Abstract
The present work deals with the preparation and characterization of the chitosan and poly-ε-caprolactone (PCL) solution-casted blended films in various proportions (chitosan–PCL ratio 90:10, 80:20, and 70:30). The films were casted and dried at 55 °C, which were characterized based on the mechanical, barrier, thermal, and microscopic properties. The film prepared from chitosan to PCL ratio 80:20 resulted in increase percentage elongation by 20.56% as compared to pure chitosan film. Fourier transform infrared spectrum indicated a shift in peak of absorption from 1743.9 to 1724.8 cm−1 due to carbonyl group of PCL indicating the miscibility and interaction between the PCL and chitosan. Scanning electron microscopy revealed that PCL appeared as a co-continuous phase with chitosan for ratio 80:20, confirmed the interaction between chitosan and PCL. The above study indicated that the properties of chitosan films can be modified with the addition of PCL and may find its versatile use in food packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food packaging plays a decisive role in achieving protection and preservation of all types of food particularly from oxidative and microbial spoilage as well as dehydration and therefore extends the shelf life of the food product. Synthetic and non-biodegradable materials are most often used for food packaging and accumulation of which poses a threat to environment. The most effective solution to this problem could be to replace such materials with environmentally friendly packaging materials based on natural polymers such as proteins, polysaccharides etc. (Reinoso et al. 2008). Chitin belongs to a family of polysaccharide obtained from the crustacean wastes, which consists of β (1-4)-linked-2-acetamide-2-deoxy-d-glucose units. The de-N-acetylation of chitin results in chitosan (Rinaudo and Domard 1989), which is a non-toxic, biodegradable, biocompatible, and commercially available material. Chitosan is employed in innumerable applications in a wide range of fields such as food/nutrition, drugs, pharmaceuticals, microbiology, immunology, and agriculture (Harish Prashanth and Tharanathan 2007). Chitosan can form transparent films, which may be employed in a variety of food packaging applications (Butler et al. 1996; Srinivasa et al. 2002). The chitosan films are usually characterized by limited elongation (Cheng et al. 2003). Hence, chitosan can be blended with other polymers to modify the properties of chitosan and to develop comparatively novel flexible material (Cheung et al. 2002; Suyatma et al. 2004; Olabarrieta et al. 2001).
Poly-ε-caprolactone (PCL) is a polymer, which is flexible and biodegradable, non-toxic, hydrophobic, and easy to process material (John et al. 2002; Cao et al. 2002; Sarasam et al. 2007). Blending of chitosan with PCL has been tested mostly for tissue engineering applications (Sarasam and Madihally 2005). Chitosan/PCL melt processed blends containing less than 70% chitosan was studied using counter rotating twin screw extruder (Correlo et al. 2005). Blends prepared by mechanical mixing 2 wt.% chitosan in 0.1 M acetic acid and PCL in glacial acetic acid solutions to obtain different ratio blends, showed phase separation (Dunia et al. 2007). Moreover, glacial acetic acid may not be very suitable for food packaging application because of its pungent odor. Honma et al. (2003) used a common solvent 1.1.1.3.3.3-hexa-fluoro-2-propanol to dissolve PCL and chitosan separately for blending. But the percent weight (PCL 1.7% and chitosan 0.2%) studied was of lower concentration, since for packaging application it requires higher concentration (1–2%) of polymers to achieve good mechanical and barrier properties. It is important to study whether polymer blends are miscible or immiscible systems. Thus, blending strategy and processing into films are important for biodegradable polymers.
The objective is to optimize the blending ratio for the preparation of PCL and chitosan blended films having good tensile strength and low water vapor permeability. The mechanical, thermal, and microscopic behaviors of blended films have been investigated.
Materials and Methods
Chitosan (85% deacetylated; viscosity 1% solution—121 cP) was obtained from India Sea Food, Cochin, India. Poly-ε-caprolactone (molecular weight ∼50,000) was procured from Solvay Interox Limited, Warrington, UK. All other chemicals and reagents used were of analytical grade.
