Abstract
This study aims to improve low intrinsic ductility of poly (lactic acid) (PLA) by using a novel bio-sourced plasticizer environmentally friendly and cost-effective and to get a fully biodegradable material with potential application in films manufacturing. For that purpose, commercial sunflower oil (SO) was epoxidized and epoxidized sunflower oil (ESO) was used as plasticizer for PLA. To investigate ESO potential as plasticizer for PLA, its plasticizing effect was compared with commercial epoxidized soya bean oil (ESBO). Bioblends based on PLA and epoxidized vegetable oils (EVO) as bioplasticizers were prepared. The plasticizers (ESO or ESBO) were respectively compounded with PLA at 10, 20, 30, and 40 wt%. Mechanical (tensile and Shore D hardness), thermal (differential scanning calorimetry (DSC), thermogravimetric analysis (TGA)) and morphological properties (optical microscopy and scanning electron microscopy (SEM)) were characterized. The results showed that the addition of ESO or ESBO to PLA decreased tensile strength and tensile modulus compared to neat PLA but increased elongation at break for which an optimum (9 %, 16 and 34 % for ESBO, ESO5.5 % and ESO6.5 % respectively) was reached at a content of 20 wt% of plasticizer. The structures of the obtained plasticized PLA were confirmed by FTIR spectroscopy. The thermal properties (DSC), such as glass transition temperature (Tg) and melting temperature (Tm) were slightly decreased by addition of plasticizer into PLA, indicating that plasticizer increases the chain mobility and SEM analysis proved successful modification on the PLA brittle morphology with addition of EVO. On the other hand, TGA results revealed increase in the thermal stability. Also the results showed the effect of the EVO weight and the epoxy content (O.O value) on the improvement of the properties of PLA. ESO6.5 % at 20wt% was an efficient plasticizer for PLA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research in biodegradable polymers has received increased attention in recent years because of their wide application in environmental friendly packaging. The most popular and biodegradable polymers are aliphatic polyesters, such as polylactic acid (PLA), polycaprolactone (PCL), poly (butylene adipate terephthalate) (PBAT) and polyhydroxybutyrate (PHB).
PLA is a polyester that can be produced from lactic acid derived from renewable resources. It is one of the most frequently used biodegradable polymers, especially in food packaging and biomedical applications. However, there are still some drawbacks to PLA which have bounded its applications. The poor mechanical properties of PLA, such as low tensile strength and modulus, stiffness, brittleness, evidenced by the limited elongation at break, are some of the main limitations to its industrial applications [1]. Several modification methods have been employed to improve stiffness, processability and the mechanical properties of PLA including blending PLA with other polymers, addition plasticizers to polymer blending systems or adding various types of reinforcing fillers [1,2,3] so as to compete with low cost and flexible commodity polymers.
Plasticizers are widely used to enhance processability, flexibility and ductility of PLA. Many kinds of plasticizers have been studied such as citrate esters, polyethylene glycol (PEG), polypropylene glycol (PPG), phthalates and epoxy derivatives [4–6]. Based on literature, one of the main trends in the synthesis of new plasticizers, as alternatives to cost, toxic and non-degradable plasticizers, is plasticizers made from renewable raw materials, e.g., vegetable oils and fats, which constitute the single, largest, renewable, low cost, non-toxic and biodegradable family yielding materials. In recent years, a lot of research has been devoted to the synthesis and the possibilities of using vegetable oil-based plasticizers such as epoxidized soybean oil (ESBO), epoxidized cottonseed oil (ECO), epoxidized broccoli oil (EBO), epoxidized palm oil (EPO), epoxidized jatropha oil (EJO) and fatty acid esters (FAEs) [7–13]. Epoxidized vegetable oils have received much attention because they act as reactive plasticizers through the reactivity between epoxy functional groups and the –OH and –COOH end-groups of PLA (Scheme 1) [14–17].
Sunflower oil is a renewable resource which was modified by epoxidation. Epoxidized sunflower oil was used as not toxic and environment-friendly additive (thermal stabilizer and plasticizer) in polymer formulations and specially poly vinyl chloride [20,21,22,23,24].
In this study, commercial sunflower oil was epoxidized. The plasticizing effects of epoxidized sunflower oil (ESO) on the properties of PLA were investigated. ESO is a novel bio-sourced plasticizer environmentally friendly, cost-effective and technical solution to overcome the high intrinsic fragility of PLA and widen its industrial applications. Performances of ESO on PLA are compared to those of epoxidized soya bean oil (ESBO) in the same conditions with the aim to present ESO as another sustainable plasticizer alternative to ESBO appropriate for food packaging applications.
