Abstract
As a main component of waste electrical and electronic equipment (WEEE) plastics, polycarbonate (PC) shows an important recycling significance. However, separating PC from WEEE plastics is difficult due to their similar hydrophobic surfaces and densities. Herein, a novel surface modification method using Fenton treatment combined with ultrasonic was proposed to selectively separate PC from acrylonitrile–butadiene–styrene (ABS) and polyvinylchloride (PVC) by froth flotation. The effects of surface modification and flotation conditions on the flotation recovery of plastics were investigated to determine the optimum separation conditions for PC. The optimum conditions are ultrasonic power 240 W, ultrasonic time 8 min, molar ratio (H2O2/Fe2+) 100, hydrogen peroxide concentration 0.3 mol/L, pH 3, frother concentration 20 mg/L, stirring rate 1800 rpm and flotation time 4 min. Under optimum conditions, the recovery and purity of PC with different mass ratios exceed 97.97% and 99.18%, respectively. Contact angle and Fourier transform infrared spectroscopy were used to ascertain the mechanism of surface modification. The surface of PC becomes more hydrophilic due to the introduction of oxygen-containing groups induced by surface modification. Consequently, this study provides a practical surface modification method for the separation of PC from WEEE plastics, which will effectively promote the recycling of PC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics have been extensively utilized in the electronics industry since they possess remarkable characteristics such as light, low cost and high chemical stability [1]. Driven by technological innovation, the service life of electronic products has gradually shortened, leading to a large accumulation of waste electrical and electronic equipment (WEEE or e-waste) [2]. According to “The Global E-waste Monitor 2020” issued by the United Nations, the total amount of e-waste generated globally reached 53.6 million tons in 2019 [3]. E-waste has become one of the fastest-growing waste streams in the world.
Plastics and metals are two main types of recyclable resources in e-waste. However, in the process of recycling e-waste, people pay more attention to the recycling of rare and precious metals, while the recycling of plastics is not getting enough attention. Plastics account for about 10–30% of WEEE [4]. The environment pollution caused by waste plastics is becoming more and more serious since plastics are difficult to degrade naturally. Most of the plastics separated from WEEE are disposed by landfill and incineration, while only 10% are recycled [5, 6]. Landfill occupies a lot of land resources, and it is easy to cause potential pollution to soil and groundwater [7, 8]. Incineration of plastics can release harmful substances, such as dioxins, hydrogen chloride and polycyclic aromatic hydrocarbons, which can enter the food chain through rainfall and threaten human health [9, 10]. Life Cycle Assessment (LCA) shows that replacing virgin plastics with recycled plastics can effectively reduce the negative impact of waste plastics on the environment [11]. Therefore, the recycling of waste plastics becomes an effective disposal method, which meets the requirements of sociallly sustainable development and cleaner production [12]. Unfortunately, plastic mixtures cannot be recycled directly due to the differences in additives, physical and chemical properties [13]. In other words, waste plastic mixtures must be separated into individual component before recycling.
As an important part of WEEE plastics, PC is very suitable for recycling [14]. However, the recycling of waste PC is challenging because it is usually mixed with other plastics, such as ABS and PVC [15]. In general, ABS, PVC and PC in WEEE can account for about 8 to 30%, 3–17% and 3–10%, respectively [16]. Currently, several prospective methods used for the separation of plastic including density separation [17, 18], electrostatic separation [19, 20], near-infrared (NIR) separation [21] and froth flotation [22, 23]. However, it is difficult to separate plastics with similar density by density separation. Electrostatic separation is not suitable for plastics with high humidity and small electrification differences. Near-infrared separation cannot sort dark plastics. In recent years, the application of flotation in plastic sorting has attracted widespread attention due to its cost-effective and high efficiency in the separation of plastic mixtures with similar properties [24]. The low surface energy of plastics determines that most plastics are naturally hydrophobic. Selective wetting of components to be separated is the key to realize plastic flotation separation [25,26,27]. In general, plastic flotation methods can be roughly divided into three categories according to the wetting mechanism: adsorption of wetting agents, physical regulation and chemical modification. Several wetting agents used in the flotation process include tea saponin [28], lignin sulfonate [29] and tannic acid [30]. Physical regulation methods used in the field of plastic flotation include Boiling treatment [31], plasma treatment [32], mild heat treatment [13], etc. And chemical modification methods include ammonia treatment [33], Fenton treatment [26], surface alkoxylation pretreatment [8], etc. The adsorption of wetting agents and physical regulation will not change the chemical composition of plastic while chemical modification focus on the surface reactions of plastic.