Preparation of Films
Chitosan was dissolved in 1% acetic acid solution to prepare 2% (w/v) chitosan solution and PCL in chloroform to prepare 5% (w/v) PCL solution. The chitosan solution was kept in a water bath maintained at 55 °C under stirring. Different amounts of PCL solution such as 10 ml, 20 ml, and 30 ml were added drop wise to 90, 80, and 70 ml of chitosan solutions, respectively to obtain chitosan/PCL blends, which are designated as chitosan, CH90, CH80, and CH70. The final mixture was stirred for 30 min. The conical flasks containing the solution were sealed with a rubber stopper and degassed using a vacuum pump in order to avoid the formation of air bubbles during casting. After degassing, 100 ml of the solution was casted on to a Teflon-coated plate (25 × 18 cm). Film thickness was controlled by pouring a constant amount of solution during wet casting. The solution was poured onto the center of the plate and spread uniformly. The casted film was kept overnight (∼12 h) at ambient conditions and further dried in a hot air oven at 55 °C for 3 h. The exposure of the blended film at room temperature allows the reduction of the residual acetic acid odor.
Mechanical Properties
Tensile strength (TS) and elongation at break point were determined according to the ASTM procedure D 882 (ASTM 1995) using LLOYDS Universal Testing (LLOYDS-50 K, London, UK) instrument. Samples (100 × 15 mm) were cut from the film and thickness was measured at five points using a micrometer (Testing Machines, Minneapolis). The data were collected from ten samples and average values are reported. The initial grip separation and crosshead speed were set at 50 mm and 50 mm/min, respectively. Tensile strength was expressed as the ratio of maximum load for breaking the film to initial cross-sectional area of the sample and is expressed in MPa. Percent elongation was expressed as a ratio of the elongation at the break point to the initial length of the sample multiplied by 100.
Water Vapor Permeability
Water vapor permeability (WVP) of films was determined according to ASTM E 96-97 procedure (ASTM, 1980). A film of 50 cm2 was mounted on the top of the aluminum cup filled with fused calcium chloride and sealed with blend of microcrystalline and paraffin waxes. The cups were placed in a humidity chamber (Laboratory Thermal Equipment, Glasgow, UK) maintained at 38 °C, RH 90% and weighed every hour for 6 h. A slope of linear portion of the weight gained versus time was used to determine the water vapor transmission rate (WVTR), which corresponded to the amount of water vapor diffused through the film per unit time (g/h) at a steady state. The WVTR and WVP were calculated according to the equation, WVTR = slope/film area and WVP = WVTR/(P 2 − P 1) × L where, P 1 = partial pressure inside the cup (kPa), P 2 = water vapor partial pressure at the film outer surface (kPa), and L = average film thickness (mm). The values of WVP are expressed in g m−1 day−1 atm−1 (Chinnan and Park, 1995).
Oxygen Permeability
Oxygen permeability (OP) of films was determined volumetrically using permeability cell (Customs Scientific Instruments, New Jersey, USA) according to the ASTM D 1434 procedure (ASTM, 1983). The change in volume of the oxygen permeated (inferred from the displacement of mercury) was plotted as a function of time. The slope of the obtained straight line was derived by using simple linear equation. The oxygen transmission rate (OTR) under standard experimental condition was calculated using the formula, OTR = 34,029 × slope/pressure, where 34,029 is capillary constant. Oxygen permeability was calculated by multiplying the oxygen transmission rate and thickness of the film and reported in cc m−1 day−1 atm−1.
Differential Scanning Calorimetry
Thermal properties of chitosan, PCL and chitosan/PCL-blended films were characterized using Mettler DSC-30 (Switzerland) equipment. Calibration was carried out using a pure Indium Standard. Exactly weighed 5 mg of finely cut dried film sealed in an aluminum pan and heated from 30 to 200 °C at a rate of 10 °C/min. Heating regime of the samples was analyzed (Dunia et al. 2007; Sarasam and Madihally 2005).