Experimental
Materials
PLA (Nature Works 2002D) (MFI = 6.18 g/10 min, density = 1.23, Tg= 63 °C, Tm= 154 °C, Mw = 215 000, Mw/Mn= 1.9 and D-isomer = 4.2 % [25]) was used. Sunflower oil is a commercial product from the company Cévital (Béjaia, Algeria). It was used as received. Epoxidized sunflower oil was especially prepared as described previously [21]. Its level of oxirane oxygen (O.O) is 5.5 and 6.5 %. Commercial epoxidized soya bean oil (ESBO) from Akdeniz Kimya A.S. (Turkey) with O.O of 5.9 % was used for comparison as plasticizer for PLA. Formic acid (98 %) was obtained from Panreac Química SA, hydrogen peroxide (30 wt%) and hydrogen bromide (40 wt%) were purchased from Biochem Chemophama, glacial acetic acid (99.9 %) was purchased from ChemLab NV and chloroform analytical grade from Aldrich Co.
Sunflower Oil and Epoxidized Sunflower Oil Characterization
Characterization of SO and ESO was carried out according to American Oil Chemists’ Society (AOCS) Official Method. The acid value (AV), saponification value (SV) and iodine value (IV) were determined according to the AOCS Official Method CD 3 A-63, AOCS Official Method CD 3 C-91 and AOCS Official Method CD 1D-92, respectively. The viscosity was determined according to dynamic viscosity test method (ASTM D 445–79) using a DV-II + Pro Viscometer, Brookfeld Engineering Labs. Inc. Middleboro at 100 rpm with S2, S1 spindles at different temperatures. The relative density was determined according to ASTM D891-09 Test Method B by means of a pycnometer, water content was measured by Karl Fischer titration. Titration of oxirane groups (oxirane index) was determined using analytical method [26]. Characteristics of SO and ESO are given respectively in Tables 1 and 2.
Preparation of Plasticized Poly (Lactic Acid)
Plasticized PLA films were prepared by solution casting method. PLA was first dried overnight in an oven at 40 °C. Plasticized PLA films were obtained by dissolving the required amount of PLA in chloroform at room temperature in a stirred flask for 1 h, followed by the addition of the plasticizer, with a dropper and continued stirring for 3 at 4 h. The PLA / plasticizer mixtures were poured into ceramic dishes with appropriate dimensions (50 × 100 × 60 mm3). Chloroform was evaporated. The plasticizers (ESO (O.O = 5.5 or 6.5 %) or ESBO) were respectively compounded with PLA at 10, 20, 30, and 40 wt %.
Plasticized PLA Characterization
Tensile Testing
Tensile properties were performed at room temperature using MTS Criterion Electromechanical Universal Test System, Model 41 Series 40. The 1 mm plasticized PLA sheets were cut into a dumbbell shape based on ASTM D638 (type V) standard. The test was conducted with a 1.0 kN load cell and a constant crosshead speed of 10 mm.min− 1. Tensile strength, tensile modulus, and elongation at break were measured. The results obtained represent the average of three measurements for each sample at least.
Hardness Shore D Characterization
The shore D hardness test was carried out according to ISO 868 using a Bareiss Shore D Tester. Sheets of 4mm thickness were used for hardness measurements. Three measurements were made on each sample type.
Fourier Transform Infrared Spectroscopy Analysis
In order to identify the surface functional groups on the prepared samples, Fourier transform infrared spectroscopy was carried out on a Nicolet iS10 spectrophotometer at high resolution and over 32 scans in the wavelength range between 4000 and 500 cm− 1.
Differential Scanning Calorimetry Analysis
Differential scanning calorimetry was conducted with a NETZSCH STA 409PC/PG instrument in nitrogen atmosphere. Each sample was equilibrated at 20 °C and then the temperature was increased at 10 °C/min to 200 °C. At this temperature, an isotherm was maintained for one minute, and then the sample was cooled at the same rate and heated again to 200 °C at 10 °C/min. The first heating scan was used to erase any prior thermal history while the second heating scan was used to determine glass transition temperatures (Tg). OriginPro 2018 was used to analyze the curves.
Thermogravimetric Analysis
Thermogravimetric analysis measurements were performed using a NETZSCH STA 409PC/PG instrument from 20 to 500 °C at a heating rate of 10 °C.min− 1 in nitrogen atmosphere.
Optical Microscopy Analysis
Samples were analyzed by optical microscopy with an OPTIKA® type apparatus piloted by a computer at a magnification of 100 times using a 10/0.40 compound lens and transmitted light.
Scanning Electron Microscopy Analysis
The fracture surfaces of tensile failed sample were studied under a FEI Quanta 650 scanning electron microscopy (SEM) instrument.