Advanced oxidation process (AOP), which is mainly based on the generation of highly reactive hydroxyl radicals, has been widely used in sewage treatment, soil remediation and landfill leachate [34, 35]. The common advanced oxidation processes include Fenton treatment, ozone oxidation, photochemical oxidation, etc. In recent years, the combination of Fenton treatment with other technologies has attracted more and more attention. Especially the synergistic effect of Fenton treatment and ultrasound technology can adequately decompose many organic substances which are difficult to degrade. Previous researches have shown that AOP is an effective surface modification method for plastic, which can achieve changes in hydrophilicity by oxidizing the surface of some plastics [14]. However, Fenton treatment combined with ultrasonic was never applied to the surface modification of plastics. Thus, it will be a meaningful attempt to study the influence of it on the surface of plastics.
Herein, we put forward a novel surface modification method using Fenton treatment combined with ultrasonic to separate PC from WEEE plastics. In this work, the effects of ultrasonic power, ultrasonic time, molar ratio (H2O2/Fe2+), H2O2 concentration, pH, frother concentration, stirring rate and floatation time on floatability of plastics were investigated to identify the optimum conditions for separation. In addition, different mass ratios of PC were studied to determine the suitability of surface modification for diverse waste streams. Contact angle and Fourier transform infrared spectroscopy (FT-IR) were conducted to ascertain the mechanism of surface modification.
Materials and methods
Materials and chemicals
Plastic samples used in this study, including acrylonitrile butadiene styrene (ABS), polycarbonate (PC) and polyvinyl chloride (PVC), were obtained from commercial source (SK, Holdings, Korea). The average density of ABS, PC and PVC in this study is 1.05 g/cm3, 1.20 g/cm3 and 1.35 g/cm3, respectively. In addition, to avoid the influence of surface pollution and aging degree after consumption on flotation results, virgin materials were used. The colors of ABS, PC and PVC are different, which makes it easy to sort manually after flotation experiments. The plastic samples were smashed to particles with the size of 3–5 mm by a Miniature multifunctional crusher (DFT-200A, JIUPIN, China). According to the previous research, the optimum size fraction in plastic flotation was 1–5 mm [16, 36, 37]. Thus, the size of the plastic particle selected in this study was 3–5 mm. The obtained plastic particles were then washed with tap water and dried at room temperature (25 ± 2 ℃).
Ferrous sulfate (FeSO4·7H2O) was obtained from Kermel Chemical Reagent Co., Ltd. in Tianjin, China. Hydrogen peroxide (H2O2, 30%) was purchased from Damao Chemical Reagent Factory in Tianjn, China. Dilute sulfuric acid and sodium hydroxide were used as pH adjusters. Terpineol was frother and tap water was the flotation medium in all flotation experiments. Terpineol was chosen as the frother because of its advantages of convenient preparation, low cost and strong foaming. All chemical reagents were of analytical grade.
Surface modification
ABS (5 g), PC (5 g) and PVC (5 g) were mixed manually and added to a 100 mL beaker containing 50 mL Fenton reagent. Then, surface modification was carried out in a numerical control ultrasonic cleaner (KQ-300DE, Kunshan Ultrasonic Instrument Co., Ltd., China) with the power of 0–300 W, reaction time of 0–10 min and ultrasound frequency 40 kHz. After surface modification, plastic particles were washed three times with tap water, and then flotation experiments were performed to investigate the effect of related parameters in surface modification on the floatability of ABS, PC and PVC.
