10.1016/S0032-3861(01)00086-6
Darie-Niţӑ RN, Vasile C, Irimia A, Lipşa R, Rȃpȃ M (2016) Evaluation of some eco-friendly plasticizers for PLA films processing. J Appl Polym Sci 133:43223–43233. https://doi.org/10.1002/app.43223
Hassouna F, Raquez J-M, Addiego F, Dubois P, Toniazzo V, Ruch D (2011) New approach on the development of plasticized polylactide (PLA): Grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur Polym J 47:2134–2144. https://doi.org/10.1016/j.eurpolymj.2011.08.001
Hassouna F, Raquez J-M, Addiego F, Toniazzo V, Dubois P, Ruch D (2012) New development on plasticized poly(lactide): chemical grafting of citrate on PLA by reactive extrusion. Eur Polym J 48:404–415. https://doi.org/10.1016/j.eurpolymj.2011.12.001
Shirai MA, Müller CMO, Grossmann MVE, Yamashita F (2015) Adipate and citrate esters as plasticizers for poly(lactic acid)/thermoplastic starch sheets. J Polym Environ 23:54–61. https://doi.org/10.1007/s10924-014-0680-9
Avolio R, Castaldo R, Gentile G, Ambrogi V, Fiori S, Avella M, Cocca M, Errico ME (2015) Plasticization of poly(lactic acid) through blending with oligomers of lactic acid: effect of the physical aging on properties. Eur Polym J 66:533–542. https://doi.org/10.1016/j.eurpolymj.2015.02.040
Erikson DR (1995) Practical handbook of soybean processing and utilization. AOCS Press, Illinois
Tran P, Graiver D, Narayan R (2005) Ozone-mediated polyol synthesis from soybean oil. J Am Oil Chem Soc 82:653–659. https://doi.org/10.1007/s11746-005-1124-z
Garrison TF, Kessler MR (2016). In: Madbouly S, Zhang C, Kessler MR (eds) Bio-based plant oil polymers and composites. Elsevier, Oxford
Vasanthan N, Ly O (2009) Effect of microstructure on hydrolytic degradation studies of poly(l-lactic acid) by FTIR spectroscopy and differential scanning calorimetry. Polym Degrad Stabil 94:1364–1372. https://doi.org/10.1016/j.polymdegradstab.2009.05.015
Uzuna H, Kaynak EG, Ibanoglu E, Ibanoglu S (2018) Chemical and structural variations in hazelnut and soybean oils after ozone treatments. Grasas Aceites 69:e253. https://doi.org/10.3989/gya.1098171
Mahou R, Wandrey C (2012) Versatile route to synthesize heterobifunctional poly(ethylene glycol) of variable functionality for subsequent pegylation. Polym 4:561–589. https://doi.org/10.3390/polym4010561
Wu M, Church DF, Mahier TJ, Barker SA, Pryor WA (1992) Separation and spectral data of the six isomeric ozonides from methyl oleate. Lipids 27:129–135. https://doi.org/10.1007/BF02535812
Soriano UN Jr, Migo VP, Matsumura M (2003) Functional group analysis during ozonation of sunflower oil methyl esters by FT-IR and NMR. Chem Phys Lipids 126:133–140. https://doi.org/10.1016/j.chemphyslip.2003.07.001
Al-Mulla EAJ, Ibrahim NAB, Shameli K, Ahmad MB, Yunus WMZW (2014) Effect of epoxidized palm oil on the mechanical and morphological properties of a PLA–PCL blend. Res Chem Intermed 40:689–698. https://doi.org/10.1007/s11164-012-0994-y
Dai X, Xiong Z, Na H, Zhu J (2014) How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos Sci Technol 90:9–15. https://doi.org/10.1016/j.compscitech.2013.10.009
Tee YB, Talib RA, Abdan K, Chin NL, Basha RK, Yunos KFM (2016) Comparative study of chemical, mechanical, thermal, and barrier properties of poly(lactic acid) plasticized with epoxidized soybean oil and epoxidized palm oil. BioResources 11:1518–1540. https://doi.org/10.15376/biores.11.1.1518-1540
Ali F, Chang YW, Kang SC, Yoon JY (2009) Thermal, mechanical and rheological properties of poly(lactic acid)/epoxidized soybean oil blends. Polym Bull 62:91–98. https://doi.org/10.1007/s00289-008-1012-9
Hachana N, Wongwanchai T, Chaochanchaikul K, Harnnarongchai W (2017) Influence of crosslinking agent and chain extender on properties of gamma-irradiated PLA. J Polym Environ 25:323–333. https://doi.org/10.1007/s10924-016-0812-5
Tábi T, Sajó IE, Szabó F, Luyt AS, Kovács JG (2010) Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym Lett 4:659–668. https://doi.org/10.3144/expresspolymlett.2010.80
Acknowledgements
This research work was financially supported by Rajamangala University of Technology Phra Nakhon (RMUTP) [Grant No. 59110503/2]. The authors also are grateful to King Mongkut’s University of Technology Thonburi (KMUTT) for supporting ozone generator.
Funding
Financial support for the research was provided by Rajamangala University of Technology Phra Nakhon (RMUTP) [Grant No. 59110503/2].
Author information
Authors and Affiliations
Contributions
1. KC: Conceptualization, Methodology, Writing—Original Draft, Writing—Review & Editing. 2. PP: Methodology, Validation, Writing—Original Draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaochanchaikul, K., Pongmuksuwan, P. Influence of Ozonized Soybean Oil as a Biobased Plasticizer on the Toughness of Polylactic Acid. J Polym Environ 30, 1095–1105 (2022). https://doi.org/10.1007/s10924-021-02264-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02264-6