Abstract
The use of fully bio-based and biodegradable materials for massive applications, such as food packaging, is an emerging tendency in polymer research. But the formulations proposed in this way should preserve or even increase the functional properties of conventional polymers, such as transparency, homogeneity, mechanical properties and low migration of their components to foodstuff. This is not always trivial, in particular when brittle biopolymers, such as poly(lactic acid) (PLA), are considered. In this work the formulation of innovative materials based on PLA modified with highly compatible plasticizers, i.e. oligomers of lactic acid (OLAs) is proposed. Three different synthesis conditions for OLAs were tested and the resulting additives were further blended with commercial PLA obtaining transparent and ductile materials, able for films manufacturing. These materials were tested in their structural, thermal and tensile properties and the best formulation among the three materials was selected. OLA with molar mass (Mn) around 1,000 Da is proposed as an innovative and fully compatible and biodegradable plasticizer for PLA, able to replace conventional plasticizers (phthalates, adipates or citrates) currently used for films manufacturing in food packaging applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the introduction of synthetic polymers, they have been extensively used as packaging materials by their advantageous properties, such as softness, lightness and transparency [1]. Over the last decade, environmental, economic and safety challenges have induced the use of biodegradable polymers to partially replace conventional petrochemical-based materials in packaging applications [2].
Poly(lactic acid) (PLA) is one of the most studied biodegradable polymers, since it is a compostable and non-toxic thermoplastic polyester obtained from the controlled polymerization of lactic acid [1]. PLA shows excellent biocompatibility, processability, it is less energy-dependent and provides excellent properties at a competitive price in packaging, consumer goods, fibers and in biomedical devices [3, 4].
Poly(lactic acid) is classified as GRAS (Generally Recognized As Safe) by the US Food and Drug Administration (FDA) for food packaging applications [1]. It is currently commercially available only in form of rigid cups and containers for short shelf-life food, since some poor properties, such low glass transition temperature, weak thermal stability and high brittleness limit its use in flexible films manufacturing. Several modifications have been proposed to overcome this drawback, such as copolymerization, blending with other polymers or plasticization. The addition of plasticizers to PLA has been subject of numerous studies and maybe it is an economical way to increase the flexibility of PLA [2–4]. Concerning food contact materials, there are several requirements to be satisfied by additives, such as being non-toxic substances, showing good miscibility with the polymer, providing high tensile strength, ductility, and suitable thermal properties. Besides transparency, low volatility and low migration into foodstuff are also relevant properties [2].
Some compounds have been tested as potential plasticizers for PLA, such as triacetin, citrate esters [5–7], glucose monoesters, partial fatty acid esters [8], glycerol, poly(ethylene glycol) [9], poly(propylene glycol) [10] or polyadipates [11, 12], but their compatibility with PLA is limited and the possibility of incorporation with no phase separation is restricted to concentrations lower than 20 wt% limiting their potential properties in food packaging. In general, it has been accepted that amounts from 10 to 20 wt% of plasticizers are required to provide a substantial reduction of the PLA glass transition temperature (Tg) as well as to obtain adequate mechanical properties for films manufacturing. The ideal approach to increase miscibility between PLA and potential plasticizers would be to add a compound with similar chemical structure and a relatively high molar mass to reduce migration rate and detrimental changes in the material properties over time.
The possibility of synthesizing oligomers of lactic acid (OLA) from the monomer, changing the polymerization conditions to obtain compounds with different molar masses, could be an alternative to get plasticizers with high compatibility with PLA by their close chemical structure. Martin and Avérous [9] made a preliminary study of oligomeric lactic acid as a PLA plasticizer at 20 wt% content. They reported an improvement in ductility with a significant decrease in Tg, but the low Tg limits the applicability of the blend and could affect the stability over time, resulting in a loss of mechanical properties.