Results and Discussion
Mechanical Properties
Tensile properties combined with DSC evaluation are the most common used indicators of changes caused by plasticization. Results of the tensile measurements, including tensile strength, tensile modulus and elongation at break are displayed graphically in Figs. 1, 2 and 3. Stiffness, brittleness and limited extendibility are the main features of PLA based materials [1]. The possible reason for the high tensile strength for low or non plasticizer content is the domination of strong hydrogen bonds, Van Der Waals or ionic forces produced by polymer-polymer intermolecular interactions over polymer–plasticizer attraction. Plasticizing PLA with epoxidized oil in this work demonstrated markedly improved ductility. However, tensile strength of PLA/EVO 20 wt% was lower than that of neat PLA (13 MPa) and was at around 11 MPa, 10 MPa and 8 MPa for PLA/ESO6.5 %, PLA/ESBO and PLA/ESO5.5 % respectively. The tensile strength of plasticized PLA decreased with increasing amount of plasticizers. The drops in the tensile strength may be caused by the plasticizer-plasticizer interaction [27]. PLA/ESO6.5 % 20wt% exhibited higher flexible mechanical properties than that of PLA modified by ESBO and ESO5.5 %, this indicates the effect of the epoxy content of the EVO (O.O value). At higher plasticizer loading, tensile strength of the blends decreased with the incorporation of plasticizers. In general, plasticizer is introduced to a polymer matrix to overcome the brittleness caused by extensive intermolecular interactions. Thus, the presence of plasticizers ESO and ESBO decreases these intermolecular forces and enhances the mobility of PLA polymer chains, causing an increase in flexibility and extensibility of the PLA. Decrease in the tensile strength of plasticized PLA with increase in the concentration of the plasticizer has been reported by many authors [28,29,30].
The effect of plasticizer concentration on the tensile modulus values of PLA plasticized samples is shown in Fig. 2. Tensile modulus stands for the resistance of the sample to elastic deformation and this can be perceived as reflecting the stiffness and strength of the sample [30, 31]. Low tensile modulus value corresponds to flexible sample. The tensile modulus values regularly decreased with the addition of plasticizers contents, meaning that PLA samples lost their stiffness and became more flexible with the addition of the plasticizer. Analogous decrease of tensile modulus in plasticized blends has been reported [30, 32].
The effect of plasticizer content on the elongation at break of plasticized PLA is shown in Fig. 3. The plasticizing efficiency can be evaluated taking into account the flexibility of PLA or elongation at break of 5 %. The increasing of plasticizer content from 0 wt% to 20 wt% led to a slight increase in the sample elongation, from 5 to 9 % for PLA/ESO5.5 %, which was 2 times higher than that of neat PLA. For PLA/ESO6.5 % and PLA/ESBO, the elongation increased significantly from 5 to 16 and 34 %, respectively; it was 3 times and 6.7 times higher than that of neat PLA, respectively. This represents an overall percentage increase of around 80 %, 211 and 573 %, for PLA/ESO5.5 %, PLA/ESO6.5 % and PLA/ESBO respectively, regarding to neat PLA, calculated as the percentage ratio of the variation in elongation at break (9 − 5 %, 16 − 5 % and 34 − 5 % respectively) to the elongation at break of neat PLA (5 %). It is apparent that the blends containing 20 wt% plasticizers had the highest elongation at break. This is, therefore, the optimum plasticizer content, and it was used for subsequent studies. These results are in accordance with other works regarding PLA plasticization with epoxidized vegetable oils [28,29,30, 32, 33]. The comparison of ESO to ESBO at the optimum plasticizer content (20 wt%) shows that ESBO presents best elongation at break because of its lower molecular and its good compatibility with PLA, while ESO6.5 % exhibits better elongation at break than ESO5.5 %. The increasing in films elongation can be explained by the fact that plasticizers decrease the intermolecular bonds between PLA chains (polymer-polymer interaction) by filling the space between the polymer chains and substitute them with hydrogen bonds formed between plasticizer and polymer chains (plasticizer-polymer interaction). Such disruption and reconstruction of polymer chains interactions reduce the rigidity and promote the ductile behavior of films by allowing more chains mobility [34]. The addition of more than 20 wt% of plasticizer to PLA leads to a decrease of elongation at break because PLA was saturated with plasticizer and phase separation occurred, contributing to PLA-rich and EVO-rich phase formation within the EVO-plasticized PLA. Ferri et al. [35] reported that once the optimum plasticizer content was reached, a plasticizer excess leads to lower elongation at break values due to a possible phase separation. The tensile strength decreases gradually with the content of ESO6.5 %, whereas the elongation at break increases, this is attributed to the level of epoxy content (O.O value) which promote the chains mobility. The comparison of ESO6.5 % to ESBO at the chose content 20wt% shows that ESO presents similar mechanical properties than that of ESBO.
Figure 4 shows the evolution of Shore D hardness as a function of plasticizers contents (ESBO and ESO). The addition of plasticizers leads to softer materials with decreasing hardness as the plasticizer content increases. Nevertheless, at the level of 20 wt% taken as optimum, the best efficiency can be observed for PLA/ESO6.5 % by a decrease from 71 to 46. These results are in total agreement with previous mechanical characterization, thus indicating the high efficiency of ESO6.5 % versus ESO5.5 % and ESBO for PLA plasticization.
It can be seen that the mechanical properties are affected by the weight of EVO and the level of the epoxy content (O.O) value, reduced the intermolecular interactions between the polymeric chains and increased their chains mobility improving the flexibility of PLA. The plasticizing efficiency can be evaluated taking into account the flexibility of samples. The values of tensile strength, elongation at break, and shore D hardness presents ESO6.5 % as an efficient plasticizer against ESO5.5 % and ESBO. In the rest of this study the effect of plasticizers at 20 wt % was investigated.