Fourier Transform Infrared Spectroscopic Analysis
The film samples used for Fourier transform infrared (FTIR) spectroscopic measurement were thin enough (25–30 μm) to ensure that the observed absorption was within the linearity range of the detector and was recorded by fixing films onto metallic slit in air medium on a FTIR spectrophotometer (Perkin-Elmer 2000 USA) with the transmission mode at room temperature in the range from 4,000–400 cm−1 with an accumulation of ten scans (Honma et al. 2003).
Scanning Electron Microscopy
The samples were coated with conductive layer of sputtered gold (Dunia et al. 2007) and examined under scanning electron (LEO 435 VP LEO Electron microscopy, Cambridge, UK) microscope. The micrographs were taken at an accelerating voltage of 15 KV to ensure a suitable image resolution (×500).
Statistical Analysis
Data were statistically analyzed by t-test for comparison of means using Microsoft Excel for significance (95% confidence interval).
Results and Discussion
Mechanical Properties
The tensile strength and percentage elongation values of chitosan, PCL, and blended films are shown in Table 1. The tensile strength of pure chitosan and pure PCL films was found to be 25.99 and 13.62 MPa, respectively. The chitosan/PCL-blended films were found to have tensile strength of the same magnitude as that of the pure chitosan films. It may be observed that the increase in PCL proportion up to 20% (CH80) did not significantly change the tensile strength. However, the change in PCL proportion above 20% (CH70) resulted in a significant decrease in strength to 16.37 MPa, which may be due to the possible occurrence of phase separation. The mechanical property of these blended films depends on the intermolecular forces, chain stiffness, and molecular symmetry of the individual polymer used for blend or the constitutive properties obtained during blending process (John et al. 2002).
The percentage elongation for pure chitosan and pure PCL films was found to be 28.15% and 190.14%, respectively. Correlo et al. (2005) reported that the pure PCL film having percentage elongation of the order to 674%, which was decreased to 5%, when blended with 50% chitosan. The percentage elongation of PCL films was very high due to its synthetic plasticity. Compared to previous reports (Kittur et al. 1998; Suyathma et al. 2005), the percentage elongation of pure chitosan films was also quite high (28.15%), which mainly depends on the molecular weight and processing parameters. During drying of films, the loss of water is coupled with mobility of polymers chains, which results in wider space. Later, this results in moisture absorption from the atmosphere in the storage process of these films at ambient conditions. The moisture content contributes to higher plasticization of the chitosan film. It is well known that water is the most ubiquitous and uncontrollable plasticizer for most hydrocolloid-based films due to its ability to modify the structure (Suyathma et al. 2005). With an increase in the proportions of PCL from 10% (CH90) to 20% (CH80), resulted in an increase in percentage elongation from 28.69% to 33.94%. Further increase in PCL proportion up to 30% (CH70) resulted in a decrease in percentage elongation. This decrease in elongation may be attributed to possibility of phase separation as earlier indicated in the films having PCL proportions higher than 20%.
It may be noted that higher tensile strength as well as percentage elongation will be preferable for food packaging films. Considering the above fact, the chitosan/PCL-blended film with 20% PCL (CH80) with tensile strength 22.66 MPa and percentage elongation 33.94% will be the most suitable.
Water Vapor Permeability
The mean water vapor permeability (WVP) values for chitosan, PCL, and blended films are presented in Table 1. The WVP values were found to decrease with an increase in PCL content from 10% (CH90) to 30% (CH70, p ≤ 0.05). WVP value for native chitosan was 1.57, which reduced to 1.08 as the PCL concentration increased up to 30% (CH70). This could be attributed to the increase in hydrophobic nature due to increased PCL concentration. In case of blend having 20% (CH80), the WVP was found to be reduced by 28% from 1.57 to 1.13 g m−1 day−1 atm−1.