Some authors have studied the synthesis of lactic acid oligomers and their application to some fields, in particular biomedical. These OLAs, with molar masses lower than 10 kDa are typically produced by direct polycondensation [13, 14]. The preparation and characterization of polydisperse and monodisperse OLAs and their ability to form stereocomplexes to increase the yield of their synthesis reactions has been fully described [15] and a minimum amount of lactate (either in d- or l-form) was obtained by following specific routes that will be further described in the experimental section.
In the present work three oligomers based on d,l-lactic acid were first synthesized under different conditions and then were blended with commercial PLA forming fully-biodegradable films. The efficiency of these biocompatible plasticizers was evaluated in order to obtain PLA-based formulations with adequate properties for flexible films manufacturing. This innovative approach results in fully biodegradable films for food packaging with improved mechanical and functional properties reached by the high compatibility between the PLA matrix and plasticizer.
Experimental
Materials
Three oligomers of lactic acid (OLA′s) with Mn between 670 and 1,000 Da were synthesized by using the following reagents: d-l-lactic acid (90 wt% l-LA) (d,l-LA) kindly supplied by PURAC BV (Gorinchem, The Netherlands); a commercial mixture of linear alcohols (n-octanol/n-decanol with 45/55 v/v ratio) called Nafol®810D obtained from Sasol Ltd (Johannesburg, South Africa); 1,4-cyclo-hexanedimethanol and two class of catalysts [methanesulfonic acid, CH3SO3H, and dioctyl(maleate)tin oxide, C16H34OSn (DOTO)] were purchased from Sigma-Aldrich Chemical Co (Móstoles, Spain). These reagents were selected in consideration of their potential use at industrial scale in the OLAs synthesis. Poly(lactic acid) (PLA Ingeo™ 2003D; Mn = 108 kDa; 4 wt% d-isomer) from NatureWorks LLC (Minnetonka, MN, USA) was used as polymeric matrix.
Synthesis of Oligomers (OLAs)
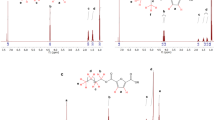
Lactic acid oligomers from d,l-LA were synthesized by direct polycondensation with the commercial mixture of n-octanol and n-decanol above described as the chain terminator, while DOTO was used as the polycondensation reaction catalyst. A previously proposed method was used in these syntheses [16]. A simplified mechanism for the polymerization of d,l-LA to form linear monosubstituted oligomers is shown in Fig. 1. Linear monosubstituted oligomers (named as OLA 00A/5 and 00A/8) were first synthesized by following the above-indicated route and further blended with PLA. d,l-LA was added along with 0.05 wt% DOTO as the catalyst and the chain terminator at different d,l-LA/Nafol®810D ratios (approximately 70/30 for 00A/5; 70/20 for 00A/8) to a 1 L round bottom glass reactor fitted with a multiconnection glass hat joined by a silicon gasket under mechanical stirring and pure nitrogen atmosphere. The temperature was slowly increased up to 110 ± 1 °C at atmospheric pressure and held for 30 min in order to eliminate the condensation water formed during the esterification reaction. The temperature was raised up to 160 ± 1 and 175 ± 1 °C for OLA 00A/5 and OLA 00A/8, respectively and pressure was decreased down to 1 kPa for 10 min. At this point, the temperature was increased to 180 ± 1 °C in order to eliminate the excess of alcohol and side reaction products and the pressure was further decreased down to 0.5 kPa for 15 additional minutes. In all cases pressure was decreased by using an oil vacuum pump controlled by an electronic vacuometer to ±0.1 kPa. Finally, when the desired parameters were reached, the product was unloaded from the reaction vessel and further characterized in their chemical parameters. The average yield percentage achieved for the reaction was 96 %.
However, some preliminary results showed that OLA 00A/5 had some residual lactic acid that could result in significant differences in crystallinity of the oligomer and consequently on properties after blending with PLA. Therefore, a third OLA (named OLA 09A/5) was synthesized with the same experimental conditions than OLA 00A/5 (d,l-LA/Nafol®810D ratio, pressures and temperatures) but with the addition of 1,4-cyclohexane-dimethanol (1.5 wt%) to permit the residual LA to be eliminated by following the route shown in Fig. 2. Polymerization catalyst was also changed (methanesulfonic acid, 0.05 wt%) in this synthesis route.