FT-IR Spectroscopy
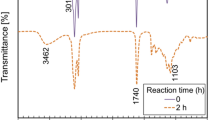
FT-IR spectroscopy was used to control the known functional group interactions of PLA with epoxidized vegetable oils and compare different plasticized PLA. Yin et al. and Guan et al. [36, 37] reported that when two or more substances are mixed, physical blends versus chemical interactions are reflected by changes in the characteristic spectral bands. Figure 5 exhibits infrared spectra of ESBO, ESO6.5 % and ESO5.5 % from 500 to 4000 cm− 1 and Fig. 6 shows the FT-IR spectra of PLA in absence and presence of 20 wt % of ESBO, ESO5.5 % or ESO6.5 % from 500 to 4000 cm− 1.
The FTIR spectra of ESBO, ESO6.5 % and ESO5.5 % (Fig. 5) exhibited the two characteristic absorptions of the oxirane ring, that are revealed at around 915 cm− 1 and 3500 cm− 1, corresponding to γC−O epoxy groupe and C-H tension of the methylene group of the epoxy ring, respectively. However, this second band is not very useful since its intensity is low and it is also very close to the strong O-H absorptions [38].
Ester group of PLA exhibits characteristic peaks at 1748, 1187 and 1090 cm− 1 corresponding respectively to carbonyl group C=O stretching vibration, the asymmetrical valence vibrations of C-O-C and symmetrical valence vibrations of C-O-C of the aliphatic chain as reported by Ristić et al. [39]. Changes in the position and intensity of these peaks were observed for the blends materials. Also, it has been reported that the peaks at 871 and 756 cm− 1 are related to the amorphous and crystalline region [40]. Similar findings were reported by Auras et al. [41].
The epoxide stretching vibration (γC−O) and the stretching vibration at 1250 cm− 1 were disappeared in plasticized PLA (Fig. 6) indicating the possibility of interaction between the EVOs and PLA. This result is similar to plasticized PLA by epoxidized palm oil (EPO) and plasticized PLA by epoxidized soybean oil (ESBO) reported by Silverajah et al. [42] and Tee et al. [43], respectively. The stretching vibration of CH2 and C = O in PLA were at 2926 cm− 1, 2852 cm− 1 and 1748 cm− 1. As for EVOs, they were slightly different at 2918 cm− 1, 2854 cm− 1 and 1752 cm− 1. With the incorporation of plasticizers into PLA, they were at 2925 cm− 1, 2853 cm− 1 and 1747 cm− 1, which may indicate some intermolecular interactions and miscibility. It is also observed a change in the intensity of these peaks.
The FTIR result is indicated that PLA-EVOs blends could form interaction via hydrogen bonding [44]. Xu et al. [45] are proposed that hydrogen bonding could form between the ester group of PLA and the oxirane group of EVO (Scheme 2). Also, the interaction between PLA and EVOs could be attributed to the possible hydrogen bonding that occurs between the carbonyl group (i.e., from ester linkage) in PLA and the epoxy group in EVOs. Furthermore, it can be seen that a relatively small peak at approximately 3500 cm− 1 (characteristic absorption region for O-H bond stretching deformation) is visible for neat PLA which indicate the presence of hydroxyl groups in the pure PLA. It is also interesting to note that this characteristic peak of PLA has disappeared with the incorporation of plasticizers, and was shifted upper to a broad peak similar to that of plasticizers. Therefore, the terminal hydroxyl group (–OH) in the PLA could be possibly interacting with oxirane groups (C–O–C) of plasticizers by hydrogen bonding as proposed by Xu et al. [45]. It is also interesting to note that the proposed possible interaction between PLA and plasticizers are reported in other studies such as Al-Mulla et al. [46].
By comparing FTIR results of PLA/ESO6.5 % with PLA/ESO5.5 % and PLA/ESBO, it is observed that the increase in intensity of the (C=O) absorption at 1747 cm− 1, and the appearance of the (-OH) absorption at 3650 cm− 1 for PLA/ESO6.5 % is stronger than the same peaks for PLA/ ESO5.5 % and PLA/ ESBO. Also, a small shift of C-O stretching peak from 1090 cm− 1 (neat PLA) to 1095 cm− 1 in.
PLA/ ESO5.5 % was observed. This shift in the absorption peak indicates the miscibility and interaction of PLA and ESO6.5 %. According to George Wypych [27], polar groups in a plasticizer (presented by epoxy groups and their epoxy content (O.O value)) improve mechanical properties and are essential for good compatibility. Regarding ESO6.5 %, it has higher polarity compared to ESO5.5 % and ESBO due to its higher level of epoxy content (6.5 %) than those of ESO5.5 % (5.5 %) and ESBO (5.9 %). Therefore, a stronger interaction between PLA and ESO6.5 % through hydrogen bonding which is influenced by the epoxy content of the EVO (O.O value) comparing to PLA-ESO5.5 % and PLA-ESBO interactions. This is a correlation: higher the epoxy content of the EVO corresponding in general to higher sites of interactions between EVO and PLA (higher hydrogen bounding). As a result, the ESO6.5 % is the efficient plasticize versus ESO5.5 % and ESBO for PLA plasticization, and this is in total accordance with previous efficiency of ESO6.5 % mechanical properties.