Oxygen Permeability
The oxygen permeability was found to increase as PCL content in film was increased. The oxygen permeability for pure chitosan film was found to be 1.8 × 10−4 cc m−1 day−1 atm−1. The increase in PCL content up to 10% (CH90) resulted slow increase in oxygen permeability (from 1.8 to 7.5 × 10−4 cc m−1 day−1 atm−1, p ≤ 0.05), whereas further increase in the proportion of the PCL resulted in steep rise in oxygen permeability (Table 1). The increase in PCL concentration in the blended film leads to alteration in inter-chain arrangement of chitosan and polymer–polymer interaction between chitosan and PCL, which results in increase in oxygen permeability.
Thermal Properties
The thermograms of chitosan, PCL, and blended films are presented in Fig. 1. Pure chitosan film does not have melting properties and hence no endothermic peaks associated to melting process were detected. The pure PCL film indicated a melting endothermic peak at 62 °C (Honma et al. 2003; John et al. 2002). The endothermic peak for the blended film with 20% PCL (CH80) was found to shift towards lower temperature at 56.88 °C, which is 5.12 °C lower than the peak temperature corresponding to the pure PCL film. This shift in temperature may be attributed to the interaction between the carbonyl group PCL and the –OH and NH2 groups of chitosan (Correlo et al. 2005).
Sarasam and Madihally (2005) also demonstrated that the shift in melting point in case of blended films indicates the miscibility of the polymers. They have observed that the blend with PCL 25% proportion resulted in increased miscibility, however, phase separation occurred as the PCL proportion was increased to 50%. Similarly, in the present case, the increased miscibility was observed when PCL proportion was 20% (CH80), which was found to decrease as the proportion was increased up to 30% (CH70), as inferred from the shift of endothermic peak temperatures (Table 2). The increase in proportion of PCL from 10% (CH90) to 30% (CH70) resulted in an increase in enthalpy for PCL melting (Table 2). Another endothermic peak appeared between 100–160 °C as a function of water evaporation due to moisture present in chitosan and its blended films (Fig. 1). Due to increase in the hydrophobic nature with an increase in PCL component, the enthalpy of water absorption was found to decrease (Table 2).
Fourier Transform Infrared Spectroscopic Analysis
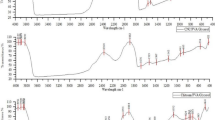
The infrared spectrum of pure PCL film has a prominent characteristic absorption peak centered around 1,744 cm−1, which is attributed to the carbonyl group absorption (Fig. 2a) (Senda et al. 2001). Two absorption peaks correspond to amide І (>CH-NH-) and amine (-NH2) deformation vibration of chitosan centered at 1,636 and 1,559 cm−1 respectively, are found in pure chitosan film (Fig. 2b). FTIR spectrum of blended film (CH80) indicated a shift in peak absorption from 1743.9 to 1724.8 cm−1 due to carbonyl group, whereas the peaks due to amide I and amine deformation appeared at the same frequency. Moreover, the peak absorption due to 732 cm−1of PCL representing –C=O rocking has shifted to lower wavelength 728 cm−1only for the CH80 blended film. This shift in the absorption peak indicates the miscibility and interaction of two polymers.
Scanning Electron Microscopy
SEM was carried out to understand the distribution of PCL and chitosan surface topologies. Pure chitosan and pure PCL films formed a continuous plane surface morphology when viewed at 10 µm (Fig. 3a, b). For the blends of PCL 10% (CH90) and 30% (CH70), the films showed uniformly dispersed PCL globules (Fig. 3c, d). However, the size of the globules was higher in case of CH70 film, due to increased PCL proportion. The morphology of CH80 sample was found to be quite different (Fig. 3e). There were depressions all over the surface instead of globule formation, which indicated the interaction of chitosan with PCL. As a result the film is more uniform when compared to other blends and appears as a co-continuous phase. This clearly explains the unique miscibility behavior of 20% PCL with chitosan without phase separation.