Preparation of PLA–OLAs Films
Poly(lactic acid) pellets were dried under vacuum overnight at 80 °C and milled with a RETSCH ZM200 Ultra Centrifugal Mill (Haan, Germany) to a final particle size of approximately 1 mm, using liquid nitrogen to avoid thermomechanical degradation. Milled PLA and oligomeric plasticizers (15 wt%) were mixed at ambient temperature by manual shaking until homogeneous mixtures were obtained. They were further melt-blended in a Haake Polylab QC, ThermoFisher Scientific (Waltham, USA) at 50 rpm during 8 min.
The temperature was set at 160 °C but it increased to 170 °C upon mixing. This blending procedure for plasticized PLA was previously optimized and reported elsewhere [11, 12]. Blends were then processed into films (14.0 × 14.0 cm2 and 220 ± 5 μm thickness) by compression molding at 170 °C in a Carver Inc. Hot Press (Wabash, IN, USA), keeping the material between the plates at atmospheric pressure for 5 min until melting and then gradually increasing the pressure during 2 min up to 5 MPa, maintained for 5 min.
Material Characterization
Several physical and chemical properties of the synthesized oligomers were determined immediately after their synthesis by using standard test methods: hydroxyl number (IOH, ASTM E1899-08) [17]; Gardner Color Scale (ASTM D1544-04) [18]; kinematic viscosity at 25 °C (υ, ASTM D445-09) [19]; refractive index (N) and density (ρ) at 25 °C (ASTM D1045-08) [20].
Average molar masses of plasticizers were characterized by Size Exclusion Chromatography (SEC) measurements using a Shimadzu liquid chromatograph (Kyoto, Japan) equipped with a RID-10A refractive index detector. The columns set used was composed of a 50 mm PLgel Guard 5 μm column, two 300 mm PLgel MIXED-C 5 μm columns and a 300 mm PLgel 5 μm-100 Å column. Chloroform was used as the mobile phase and analyses were carried out at 25 °C with a solvent flow rate of 0.8 mL min−1. Calibration was carried out with polystyrene standards from 580 to 1.6 × 106 g mol−1.
Wide angle X-ray scattering (WAXS) of materials was performed on a Bruker D8-Advance (Madison, WI, USA) diffractometer, equipped with a Cu Kα radiation source (λ = 1.546 Å), operating at 40 kV and 40 mA as the applied voltage and current, respectively. The incidence angle (2θ) was varied between 2° and 90° at a scanning rate of 2° min−1.
TGA tests were performed on a TGA/SDTA 851e Mettler Toledo thermal analyzer (Schwarzenbach, Switzerland). Samples of approximately 7 mg were heated from room temperature to 700 °C at 10 °C min−1 under a nitrogen gas flow rate of 50 mL min−1. Two replicates were scanned for each formulation and errors were lower than 3 % in all cases.
DSC tests were performed on a TA Instruments DSC Q2000 (New Castle, DE, USA) under a dry nitrogen gas flow rate of 50 mL min−1. OLAs (4–6 mg) were sealed in aluminium pans and subjected to the following thermal cycles: first heating from −90 to 150 °C at 10 °C min−1, followed by 5 min at 150 °C (in order to erase the thermal history), quenching to −90 °C and a second heating up to 150 °C at 10 °C min−1. Two replicates were tested for each formulation and errors were lower than 1 % in all cases. PLA plasticized films were analysed from −30 to 180 °C at 10 °C min−1, followed by quenching to −30 °C and further heating up to 180 °C at 10 °C min−1. Glass transition temperatures (Tg) were determined in the second heating scan as the inflection point of the region where a shift in the signal baseline was detected.