Differential Scanning Calorimetry Investigation
DSC is a very useful technique to study the glass transition temperature, crystallization temperature, and melting behavior. Figure 7 shows the DSC curves recorded during the second heating cycle of plasticized PLA.
It is worth noting a slight decrease in Tg values with a change from 64 °C (neat PLA) to 59 °C (PLA/ESBO), 61 °C (PLA/ESO5.5 % 20wt%) and 60 °C (PLA/ESO6.5 % 20wt%). The Tg of all PLA/plasticizer blends was lower than that of neat PLA, this is related to an increase in chain mobility with added plasticizer because of the penetration of plasticizers molecules into PLA matrix [48]. This same behavior was reported by Ozgur et al. [49] that employed epoxidized vegetable oils as plasticizers for biodegradable polymers. But no obvious crystallization exotherm (Tc) peak was observed, possibly because of the important content of D-isomer in PLA. It is possible to note that crystallization behavior of PLA blends was insignificantly affected by plasticizers.
As observed from Fig. 7, the DSC thermogram of neat PLA exhibits two distinct melting endotherms at 132 and 148 °C, according to Yasuniwa etal. [50], the double-melting behavior of PLA was explained by the melt-recrystalyzation model. Conversely, in the heating scan of plasticized PLA only one (Tm) peak appeared at 142 °C, 141 and 144 °C, for PLA/ESBO, PLA/ESO5.5 % and PLA/ESO6.5 % respectively. The downward shift in Tm of this blended film indicates the increased miscibility of the components in the blends as reported by Joseph et al. [51]. According to DSC curves, no trace of separate melting or crystallization of plasticizers was detected confirming that the phase separation did not occur.
Optical Microscopy Analysis
To give a clearer view of surface modification of PLA and PLA/EVO blends, surfaces were characterized by optical microscopy. Figure 8a to d show the optical images of neat PLA, PLA/ESBO, PLA/ESO5.5 % and PLA/ESO6.5 %. Neat PLA (Fig. 10a) displays smooth surface as compared with PLA/EVO blends while morphology of plasticized samples is heterogeneous and displays a skin-core distribution. EVO plasticizers are located below PLA skin as droplets in the polymer as it is clearly showed in Fig. 8b to d. These droplets cause the improvement of the mechanical properties.
Scanning Electron Microscopy Analysis
Scanning electron microscopy analysis shows the surface morphology of the fractured tensile specimens and the state of dispersion of the epoxidized vegetable oils in the PLA matrix.
PLA exhibited smooth, homogeneous and a flat surface, corresponding to its brittle behavior as reported by Silverajah et al. [29], (Fig. 9a). The addition of 20 wt% of EVO to PLA matrix revealed a marked change in morphology.
SEM micrographs of plasticized PLA (Fig. 9b and d), exhibited an uneven surfaces with presence of fibrils due to plastic stretching or deformation, those morphologies indicates a good adhesion between components without voids, producing a single phase morphology. This diffused PLA-EVO interface is related to the chemical interactions between PLA and plasticizer. Thus, the plasticizer was formed a homogeneous distribution and good miscibility.
Thermogravimetric Analysis
The study of plasticized polymers must take into account the impact of the plasticizer on the thermal stability. The thermal degradation of plasticized PLA was investigated by thermogravimetric analysis. Figure 10a and b illustrate, respectively, the weight loss and the weight loss derivative of PLA/EVO samples.
The thermal stability of a polymeric material depends on the inherent characteristics of the macromolecules as well as on the molecular interactions between the different molecules. The chain cleavage or the bond dissociation of the macromolecules takes place when the supplied thermal energy exceeds the bond dissociation energy of the respective chemical bonds [47]. From the thermogravimetric analysis (TGA) curves, addition of 20 wt% of EVOs into PLA improved he thermal stability of PLA as can be observed from the degradation onset temperatures (Tonset) of PLA/ESBO (290 °C), PLA/ESO5.5 % (288 °C) and PLA/ESO6.5 % (291 °C) which are higher than that of neat PLA (280 °C). A delay of thermal degradation is observed for PLA/EVO blends from the onset temperatures. The blends exhibited a higher degradation temperature than neat PLA. The presence of plasticizer with a good dispersion in the PLA polymer acts as a barrier sheet to prevent oxidation, as well as, hinders the permeability of volatile degradation products out from the blend materials and helps delay the thermal degradation process [52]. Also, From TG and DTG thermograms, it can be observed that all of the PLA blends showed an increase in temperature of maximum weight loss (Tmax) : (365 °C) for PLA/ESO6.5 %, (359 °C) for PLA/ESO5.5 % and (363 °C) for PLA/ESBO comparing to PLA (348 °C), it was reported in the literature that the increase in thermal stability of EVO plasticized PLA was due to their strong interaction and well-dispersion of plasticizer into PLA [53, 54]. On the other hand, the weight loss of PLA in presence of ESO5.5 % (88 %) or ESO6.5 % (88 %) is similar to that of PLA (86 %) and lower than that of PLA in presence of ESBO (90 %).