Flotation experiments
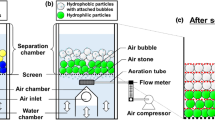
Flotation experiments were carried out on a single-slot flotation equipment (XFD‐II, China). The detailed flotation device and the experimental process can refer to our previous article [38]. After flotation experiments, the plastic particles floated and submerged were collected, washed, dried at room temperature and weighed. The separation efficiency was evaluated by recovery and purity. The recovery and purity of ABS, PC and PVC can be calculated by Eqs. (1)—(4). It should be noted that the presented recovery and purity values in this study were average values of three experimental results.
where Rif and Ris are the recovery of floated products and submerged products of i samples, respectively; Pif and Pis are the purity of floated products and submerged products of i samples, respectively. Mif and Mis are the weight of floated products and submerged products of i samples, respectively. Mf and Ms are the weight of floated products and submerged products, respectively.
Surface characterization of plastic samples
To ascertain the mechanism of Fenton treatment combined with ultrasonic, contact angle and Fourier transform infrared spectroscopy (FT-IR) were conducted under optimum surface modification conditions. Contact angles of distilled water dropped on the plastic surface were measured with a contact angle measuring instrument (SL200KS, Kino Industry Co., Ltd., USA). Besides, the contact angle value of each plastic sample was an average of five measurements. The functional groups on plastic surface were researched by Fourier transform infrared spectrometer (IL8CERNGI, PerkinElmer, USA).
Results and discussion
The effect of surface modification on plastic flotation
Effect of ultrasonic power
The influences of ultrasonic power, ultrasonic time, molar ratio (H2O2/Fe2+), H2O2 concentration and pH on the flotation recovery of ABS, PC and PVC were investigated and the results are shown in Fig. 1. It should be noticed that flotation experiments after surface modification were conducted under the conditions of frother concentration 50 mg/L, stirring rate 1800 rpm and flotation time 10 min.
The effect of a ultrasonic power, b ultrasonic time, c molar ratio (H2O2/Fe2+), d H2O2 concentration and e pH on flotation recovery of ABS, PC and PVC. The flotation experiments after surface modification were conducted under the conditions of frother concentration 50 mg/L, stirring rate 1800 rpm and flotation time 10 min
As shown in Fig. 1a, ultrasonic power has no significant effect on the flotation recovery of ABS and PVC, which is maintained at approximately 95% and 97%, respectively. When the ultrasonic power is 0, the flotation recovery of PC is 81.87%, which means that it is difficult to separate PC from the other plastics (ABS and PVC) by Fenton treatment alone. However, the flotation recovery of PC decreases rapidly with the increase of ultrasonic power, and it decreases from 81.87% to approximately 3% when ultrasonic power increases from 0 to 240 W. The subsequent mechanism analysis shows that the contact angle of PC dropped obviously after surface modification, indicating the wettability of PC surface was enhanced. And the FT-IR analysis shows that the introduction of hydrophilic oxygen-containing groups (C = O and C–O) lead to the enhancement in wettability of PC surface. Detailed analysis can be seen in 3.4 mechanism of surface modification. The enhancement of wettability for PC surface reduced the flotation recovery drastically. Therefore, the optimum ultrasonic power for separating PC from plastic mixtures is 240 W.
Effect of ultrasonic time
As seen from Fig. 1b, the flotation recovery of ABS and PVC is insensitive to ultrasonic time, while that of PC decreases significantly with the increase of ultrasonic time. The flotation recovery of ABS, PC and PVC is about 99%, 97% and 99% without Fenton treatment combined with ultrasonic, which is mainly caused by the natural hydrophobicity of ABS, PC and PVC. The natural floatability of plastics refers to the floatability of plastic particles under the action of air bubbles without adding any frother. The natural floatability data of ABS, PC and PVC were presented in Fig. S1. As shown in Fig. S1, the natural floatability of ABS, PC and PVC is 94.89%, 85.33% and 71.98% respectively, indicating the descending order of the natural floatability of the three plastics is ABS, PC and PVC. Furthermore, the addition of frother is beneficial to promote the formation of a large number of tiny bubbles, which makes plastics exist in the form of floating products easily. The flotation recovery of PC decreases to the lowest value of about 3% when the ultrasonic time increases to 8 min, which means that surface modification can effectively transform the hydrophobic surface of PC into hydrophilic surface. The difference between PC and other plastics in floatability is the largest at this moment. Therefore, the optimum ultrasonic time for separating PC from plastic mixtures is 8 min.