Elongation at break (% εtB), tensile strength (TS) and elastic modulus (E) of PLA–OLA films were determined at a crosshead speed of 10 mm min−1 using a universal test machine IBERTEST ELIB 30 (Ibertest, Madrid, Spain), equipped with a 5 kN load cell. Tests were performed according to ASTM D 882-01 standard21, with rectangular probes (100 × 10 mm2) and an initial grip separation of 50 mm. All values were the average of five measurements (±standard deviation).
A JEOL model JSM-840 (Jeol USA Inc., Peabody, MA, USA) scanning electron microscope, operated at 12 kV, was used to observe the surface and cross-sections of plasticized PLA films. Samples were previously sputtered with gold to turn them in conductive materials with a metallizer (Au)/Evaporator (C) Balzers, model SCD 004 (Oerlikon Balzers, Liechtenstein).
Results and Discussion
Characterization of Synthesized Oligomers of Lactic Acid (OLAs)
Some physical and chemical properties of the synthesized oligomers are summarized in Table 1, while SEC analysis was used to determine their average molar masses (Mn and Mw) and results are shown in Table 2. No significant differences between all OLAs were observed in their refractive index and density values determined at 25 °C. It was observed that OLA 09A/5 showed the highest hydroxyl index (IOH) since shorter chains with –OH end groups were obtained, in comparison with 00A/8 whose lowest IOH index was given by the minor proportion of chain terminator. It was also observed that all the oligomers showed a slightly yellowish color, as indicated by the Gardner Color Scale. These results are important for the use as a potential plasticizer for PLA where the use of colorless additives is normally preferred from the industrial point of view to avoid the modification of the polymer natural color. On the other hand, the kinematic viscosity of 00A/8 is clearly higher than values for the other two OLAs, which is consistent with its higher molar mass (Table 2). This is an important parameter for further blending with PLA. In general, higher viscosities result in more difficulties during blending, but it should be pointed out that the obtained value for this parameter ranges in the same order to those normally presented by polyadipates, which were successfully blended with PLA in previous works [11, 12].
The number-average molar mass (Mn) of OLAs ranged from 671 to 957 Da, with polydispersity indexes below 1.5 in all cases (Table 2). This is an indication of the successful synthesis of these compounds with a relatively low dispersion in the length of macromolecular chains. Figure 3 shows a representative SEC curve obtained for OLA 00A/8. A peak with low intensity (<2 % of the total area of the chromatogram) was observed in all oligomers at high retention times with an average Mn around 170 g mol−1. This peak could be attributed either to the formation of a certain amount of the lactic acid dimer (Mn = 162 g mol−1) during polymerization or to the presence of some residual chain terminator. It is noteworthy that the 00A/8 oligomer differed from the rest of synthesized OLAs because of its higher average molar mass that could be due to the higher temperature used in the reaction [23]. Besides, if compared with 00A/5, the formation of longer chains in 00A/8 is consistent with the lower proportion of chain terminator used in the synthesis.
The determination of the degree of polymerization (DP) of these OLAs is necessary to calculate solubility parameters. DP was calculated on the basis of the oligomers chemical structures (Fig. 1), being lactate the repeating unit (molar mass, M = 72 g mol−1) and Nafol®810 the chain terminator (M ~ 146 g mol−1), according to the following Eq. (1):
The selection of a plasticizer to be used in specific PLA compositions requires that this compound should present low volatility, resistance to migration, lack of toxicity and plasticizer efficiency resulting in a significant increase in ductile properties. This efficiency is mostly related to their chemical structure and to the compatibility between the plasticizer and the polymer matrix [7]. In general, plasticizers reduce the polymer chain-to-chain interactions by distributing homogeneously within the polymer, increasing the free volume [24]. It should be considered that the most effective plasticizers for a specific polymer could be those with similar structure to the matrix and this is characterized by their close solubility parameters [25].
In order to predict PLA–OLAs compatibility, their solubility parameters were determined by the group contribution, according to the Small′s cohesive energies calculated according to Eq. (2) [22].
where δ is the solubility parameter, ρ is the density, Mn is the number-average molar mass and ΣG is the sum of the molar attraction constants estimating the contribution of each group in their chemical structure. As shown in Table 2, all the synthesized OLAs had solubility parameters close to PLA, which indicates that they should be miscible at least up to a certain concentration of oligomer, having a plasticizing effect in their blending with PLA. Slight differences were observed in solubility parameters between oligomers that could be mainly ascribed to their different polymerization degree and molar mass.