It could be seen that ESO6.5 % exhibited better act on thermal stability of PLA than ESO5.5 % and slightly higher than ESBO, confirming its good interaction with PLA due to its level of epoxy content. Thermal investigations confirming the mechanical characterization and FT-IR spectroscopy that ESO6.5 % is the efficient plasticizer against ESO5.5 % and ESBO.
Conclusions
This paper describes plasticization of PLA by a new bio-based plasticizer (ESO) via a solution casting process using chloroform as a solvent. From the above results, the following conclusions can be derived:
Mechanical characterization of PLA/EVO samples showed a decrease in tensile strength and tensile modulus, but an increase in elongation at break for which an optimum (9 %, 16 and 34 % for ESBO, ESO5.5 % and ESO6.5 % respectively) was reached at a content of 20 wt% of plasticizer. The optimum EVO loading with enhanced mechanical properties of PLA was 20 wt %. The addition of plasticizers leads to softer materials with decreasing hardness as the plasticizer content increases. Nevertheless, the best efficiency was observed for PLA/ESO6.5 % by a decrease from 71 to 46 at a level of 20 wt %. FTIR spectroscopy shows some molecular interactions by intermolecular hydrogen bonding between PLA and ESO. According to thermal and mechanical properties, ESO6.5 % was more effective plasticizer than ESO5.5 %, due to more hydrogen bonds with hydroxyl groups of PLA, and it have similar performances to those of ESBO. Furthermore, Tg values decreased from 64 °C (neat PLA) to 59 °C (PLA/ESBO), 61 °C (PLA/ESO5.5 %) and 60 °C (PLA/ESO6.5 %) at the level of 20 wt% plasticizer. SEM analysis showed successful modification of the PLA brittle morphology with addition of EVO. On the other hand, the PLA blends exhibited a higher degradation temperature than neat PLA, confirmed a higher ability to resist thermal degradation for PLA blends when compared to neat PLA.
Globally, the results showed that the performances of the epoxidized sunflower oil are similar to those of epoxidized soya bean oil.
Based on the results of this study, ESO exhibited promising results regarding the improvement of the brittleness and overall properties of PLA and can therefore be considered as a potential plasticizer.
References
Sin LT, Tueen BS (2019) Polylactic acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA. Plastics Design Library. 2:273–305. https://doi.org/10.1016/B978-0-12-814472-5.00008-X
Sin LT, Rahmat A, Abdul rahman W A W, (2013) Polylactic Acid: PLA Biopolymer Technology and Applications. Plastics Design Library. 1:177–219. https://doi.org/10.1016/B978-1-4377-4459-0.00005-6
Sin LT, Rahmat A, Abdul rahman W A W (2013) Overview of poly(alctic acid). Handbook of Biopolymers and Biodegradable Plastics. Plastics Design Library. 11–54. https://doi.org/10.1016/B978-1-4557-2834-3.00002-1
Farah S, Anderson DG, Langer R (2016) Physical and Mechanical Properties of PLA, and their Functions in Widespread Applications - a Comprehensive Review. Adv Drug Deliv Rev 107:367–392. https://doi.org/10.1016/j.addr.2016.06.012
Godwin AD (2000) Plasticizers. Applied polymer science: 21st century. 157–175. https://doi.org/10.1016/B978-008043417-9/50011-8
Sastri VR (2010) Polymer additives used to enhance material properties for medical device applications Plastics in medical devices: properties, requirements, and applications. Plastics design library https://doi.org/10.1016/B978-0-8155-2027-6.10005-4
Langer E, Bortel K, Waskiewscz S, Lenartowicz-klik M (2020) Research Trends in Plasticizer Production. Plasticizers derived from post-consumer PET: Research trends and potential applications. Plastics design library. 101–126. https://doi.org/10.1016/B978-0-323-46200-6.00004-0
Jia P, Zhang M, Hu L, Zhou Y (2016) Green plasticizers derived from soybean oil for poly(vinyl chloride) as a renewable resource material. Korean journal of chemical engineering 33:1080–1087. https://doi.org/10.1007/s11814-015-0213-9
Dinda S, Patwardhan AV, Goud VV, Pradhan NC (2008) Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour Technol 99(9):3737–3744. https://doi.org/10.1016/j.biortech.2007.07.015
Jean-Luc A, Loıc L, Yves-Marie A (2014) Thermal and mechanical properties of a polyhydroxyalkanoate plasticized with biobased epoxidized broccoli oil. J Appl Polym Sci 131(6):39983. https://onlinelibrary.wiley.com/doi/epdf/10.1002/app.39983
Al-Mulla EAJ, Yunus W M Z W, Ibrahim NAB, Abd Rahmad MZ (2010) Properties of epoxidized palm oil plasticized polytlactic acid. Journal of materials science 45:1942–1946. https://doi.org/10.1007/s10853-009-4185-1
Kandula S, Stolp L, Grass M, Woldt B, Kodali D (2015) Functionalization of soy fatty acid alkyl esters as bioplasticizers. Vinyl additive technology 23(2):93–105. https://doi.org/10.1002/vnl.21486