Conclusion
Out of the chitosan and PCL-blended films were prepared in which chitosan to PCL ratio 80:20 resulted in retention of mechanical and improvement of barrier properties. The change in the properties of the blended film was due to interaction of PCL with chitosan, which was evident from the FTIR spectrum, differential scanning calorimetric studies as well as microscopic examination. The present study indicated that the properties of the blended chitosan/PCL films could be modified by changing the proportion of PCL. The incorporation of PCL (hydrophobic polymer) with chitosan (hydrophilic polymer) resulted in the increase in hydrophobic nature of blended films. Therefore, the blended films will have low water vapor transmission rate as compared to native chitosan film. Due to this feature, fruits and vegetables packaged in such film is expected to have extended storage life.
References
ASTM. (1980). Standard test methods for water vapor transmission of materials. In Annual Book of ASTM standards (pp. 760–767). Philadelphia, PA, USA: American Society for Testing and Materials.
ASTM. (1983). Standard test methods for gas transmission of materials. In Annual Book of ASTM standards (pp. 632–647). Philadelphia, PA, USA: American Society for Testing and Materials.
ASTM. (1995). Standard test methods for tensile properties of thin plastic sheeting. In Annual Book of ASTM standards (pp. 159–167). Philadelphia, PA, USA: American Society for Testing and Materials.
Butler, B. L., Vergano, P. J., Testin, R. F., Bunn, J. M., & Wiles, J. L. (1996). Mechanical and barrier properties of edible chitosan films as affected by composition and storage. Journal of Food Science, 61, 953–961. doi:10.1111/j.1365-2621.1996.tb10909.x.
Cao, A., Okamura, T., Ishiguro, C., Nakayama, K., Inoue, Y., & Masuda, T. (2002). Studies on synthesis and physical characteristics of biodegradable aliphatic poly (butylene succinate-co-ε-caprolactone). Polymer (Guildf)., 43, 671–679. doi:10.1016/S0032-3861(01)00658-9.
Cheng, M., Deng, J., Yang, F., Gong, Y., Zhao, N., & Zhang, X. (2003). Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials, 24, 2871–2880. doi:10.1016/S0142-9612(03)00117-0.
Chinnan, M. S., & Park, H. J. (1995). Effect of plasticizer and temperature on the water vapour transmission of cellulose based edible films. Journal of Food Process Engineering, 18, 417–429. doi:10.1111/j.1745-4530.1995.tb00375.x.
Cheung, M. K., Wan, K. P. Y., & Yu, P. H. (2002). Miscibility and morphology of chiral semicrystalline Poly-(R)-(3-hydroxy butyrate)/chitosan and Poly-(R)-(3-hydroxy butyrate-co-3-hydroxy valerate)/chitosan blends studied with DSC, 1HT 1 and T 1ρ CRAMPS. Journal Applied Polymer Science, 86, 1253–1258. doi:10.1002/app.11091.
Correlo, V. M., Boesel, L. F., Battacharya, M., Mano, J. F., Neves, N. M., & Reiz, R. L. (2005). Properties of melt processed chitosan and aliphatic polyester blends. Material Science Engineering A, 403, 57–68. doi:10.1016/j.msea.2005.04.055.
Dunia, M., Cruz, G., Jose, L., Ribelles, G., & Sanchez, M. S. (2007). Blending polysaccharides with biodegradable polymers. 1. Properties of chitosan/polycaprolactone blends. Journal Biomedical Materials Research, Part B, Applied Biomaterials, 85, 303–313.
Harish Prashanth, K. V., & Tharanathan, R. N. (2007). Chitin/chitosan: modifications and their unlimited application potential—an overview. Trends Food Science Technology, 18, 117–131. doi:10.1016/j.tifs.2006.10.022.