Thermal stability of OLAs was studied by TGA under nitrogen atmosphere. Table 3 shows the initial decomposition temperature (T5), defined as the temperature at which 5 % of the total mass has been lost; the temperature at the maximum degradation rate (Tmax), determined as the peak maximum from the first derivative of TGA curves and the final decomposition temperature (T95), corresponding to the temperature at which 95 % of the total mass has been lost.
It was observed in TGA curves (not shown) that thermal degradation of all OLAs occured in a single step. Intermolecular and intramolecular trans-esterification reactions could occur during thermal degradation, such as in the case of PLA, resulting in the formation of the monomer and low molar mass lactides [26, 27].
When comparing TGA results for the three studied OLAs, 00A/8 exhibited the highest thermal stability, since T5 value was 25 and 35 °C higher than those calculated for 00A/5 and 09A/5, respectively (Table 3). Moreover, a clear relation between the thermal stability and the average molar mass of the oligomers was observed. When comparing thermal degradation parameters with SEC results (Table 2) it could be concluded that an increase in molar mass produced a more stable material upon heating. Besides, in the case of 00A/5 and 09A/5, shorter chains were obtained since a higher proportion of alcohol was used in their syntheses. The presence of hydroxyl end groups in the PLA oligomer chains was found to be critical for degradation, initiating the chain-scission and decreasing the thermal stability [28, 29]. In the case of 09A/5, the highest number of hydroxyl end groups derived from 1,4-cyclohexanedimethanol could explain the low thermal stability compared with 00A/5 and 00A/8 (with T5 value 10 and 35 °C lower, respectively). On the other hand, Tmax values for 00A/8 and 00A/5 were very similar, just slightly higher for 00A/8; while 09A/5 showed a Tmax value 32 °C lower than 00A/8. A similar behavior was observed for the final decomposition temperature (T95), but differences between oligomers were even higher. While 00A/8 showed the highest T95, 00A/5 and 09A/5 showed T95 values 29 and 58 °C lower than 00A/8, respectively.
In summary, the higher thermal stability of 00A/8 is an advantage when considering its potential use as PLA plasticizer, since it could avoid an important mass loss during processing at high temperatures. This hypothesis will be discussed later.
Only one glass transition temperature (Tg) was observed for each OLA during the second heating scan in DSC analysis (Table 3) indicating the high homogeneity of all materials and the success in the synthesis strategies. As expected, the Tg values of each OLA increased with their molar mass. The two lower molar mass compounds (00A/5 and 09A/5) showed similar Tg values, 20 °C lower than the observed result for 00A/8. On the other hand, DSC curves (not shown) did not reveal any endothermic (melting) or exothermic transitions (cold crystallization) during the first heating scan, giving a clear indication of the amorphous nature of all raw materials. This result is in agreement with PLA that is either amorphous or semicrystalline at room temperature depending on the amount of l-lactide in its structure. PLA containing 50–93 % l-lactide is strictly amorphous whereas PLA with >93 % l-lactide is semicrystalline [35]. The amorphous structure of OLAs indicated in DSC analysis was confirmed in XRD patterns (Fig. 4) since no diffraction peaks were observed but only an amorphous halo.
Characterization of PLA–OLAs Films
In order to evaluate the ability of OLAs as potential plasticizers for PLA, films with OLAs (15 wt%) and the un-plasticized PLA matrix were prepared by compression molding after melt-blending. Processing conditions were those previously optimized for PLA-polyadipate blends and reported elsewhere [11] in order to avoid the plasticizer loss as well as thermal degradation of materials during melt-blending at high temperatures. However, a significant release of vapors was detected during blending of PLA with 00A/5 and 09A/5 oligomers, suggesting that there was some volatilization of these compounds during processing. It should be noted that temperatures as high as 170 °C were registered in some moments of the melt-blending process. This is in agreement with TGA analysis of raw OLAs, since at such temperature, 00A/5 and 09A/5 showed a mass loss of 2.5 and 2.9 %, respectively. However, no vapors were observed during melt-blending of PLA with 00A/8, also in agreement with TGA results, showing the stability of this compound at 170 °C with no apparent mass losses.