Chieng BW, Ibrahim NA, Then YY, Loo YY (2017) Epoxidized jatropha oil as a sustainable plasticizer to poly(lactic acid). Polymers 9(6):204–214. https://doi.org/10.3390/polym9060204
Anakabe J, Zaldua Huici AM, Eceiza A, Arbelaiz A (2016) The effect of the addition of poly (styrene-co-glycidyl methacrylate) copolymer on the properties of polylactide/poly (methyl methacrylate) blend. J Appl Polym Sci 133(37). https://doi.org/10.1002/app.43935
Broström J, Boss A, Chronakis IS (2004) Biodegradable films of partly branched poly (L-lactide)-co-poly (ε-caprolactone) copolymer: Modulation of phase morphology, plasticization properties and thermal depolymerization. Biomacromol 5(3):1124–1134. https://doi.org/10.1021/bm049920q
Dai X, Xiong Z, Na H, Zhu J (2014) How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos Sci Technol 90:9–15. https://doi.org/10.1016/j.compscitech.2013.10.009
Mihai M, Huneault MA, Favis BD (2010) Rheology and extrusion foaming of chain-branched poly (lactic acid). Polym Eng Sci 50(3):629–642. https://doi.org/10.1002/pen.21561
Mehta R, Kumar V, Bhunia H, Upadhyay SN (2005) Synthesis of poly(lactic acid): a review. Journal of macromolecular science part C: polymer reviews 45:325–349. https://doi.org/10.1080/15321790500304148
Garlotta D (2001) A literature review of poly(lactic acid). Journal of polymers the environment 9(2):63–84. https://doi.org/10.1023/A:1020200822435
Benaniba MT, Belhanache-Bensemra N, Gelbard G (2008) Epoxidation of sunflower oil with peroxoacetic acid in presence of ion exchange resin by various processes. Energy education science technology 21(2):71–82
Benanibaa MT, Belhaneche-Bensemra N, Gelbard G (2001) Stabilizing effect of epoxidized sunflower oil on the thermal degradation of poly(vinyl chloride). Polymer degradation stability 74(3):501–505. https://doi.org/10.1016/S0141-3910(01)00170-7
Benaniba MT, Massardier-Nageotte V (2010) Evaluation effects of biobased plasticizer on the thermal, mechanical, dynamical mechanical properties, and permanence of plasticized PVC. J App Polym Sci 118(6):3499–3508. https://doi.org/10.1002/app.32713
Bouchareb B, Benanibaa MT (2008) Effects of epoxidized sunflower oil on the mechanical and dynamical analysis of the plasticized poly(vinyl chloride). Applied polymer 107(6):3442–3450. https://doi.org/10.1002/app.27458
Rouane A, Zerrouki D, Benanibaa MT (2014) Effect of Sunflower Oil on the Mechanical Permanence and the Thermal Properties of Poly (Vinyl Chloride). Energy procedia 50:285–289. https://doi.org/10.1016/j.egypro.2014.06.035
Murariu M, Dubois P (2016) PLA composites: From production to properties. Adv Drug Delivery Rev 107:17–46. https://doi.org/10.2016/j.addr.2016.04.003
AOCS (2017) Official Method Cd 9–57, oxirane oxygen in epoxidized materials
Wypych G (2004) Handbook of Plasticizers, ChemTec Publishing, Ontario, Canada. 112–117
Silverajah VSG, Ibrahim NA, Yunus W M Z W, Abu Hassan H, Woei CB (2012) A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. International journal of molecular science 13:5878–5898.
Prempeh N, Li J, Liu D, Das K, Maiti S, Zhang Y (2014) Plasticizing Effects of Epoxidized Sun Flower Oil on Biodegradable Polylactide Films: A Comparative Study. Polymer science, serie A. 56(6):856–863. https://doi.org/10.1134/S0965545X14060182
Omelczuk MO, McGinity JW (1992) The influence of polumer glass transition temperature and molecular weight on drug release from tablets containing poly (DL-lactic acid). Pharmaceutical research 9:26–32. https://doi.org/10.1023/A:1018967424392
Roberts RJ, Rowe RC (1987) The Young’s modulus of pharmaceutical materials. International journal of pharmaceutics 37(1–2):15–18. https://doi.org/10.1016/0378-5173(87)90004-4
Al-Mulla EAJ, Ibrahim NAB, Shameli K, Ahmed MB, Yunus WM, Z W (2014) Effect of epoxidized palm oil on the mechanical and morphological properties of a PLA–PCL blend. Research on chemical international 40:689–698. https://doi.org/10.1007/s11164-012-0994-y
Carbonell-Verdu A, Samer MD, GarciaGarcia D, Sancher-Nacher L, Balar R (2017) Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Industrial crops products 104:278–286. https://doi.org/10.1016/j.indcrop.2017.04.050
Daniels PH (2009) A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. Journal of vinyl additive technology 15(4):219–223. https://doi.org/10.1002/vnl.20211
Ferri JM, Samper M, Garcia-Sanguera D, Reig MJ (2016) Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J Mater Sci 51(11):5356–5366. https://doi.org/10.1007/s10853-016-9838-2
Yin YJ, Yao KD, Cheng GX, Ma JB (1999) Properties of polyelectrolyte complex films of chitosan and gelatin. Polym Int 48:429–433.