Effect of molar ratio (H2O2/Fe2+)
As seen from Fig. 1c, the flotation recovery of PC is remarkably affected by the decrease of molar ratio (H2O2/Fe2+), while that of ABS and PVC is not affected. The flotation recovery of PC decreases from 74.54% to 3.61% when the molar ratio (H2O2/Fe2+) decreases from 500 to 100, indicating that a significant fraction of PC submerged. The flotation recovery of ABS and PVC stays at about 98% and 97%, respectively. It can be explained that Fe2+ can catalyze H2O2 to produce ·OH. When the amount of Fe2+ is too low, it will limit the production rate of ·OH, and thus inhibit the oxidation reaction on the PC surface [39]. Therefore, the optimum molar ratio (H2O2/Fe2+) for separating PC from plastic mixtures is 100.
Effect of H2O2 concentration
As seen from Fig. 1d, the flotation recovery of ABS, PC and PVC is close to 100% when the H2O2 concentration is 0. The results indicate that PC cannot be separated from plastic mixtures by ultrasonic treatment alone. The flotation recovery of PC decreases significantly to approximately 3% in the H2O2 concentration range of 0–0.3 mol/L, while the flotation recovery of ABS and PVC is insensitive to the change of H2O2 concentration. It shows that the floatability of PC was changed after surface modification, while ABS and PVC were not affected. Therefore, the optimum H2O2 concentration for separating PC from plastic mixtures is 0.3 mol/L.
Effect of pH
As seen from Fig. 1e, pH has no significant effect on the flotation recovery of ABS and PVC, which maintains at about 98% and 97%, respectively. The flotation recovery of PC is 84.41% when pH is 1. It is because that when the pH is low, H+ will inhibit the reaction of H2O2 with Fe2+ and reduce the production of •OH. When the pH increases to 3, the flotation recovery of PC drops to the lowest value of about 3%. When the pH continues to increase from 3, the flotation recovery of PC has a slight upward trend. It can be explained that the increase of pH will form hydroxide precipitation in solution, resulting in a decrease in the oxidizing capacity of system [40]. Therefore, the optimum pH for separating PC from plastic mixtures is 3.
The effect of flotation conditions on plastic flotation
Effect of frother concentration
Under optimal surface modification conditions, the influences of frother concentration, stirring rate and flotation time on the floatability of ABS, PC and PVC were further investigated, and the results are shown in Fig. 2. Frother concentration has a great influence on the generation of homogeneous bubbles and further affects the stability of flotation [41]. As shown in Fig. 2a, with the increase of frother concentration, the flotation recovery of ABS and PVC gradually increases and reaches the maximum at 20 mg/L, while the flotation recovery of PC maintains at around 3%. Therefore, the optimum frother concentration for separating PC from plastic mixtures is 20 mg/L.
Effect of stirring rate
The effect of the stirring rate on plastic flotation separation is presented in Fig. 2b. The flotation recovery of ABS is not affected by the stirring rate, which maintains at about 99%. As for PVC, with the increase of stirring rate, the floatation recovery gradually increases to about 99% when the stirring rate increases to 1800 rpm. When the stirring rate is less than 1800 rpm, the flotation recovery of PC maintains at about 3%. However, when the stirring rate increases from 1800 to 2200 rpm, the flotation recovery of PC increases rapidly to 80.76%. Under the optimum conditions, PC surface is completely oxidized and air bubbles will not be adsorbed on its surface. However, at 1800 + rpm, the entire flotation system is in an enormously turbulent state and PC will float up under the action of mechanical entrainment due to its relatively low density. It is not good for the whole separation process. Therefore, the optimum stirring rate for separating PC from plastic mixtures is 1800 rpm.
Effect of flotation time
As shown in Fig. 2c, the flotation recovery of ABS and PVC increases sharply with flotation time. When flotation time increases to 4 min, the flotation recovery of ABS and PVC reaches about 99% and tends to be stable. However, the flotation recovery of PC maintains at around 3% in the range of 2–10 min. Therefore, the optimum flotation time for separating PC from plastic mixtures is 4 min.