It should be pointed out that all films were transparent with homogeneous morphologies. Typical micrographs of fracture surfaces of these plasticized materials are shown in Fig. 5. These results suggest that no further evaporation of plasticizers during the formation of films at high temperatures and pressures was observed or at least that the vapor release from PLA-00A/5 and PLA-09A/5 blends was not enough to generate a porous morphology in these films. No apparent differences were observed when comparing with the PLA-00A/8 film.
The effect of plasticizers on the thermal stability of PLA was evaluated by performing TGA tests. The main thermal parameters obtained from this study are shown in Table 4. PLA–OLA films showed a single step degradation process in all cases, with Tmax values similar to those of the neat polymer, which was 365 °C, in agreement with previously reported values [11, 31, 32]. However, the thermal stability of these materials was reduced by the addition of OLAs. The low molar mass of these compounds produced a decrease in the initial decomposition temperature (T5) of blends, compared to results for neat PLA. Since 09A/5 is more volatile than 00A/5 and 00A/8, a higher decrease in the thermal stability of the PLA-09A/5 film was observed. As expected, these T5 values were higher than those observed for the pure oligomers (Table 3). This fact could be explained by the superposition of two factors. The first one would be the high miscibility of the blend components due to the strong interactions between them. The second reason would be a protective effect of the matrix on the additives that are homogeneously distributed, delaying their degradation.
DSC tests were carried out to investigate the thermal transitions of plasticized PLA films and the main results are also indicated in Table 4. As expected from the solubility parameters previously calculated and discussed in this work and their similar chemical structures, all OLAs showed good miscibility with PLA at 15 wt% content since only one Tg value was observed in the DSC thermograms of all blends. Since Tg is an excellent indicator of the chain mobility, the efficiency of OLAs as potential PLA plasticizers was evaluated by determining the decrease of Tg compared to its value for the neat PLA matrix. It was previously reported that the addition of 10–20 wt% of OLA to PLA induced a significant decrease in Tg values (from 58 to 37 °C and 18 °C, respectively) by increasing molecular mobility, showing their plasticizing effect on the matrix [9]. As expected from Tg values of pure OLAs (Table 3), this effect was clearly less pronounced for PLA-00A/8 blends due to its higher molar mass. It should be emphasized that the reduction in Tg values for these PLA–OLA blends was higher than those reported for PLA-polyadipates and PLA-citrates formulations at the same concentration levels [6, 32].
The evaluation of the incorporation of OLAs to the PLA matrix in the blends was carried out by comparison of the experimental Tg values determined from DSC curves with the theoretical values expected for binary miscible blends, calculated by following the Fox Eq. (3) [33].
where w is the weight fraction of the blend components (1 and 2) and Tg values of the pure components are those obtained from DSC tests (Table 3). Results are also shown in Table 4. The slight deviations observed between predicted and experimental data could be mainly due to the OLAs evaporation at the blending temperature. It is noticeable that theoretical and experimental Tg values approached as the molar mass of OLAs increased, confirming the above-mentioned assumption. As can be observed in Table 4, the experimental Tg value obtained for PLA-00A/8 blends is in good agreement with the theoretical prediction (only 0.4 °C difference), suggesting once again the excellent compatibility of 00A/8 with PLA. However, for PLA-00A/5 and PLA-09A/5 blends, there were differences of 2.7 and 2.8 °C between experimental and theoretical Tg values respectively. This result is in agreement with the vapors release observed during processing, leading to the decrease in OLA concentration in the final material.