Guan Y, Liu X, Zhang Y, Yao K (1998) Study of phase behavior on chitosan/viscose rayon blend film. Applied polymer. 67(12):1965–1972.
Lee Smith A, Elving PJ, Winefordner JD, Kolthoff IM (1979) Applied infrared spectroscopy. Fundamentals, techniques and analytical problem solving. Published by Wiley
Ristić IS, Tanasić J, Nikolić LB, Cakić SM, Ilić OZ, Radičević R, Budinski-Simendic ZJK (2011) The properties of poly(L-lactide) prepared by different synthesis procedure. J Polym Environ 19:419–430. https://doi.org/10.1007/s10924-011-0297-1
Hani Y. Cohn D (1988) Phase separation in poly(ethylene glycol)/poly(lactic acid) blends. Eur Polym J 24(8):765–773
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromolecular Bioscience 4(9):835–864. https://doi.org/10.1002/mabi.200400043
Silverajah VSG, Ibrahim NA, Zainiddin N, Yunus WMZW, Abu Hassan H (2012) Mechanical, Thermal and Morphological Properties of Poly(lactic acid)/Epoxidized Palm Olein Blend. Molecules 17(10):11729–11747. https://doi.org/10.3390/molecules171011729
Tee YB, Talib RA, Abdan K, Chin NL, Basha RK, Md Yunos KF (2016) Comparative study of chemical, mechanical, thermal, and barrier properties of poly(lactic acid) plasticized with epoxidized soybean oil and epoxidized palm oil. Bioressources 11(1):1518–1540. https://doi.org/10.15376/biores.11.1.1518-1540
Garlotta D, Doane W, Shogren R, Lawton J, Willet JL (2003) Mechanical and thermal properties of starch-filled poly(D,L‐lactic acid)/poly(hydroxy ester ether) biodegradable blends. Journal of applied polymer science 88(7):1775–1786. https://doi.org/10.1002/app.11736
Xu Y-Q, Qu J-P (2009) Mechanical and rheological properties of epoxidized soybean oil plasticized poly(lactic acid). J Appl Polym Sci 112(6):3185–3191. https://doi.org/10.1002/app.29797
Al-Mulla EAJ, Yunus W M Z W, Ibrahim NAB, Abd Rahman MZ (2010) Properties of epoxidized palm oil plasticized polytlactic acid. Journal of materials science 45(7):1942–1946. https://doi.org/10.1007/s10853-009-4185-1
Ray D, Roy P, Sengupta S, Sengupta SP, Mohanty AK (2009) A study of dynamic mechanical and thermal behavior of starch/poly(vinylalcohol) based films. J Polym Environ 17:49–55. https://doi.org/10.1007/s10924-009-0116-0
Pluta M, Galeski A (2002) Crystalline and supermolecular structure of polylactide in relation to the crystallization method. J App Polym Sci 86:1386–1395
Özgür SM, Misra M, Mohanty A (2010) Synergistic improvements in the impact strength and % elongation of polyhydroxybutyrate-co-valerate copolymers with functionalized soybean oils and POSS. International journal of plastics technology 14(1):1–16. https://doi.org/10.1007/s12588-010-0005-3
Yasuniwa M, Tsubakihara S, Sugimoto Y, Nakafuku C (2004) Thermal analysis of the double-melting behavior of poly(L-lactide). Journal of polymer science: part B: polymers physics 42:25–32. https://doi.org/10.1002/polb.10674
Joseph CS, Prashanth KVH, Rastogi NK, Indiramma AR, Reddu SY, Raghavarao K S M S (2011) Optimum Blend of Chitosan and Poly-(ε-caprolactone) for Fabrication of Films for Food Packaging Applications. Food Bioprocess Technol 4(7):1179–1185. https://doi.org/10.1007/s11947-009-0203-1
Emad A. Jaffar Al-Mulla (2011) Preparation of New Polymer Nanocomposites Based on Poly(lactic acid)/Fatty Nitrogen Compounds Modified Clay by a Solution Casting Process. Fibers polymers 12(2):444–450. https://doi.org/10.1007/s12221-011-0444-2
Burgos N, Martino VP, Jiménez A (2013) Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polymer degradation stability 98:651–685. https://doi.org/10.1016/j.polymdegradstab.2012.11.009
Chieng BW, Ibrahim NA, Then YY, Loo YY (2014) Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 19:16024–16038. https://doi.org/10.3390/molecules19101624
Acknowledgements
This work was supported by DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique, Algeria).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouti, M., Irinislimane, R. & Belhaneche-Bensemra, N. Properties Investigation of Epoxidized Sunflower Oil as Bioplasticizer for Poly (Lactic Acid). J Polym Environ 30, 232–245 (2022). https://doi.org/10.1007/s10924-021-02194-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02194-3