Flotation separation of PC with different mass ratios
The content of different types of plastics in diverse waste streams varies greatly [36], and thus the effect of mass ratio on separation of PC was investigated to verify the feasibility for different waste streams. Plastic mixtures were treated under optimum surface modification and flotation conditions. The mass ratios of PC are set as 25%, 50% and 75%. In addition, the mass ratio of ABS and PVC is 1:1. The results are shown in Table 1. It should be noted that F-P and S-P in Table 1 represent floated products and submerged products, respectively. It can be seen from Table 1 that PC can be separated effectively from plastic mixtures with different mass ratios. The recovery and purity of PC exceed 97.97% and 99.18%, respectively. Especially in system 3, the recovery and purity of PC is 98.23% and 99.47%, respectively. Consequently, the separation of PC can be achieved effectively through surface modification and flotation process under different mass ratios conditions.
Reusability of surface modification solution
To minimize environmental pollution and reduce recycling costs, the reusability of surface modification solution was considered and the results are shown in Fig. 3. The experiments were conducted under the optimum conditions, but the surface modification solution was used repeatedly. In the first two uses of Fenton reagent, the recovery and purity of PC are maintained above 95%. At the second cycle, the recovery of PC drops significantly, indicating that the residual H2O2 concentration is decreased. At the 3rd, 4th and 5th cycles, the recovery of PC is less than 20%, which is due to the low or zero residual H2O2 concentration in the surface modification solution. The purity of PC gradually decreases with the number of cycles, which is related to the decrease in the recovery of PC. Therefore, in view of the reduction in separation efficiency of the second cycle and subsequent cycles, we suggest that the surface modification solution can be used twice.
Mechanism of surface modification
The mechanism of hydroxyl radical (·OH) production
The ·OH is an effective oxidizing factor in the system due to its strong oxidizing properties. The ways in which ·OH are generated and plastic surface are oxidized are shown in the following formulas (5–10). Ultrasound can induce the sonolysis of water molecules to produce ·OH [14]. At the same time, under acidic conditions, ferrous ions (Fe2+) catalyze the decomposition of hydrogen peroxide (H2O2) to produce ferric ions (Fe3+) and ·OH. The ferric ions can react with H2O2 to form Fe2+ and ·OOH, which will ensure the regeneration of Fe2+ and the continuous progress of the reaction.
The generated ·OH can be adsorbed on the surface of plastic and further react with it. According to the above formulas, organics (RH) can react with ·OH through hydrogen abstraction reaction, generating organic radicals (R·) with high reactivity which react with H2O2 easily [37]. Therefore, the surface of plastic (PC) can be oxidized and introduced some hydrophilic groups in the molecular structure of plastic, which will lead to the sedimentation of PC.
Contact angle analysis
To evaluate the change of surface hydrophilicity of ABS, PC and PVC, the contact angles of the plastic surface were measured before and after surface modification under the optimum conditions. As shown in Fig. 4, the contact angle of ABS decreases from 88.88° to 72.78° with the help of surface modification, indicating that the hydrophilicity of ABS is enhanced. It indicates that the surface of ABS may be oxidized. However, ABS exists in the form of floating products after the end of the flotation experiment. That’s because the density of ABS is 1.05 g/m3, which is similar to the flotation medium (1.00 g/m3). Under the effect of low density and mechanical entrainment, ABS floats on the water. In terms of PC, the contact angle decreases from 72.51° to 49.02° after surface modification, indicating that its surface hydrophilicity is significantly increased, which is consistent with the sharp drop of flotation recovery of PC. As for PVC, the contact angles before and after surface modification are 68.88° and 68.80°, respectively. These results show that the hydrophilicity of PVC is almost unchanged, which is consistent with the fact that PVC keeps floating in flotation experiments.
FT-IR analysis
The essence of surface modification is the oxidation of hydroxyl radicals produced by the Fenton reagent. To detect the change of functional groups on the plastic surface after the combined treatment, FT-IR analysis was performed. As seen from Fig. 5a, the FT-IR spectra of untreated and treated ABS include adsorption bands at 698 and 758 cm−1 for bending vibration of C–H (mono-substituted benzenes), 1448 and 1491 cm−1 for stretching vibration of aromatic rings, 2919 cm−1 for stretching vibration of –CH3 and 965 cm−1 for bending vibration of = C–H [33, 42]. The FT-IR spectra of untreated ABS include adsorption bands at 2236 cm−1 for stretching vibration of C≡N. However, after surface modification, adsorbing bands of C≡N is reduced and stretching vibration of C = O at 1733 cm−1 is enhanced, indicating that the surface of ABS is oxidized and oxygen-containing functional groups are introduced [43]. The above results are also consistent with the reduction of the contact angle of ABS.