Figure 6 shows the DSC curves of neat PLA and PLA–OLA films during the second heating scan. The increased chain mobility of PLA due to the plasticizing effect induced by OLAs resulted in crystallization and further melting during heating, which was not observed in the case of the neat polymer. The two endothermic thermal transitions observed between 120 and 155 °C in PLA–OLA formulations provided evidence of the presence of different PLA crystals. According to the literature, both melting peaks are considered to be related with the melting phenomenon of α homocrystals developed in PLLA during heating [30, 34, 35]. The total enthalpy (ΔHtotal), calculated as the addition of melting and crystallization peak areas in the range between 80 and 155 °C, provides a method for determining the original crystallinity of materials, since it is assumed that continuous transitions are occurring in this interval [34]. The ΔHtotal values determined during the first DSC heating scan were close to zero in all cases (data not shown), indicating that crystallization exotherms and melting endotherms had similar heat contents. Therefore, it can be concluded that the cooling rate used during processing was high enough to get samples mainly amorphous. It was also found that, among the PLA-OLA blends, the cold crystallization temperature (Tcc) was higher with increasing plasticizer molar mass.
The main goal of the addition of plasticizers to polymer matrices is to get an improvement in ductile properties for flexible films manufacturing. In the case of PLA it is known that the neat polymer shows brittle behavior at room temperature with a small elongation at break (εtB %) (around 4 %), and high elastic modulus (E = 2.5 GPa) and tensile strength (TS = 56 MPa). Table 5 shows these values as well as those obtained for tensile properties of PLA–OLA formulations.
The addition of OLAs (15 wt%) lead to the expected decrease in values for tensile strength and elastic modulus. This result could be explained by considering the lower macromolecular chain cohesion produced when plasticizers penetrate through the polymer chains [36]. The elongation at break of PLA–OLA films was strongly increased (200–240 % for all plasticizers), showing an important enhancement in ductility of blends. This result is well correlated with the decreases in Tg observed for all formulations permitting to conclude that OLAs used in this work can be considered adequate plasticizers for commercial PLA. Moreover, the decrease in rigidity for the plasticized compositions was well evidenced by the lower elastic moduli, with the highest reduction around 48 % obtained for PLA-00A/5 and PLA-09A/5. It should be pointed out that the PLA-00A/8 blend showed a lower decrease in the elastic modulus (28 %) due to the highest Tg value and molar mass of 00A/8.
It should be also emphasized that the increases in elongation at break for PLA-OLA films were higher than those obtained for PLA plasticized with polyadipates, DOA and glycerol [7, 32] at the same concentration levels. All these results confirmed again the excellent plasticizing effect induced by the addition of OLAs, without significant differences between them regarding tensile properties.
Conclusions
The use of oligomers of lactic acid (OLAs) as potential plasticizers for PLA was evaluated for three compounds based on d,l-lactic acid (90 wt% l-LA). OLAs were synthesized by following a common route but other reagents, catalysts and experimental conditions were modified to obtain compounds with high compatibility with commercial PLA to form transparent and flexible films. Some differences in properties of these films were observed, mainly caused by the effect of the OLA molar mass. All these OLAs, in particular the one with the higher molar mass (called 00A/8), could lead to good performance in terms of ductility of their blends with PLA and finally to permit the preparation of fully biodegradable films based on PLA and an environmentally friendly plasticizer for several applications, particularly for food packaging.