Honma, T., Senda, T., & Inoue, Y. (2003). Thermal properties and crystallization behavior of blends of poly(ε-caprolactone) with chitin and chitosan. Polym International, 52, 1839–1846. doi:10.1002/pi.1380.
John, J., Mani, R., & Battacharya, M. (2002). Evaluation compatibility and properties of biodegradable polyester blends. Journal of Polymer Science, Polymer Chemistry, 40, 2003–2014. doi:10.1002/pola.10297.
Kittur, F. S., Kumar, K. R., & Tharanathan, R. N. (1998). Functional packaging properties of chitosan films. European Food Research and Technology, 206, 44–7.
Olabarrieta, I., Forsstrom, D., Gedde, U. W., & Hedenqvist, M. S. (2001). Transport properties of chitosan and whey blended with poly(ε-caprolactone) assessed by standard permeability measurements and microcalorimetry. Polymer (Guildf), 9, 4401–4408. doi:10.1016/S0032-3861(00)00680-7.
Reinoso, E., Mittal, G. S., & Lim, L. T. (2008). Influence of whey protein composite coatings on plum (Prunus Domestica L.) fruit quality. Food Bioprocess Technology, 1(4), 314–325. doi:10.1007/s11947-007-0014-1.
Rinaudo MR, Domard A. 1989. Chitin and chitosan: sources, chemistry, biochemistry, physical properties and applications. In: SkjakBrack G., Anthonsen, T., & Stanford, P. (Eds). Elsevier, New York, pp 71–86.
Sarasam, A., & Madihally, S. V. (2005). Characterization of chitosan/polycaprolactone for tissue engineering applications. Biomaterials, 26, 5500–5508. doi:10.1016/j.biomaterials.2005.01.071.
Sarasam, A. R., Samli, A. I., Hess, L., Ihnat, M. A., & Madihally, S. V. (2007). Blending chitosan with polycaprolactone: porous scaffolds and toxicity. Macromolecular Bioscience, 7, 1160–1167. doi:10.1002/mabi.200700001.
Senda, T., He, Y., & Inoue, Y. (2001). Biodegradable blends of poly (ε-caprolactone) with α-chitin and chitosan: specific interactions, thermal properties and crystallization behavior. Polym International, 51, 33–39. doi:10.1002/pi.793.
Srinivasa, P. C., Revathy, B., Ramesh, M. N., Harish Prashanth, K. V., & Tharanathan, R. N. (2002). Storage studies of mango packed using biodegradable chitosan films. European Food Research and Technology, 215, 504–508. doi:10.1007/s00217-002-0591-1.
Suyatma, N. E., Copinet, A., Tighzert, L., & Coma, V. (2004). Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. Journal of Polymers and the Environment, 12(1), 1–6. doi:10.1023/B:JOOE.0000003121.12800.4e.
Suyathma, N. E., Tighzert, L., & Copinet, A. (2005). Effects of hydrophilic plasticisers on mechanical, thermal and surface properties of chitosan films. Journal of Agricutural Food Chemistry, 53, 3950–3957. doi:10.1021/jf048790+.
Acknowledgements
Authors thank Dr. V. Prakash, Director, CFTRI, Mysore, for the encouragement. The authors acknowledge Department of Biotechnology, New Delhi, for financial support (No.BT/PR-7567/PID/20/296/2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
A constituent laboratory of Council of Scientific and Industrial Research, New Delhi, India
Rights and permissions
About this article
Cite this article
Swapna Joseph, C., Harish Prashanth, K.V., Rastogi, N.K. et al. Optimum Blend of Chitosan and Poly-(ε-caprolactone) for Fabrication of Films for Food Packaging Applications. Food Bioprocess Technol 4, 1179–1185 (2011). https://doi.org/10.1007/s11947-009-0203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0203-1