As seen from Fig. 5b, the FT-IR spectra of untreated and treated PC include adsorption bands at 825 cm−1 for bending vibration of C–H (mono-substituted benzenes), 1501 cm−1 for stretching vibration of aromatic rings, 2969 cm−1 for stretching vibration of –CH3, 1220 cm−1 for stretching vibration of C–O and 1770 cm−1 for stretching vibration of C = O [7, 44]. In addition, it is obvious that the intensity of C–O and C = O peaks at wavenumbers of 1220 and 1770 cm−1 are enhanced after surface modification, indicating that oxygen-containing functional groups are introduced on PC surface. The introduced oxygen-containing functional groups are hydrophilic, which are expected to enhance the surface hydrophilic of PC.
As shown in Fig. 5c, the FT-IR spectra of untreated and treated PVC include adsorption bands at 695 cm−1 for stretching vibration of C–Cl, 2854 and 2923 cm−1 for stretching vibration of –CH2, 1428 cm−1 for deformation vibration of –CH2, 958 cm−1 for rocking vibration of CH2, 1251 and 1328 cm−1 for bending vibration of C–H and 1095 cm−1 for stretching vibration of C–C [15, 45]. The FT-IR spectra of untreated and treated PVC show no significant changes, implying that surface modification has little effect on PVC.
Limitation and perspectives
This process is simple, stable and reliable. However, the limitation of this process may come from flotation behaviors and oxidation selectivity. At present, our research only stays in the laboratory stage and lacks practical application in the industry. The flotation behaviors of plastic particles in large-scale machine may different from laboratory-scale flotation machine. In the future, the flotation equipment and process can be improved, so as to establish a more complete flotation separation process. Moreover, when other plastic components are mixed in the plastic mixture (ABS, PC and PVC), its effectiveness needs further study. The strong oxidizing properties of the system may cause the surface of other plastic components to be hydrophilic and reduce the purity of PC, which can be resolved by multistage flotation separation combined with other methods.
Conclusion
In this study, we propose a novel surface modification method for plastic using Fenton treatment combined with ultrasonic, which realizes the selective flotation separation of PC from other plastics (ABS and PVC). The optimum conditions of separating PC from other plastic mixtures are ultrasonic power 240 W, ultrasonic time 8 min, molar ratio (H2O2/Fe2+) 100, H2O2 concentration 0.3 mol/L, pH 3, frother concentration 20 mg/L, stirring rate 1800 rpm and flotation time 4 min. Under different mass ratio conditions, the recovery and purity of PC exceed 97.97% and 99.18%, respectively. Surface modification solution can be used twice. The mechanism of surface modification was investigated by contact angle and FT-IR. The reduction of flotation recovery for PC is attributed to the decrease in contact angle and the introduction of hydrophilic groups (C = O and C–O) on the surface of PC, resulting in an increase in surface hydrophilicity. Therefore, this study can provide a scientific basis for promoting the recycling of PC in WEEE. The sources and types of waste plastics are various in our daily life, and hence it is necessary to verify the applicability of this method to the separation of PC in different waste streams in the future.