References
Siracusa V, Rocculi P, Romani S, Rosa MD (2008) Trends Food Sci Technol 19:634–643
Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S (2010) Comp Rev Food Sci Food Saf 9:552–571
Nampoothiri KM, Nair NR, John RP (2010) Bioresour Technol 101:8493–8501
Lim LT, Auras R, Rubino M (2008) Prog Polym Sci 33:820–852
Ljungberg N, Wesslén B (2005) Biomacromolecules 6:1789–1796
Ljungberg N, Wesslén B (2002) J Appl Polym Sci 86:1227–1234
Murariu M, Da Silva Ferreira A, Alexandre M, Dubois P (2008) Polym Adv Technol 19:636–646
Jacobsen S, Fritz HG (1999) Polym Eng Sci 39:1303–1310
Martin O, Avérous L (2001) Polymer 42:6209–6219
Kulinski Z, Piorkowska E (2005) Polymer 46:10290–10300
Martino VP, Jiménez A, Ruseckaite RA (2009) J Appl Polym Sci 112:2010–2018
Martino VP, Ruseckaite RA, Jiménez A (2009) Polym Int 58:437–444
Södergard A, Stolt M (2002) Prog Polym Sci 27:1123–1163
Harshe YM, Stort G, Morbidelli M, Gelosa S, Moscatelli D (2007) Macromol Symp 259:218–225
De Jong SJ, Van Dijk-Wolthuis WNE, Kettenes-Van den Bosch JJ, Schuyl PJW, Hennink WE (1998) Macromolecules 31:6397–6402
Fiori S, Ara P, Patent WO (2009) 2009/092825
ASTM E1899-08 (2008) Standard test method for hydroxyl groups using reaction with ptoluenesolfonyl isocyanate (TSI) and potentiometric titration with tetrabutylammonium hydroxide. Am Soc Testing Mater
ASTM D1544-04 (2004) Standard test method for color of transparent liquids (Gardner Color Scale). Am Soc Testing Mater
ASTM D445-09 (2009) Standard test method for kinematic viscosity of transparent and opaque liquids (and calculation of dynamic viscosity). Am Soc Testing Mater
ASTM D1045-08 (2008) Standard test method for sampling and testing plasticizers used in plastics. Am Soc Testing Mater
ASTM D882-01 (2001) Standard test method for tensile properties of thin plastic sheeting. Am Soc Testing Mater
Weast RC (1990) CRC handbook of chemistry and physics. 70th CRC Press, Florida, p 681
Vu DT, Kolah AK, Asthana NS, Peereboom L, Lira CT, Miller DJ (2005) Fluid Phase Equilibr 236:125–135
Rosen SL (1993) Fundamental principles of polymeric materials, 2nd edn. Wiley, New York
Hansen CM (2004) Prog Org Coat 51:109–112
Inkinen S, Hakkarainen M, Albertsson AC, Södergard A (2011) Biomacromolecules 12:523–532
Auras R, Harte B, Selke S (2004) Macromol Biosci 4:835–864
Fan Y, Nishida H, Shirai Y, Endo T (2004) Polym Degrad Stab 84:143–149
Xu L, Crawford K, Gorman CB (2011) Macromolecules 44:4777–4782
Zhang J, Tashiro K, Tsuji H, Domb AJ (2008) Macromolecules 41:1352–1357
Carrasco F, Pagès P, Gámez-Pérez J, Santana OO, Maspoch ML (2010) Polym Degrad Stab 95:116–125
Martino VP, Ruseckaite RA, Jiménez A (2006) J Therm Anal Calorim 86:707–712
Olabisi O, Robeson LM, Shaw MT (1979) Polymer–polymer miscibility. Academic Press, New York
Sarasua JR, López Arraiza A, Balerdi P, Maiza I (2005) Polym Eng Sci 45:745–753
Tsuji H, Ikada Y (1996) Macromol Chem Phys 197:3483–3499
Lemmouchi Y, Murariu M, Dos Santos AM, Amass AJ, Schacht E, Dubois P (2009) Eur Polym J 45:2839–2848
Acknowledgments
Authors would like to thank PURAC BV (Gorinchem, The Netherlands) for kindly supplying d,l-LA. It is also acknowledged the financial support obtained from the Spanish Ministry of Economy and Competitiveness (Ref. MAT-2011-28468-C02-01) and CDTI (Ref. IDI-20110558).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burgos, N., Tolaguera, D., Fiori, S. et al. Synthesis and Characterization of Lactic Acid Oligomers: Evaluation of Performance as Poly(Lactic Acid) Plasticizers. J Polym Environ 22, 227–235 (2014). https://doi.org/10.1007/s10924-013-0628-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-013-0628-5