References
Leal Filho W, Saari U, Fedoruk M, Iital A, Moora H, Klöga M, Voronova V (2019) An overview of the problems posed by plastic products and the role of extended producer responsibility in Europe. J Clean Prod 214:550–558. https://doi.org/10.1016/j.jclepro.2018.12.256
Zeng X, Gong R, Chen WQ, Li J (2016) Uncovering the recycling potential of “New” WEEE in China. Environ Sci Technol 50(3):1347–1358. https://doi.org/10.1021/acs.est.5b05446
Forti V, Baldé C, Kuehr R, Bel G (2020) The Global E-waste Monitor 2020. Quantities, flows, and the circular economy potential [M]
Martinho G, Pires A, Saraiva L, Ribeiro R (2012) Composition of plastics from waste electrical and electronic equipment (WEEE) by direct sampling. Waste Manag 32(6):1213–1217. https://doi.org/10.1016/j.wasman.2012.02.010
Wu C, Nahil MA, Miskolczi N, Huang J, Williams PT (2014) Processing real-world waste plastics by pyrolysis-reforming for hydrogen and high-value carbon nanotubes. Environ Sci Technol 48(1):819–826. https://doi.org/10.1021/es402488b
Wang Y, Zhang FS (2012) Degradation of brominated flame retardant in computer housing plastic by supercritical fluids. J Hazard Mater 205–206:156–163. https://doi.org/10.1016/j.jhazmat.2011.12.055
Wang H, Wang J, Zou Q, Liu W, Wang C, Huang W (2018) Surface treatment using potassium ferrate for separation of polycarbonate and polystyrene waste plastics by froth flotation. Appl Surf Sci 448:219–229. https://doi.org/10.1016/j.apsusc.2018.04.091
Du Y, Zhang Y, Jiang H, Li T, Luo M, Wang L, Wang C, Wang H (2020) Hydrophilic modification of polycarbonate surface with surface alkoxylation pretreatment for efficient separation of polycarbonate and polystyrene by froth flotation. Waste Manag 118:471–480. https://doi.org/10.1016/j.wasman.2020.09.006
Zhao Y, Mishra P, Han F, Liu X, Shen Z (2019) Surface micro-alcoholysis treatment: A novel approach towards froth flotation based separation for binary mixtures of polyethylene terephthalate and polyvinyl chloride. J Clean Prod 232:848–857. https://doi.org/10.1016/j.jclepro.2019.06.031
Yang X, Sun L, Xiang J, Hu S, Su S (2013) Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): a review. Waste Manag 33(2):462–473. https://doi.org/10.1016/j.wasman.2012.07.025
Gu F, Guo J, Zhang W, Summers PA, Hall P (2017) From waste plastics to industrial raw materials: A life cycle assessment of mechanical plastic recycling practice based on a real-world case study. Sci Total Environ 601–602:1192–1207. https://doi.org/10.1016/j.scitotenv.2017.05.278
Wen Z, Zhang C, Ji X, Xue Y (2015) Urban mining’s potential to relieve china’s coming resource crisis. J Ind Ecol 19(6):1091–1102. https://doi.org/10.1111/jiec.12271
Thanh Truc NT, Lee BK (2016) Sustainable and selective separation of PVC and ABS from a WEEE plastic mixture using microwave and/or mild-heat treatment with froth flotation. Environ Sci Technol 50(19):10580–10587. https://doi.org/10.1021/acs.est.6b02280
Nguyen TD, Al Tahtamouni TM, Huong PT, Thang PQ (2019) Selective flotation separation of ABS/PC from ESR plastic wastes mixtures assisted by ultrasonic catalyst/H2O2. J Environm Chem Eng. https://doi.org/10.1016/j.jece.2019.103354
Zhang Y, Jiang H, Wang H, Wang C (2020) Separation of hazardous polyvinyl chloride from waste plastics by flotation assisted with surface modification of ammonium persulfate: Process and mechanism. J Hazard Mater 389:121918. https://doi.org/10.1016/j.jhazmat.2019.121918
Wang H, Zhang Y, Wang C (2019) Surface modification and selective flotation of waste plastics for effective recycling——a review. Sep Purif Technol 226:75–94. https://doi.org/10.1016/j.seppur.2019.05.052
Li X, Guo YW, Ruan JL, Qiao Q, Zhang JQ (2015) Impacts of different wetting agents on the density separation of waste plastic mixtures. Appl Mech Mater 768:418–425. https://doi.org/10.4028/www.scientific.net/AMM.768.418
Moroni M, Lupo E, La Marca F (2017) Hydraulic separation of plastic wastes: analysis of liquid-solid interaction. Waste Manag 66:13–22. https://doi.org/10.1016/j.wasman.2017.04.045
Rezoug M, Aksa W, Boukhoulda MF, Medles K, Dascalescu L (2018) A novel tribo-electrostatic device for the treatment of granular waste plastics mixtures. Int J Environ Stud 76(2):225–235. https://doi.org/10.1080/00207233.2018.1551957
Singh N, Hui D, Singh R, Ahuja IPS, Feo L, Fraternali F (2017) Recycling of plastic solid waste: a state of art review and future applications. Compos B Eng 115:409–422. https://doi.org/10.1016/j.compositesb.2016.09.013
Beigbeder J, Perrin D, Mascaro J-F, Lopez-Cuesta J-M (2013) Study of the physico-chemical properties of recycled polymers from waste electrical and electronic equipment (WEEE) sorted by high resolution near infrared devices. Resour Conserv Recycl 78:105–114. https://doi.org/10.1016/j.resconrec.2013.07.006
Heidarpour M, Movahed SO, Jourabchi S (2021) The effect of microwave irradiation on the flotation of the selected polymers as a potential solution for plastic recycling. J Polym Environ. https://doi.org/10.1007/s10924-021-02105-6
Reddy MS, Okuda T, Kurose K, Tsai T-Y, Nakai S, Nishijima W, Okada M (2010) Surface ozonation of polyvinyl chloride for its separation from waste plastic mixture by froth floatation. J Mater Cycles Waste Manage 12(4):326–331. https://doi.org/10.1007/s10163-010-0305-x
Mallampati R, Srinivasa, Chi-Hyeonlee, Nguyenthithanhtruc, Byeong-Kyulee. Hazardous PVC plastics separation from ASR by Froth floatation after microwave assisted surface modification; proceedings of the 2015 international conference on advances in environment research, F]
Wang J, Wang H, Yue D (2019) Optimization of surface treatment using sodium hypochlorite facilitates coseparation of ABS and PC from WEEE plastics by flotation. Environ Sci Technol 53(4):2086–2094. https://doi.org/10.1021/acs.est.8b06432
Wang J-c, Wang H (2017) Fenton treatment for flotation separation of polyvinyl chloride from plastic mixtures. Sep Purif Technol 187:415–425. https://doi.org/10.1016/j.seppur.2017.06.076
Guo C, Zou Q, Wang J, Wang H, Chen S, Zhong Y (2018) Application of surface modification using sodium hypochlorite for helping flotation separation of acrylonitrile-butadiene-styrene and polystyrene plastics of WEEE. Waste Manag 82:167–176. https://doi.org/10.1016/j.wasman.2018.10.031
Zhao Y, Han F, Abdelaziz IIM, Liu X, Ghazali KH, Mishra P (2019) Application of biosurfactant tea saponin in flotation separation for ternary plastic mixtures: Statistical optimization and mechanism analysis. J Clean Prod 232:499–507. https://doi.org/10.1016/j.jclepro.2019.06.002
Wang H, Wang CQ, Fu JG, Gu GH (2014) Flotability and flotation separation of polymer materials modulated by wetting agents. Waste Manag 34(2):309–315. https://doi.org/10.1016/j.wasman.2013.11.007
Zhao Y, Yang S, Wen H, Shen Z, Han F (2019) Adsorption behavior and selectivity mechanism of flotation reagents applied in ternary plastic mixtures. Waste Manag 87:565–576. https://doi.org/10.1016/j.wasman.2019.02.044
Wang CQ, Wang H, Wu BX, Liu Q (2014) Boiling treatment of ABS and PS plastics for flotation separation. Waste Manag 34(7):1206–1210. https://doi.org/10.1016/j.wasman.2014.02.005
Zhao Y, Han F, Guo L, Singh S, Zhang H, Zhang J (2021) Flotation separation of hazardous polyvinyl chloride from waste plastics based on green plasma modification. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.128569
Wang CQ, Wang H, Gu GH, Lin QQ, Zhang LL, Huang LL, Zhao JY (2016) Ammonia modification for flotation separation of polycarbonate and polystyrene waste plastics. Waste Manag 51:13–18. https://doi.org/10.1016/j.wasman.2016.02.037
Cruz A, Couto L, Esplugas S, Sans C (2017) Study of the contribution of homogeneous catalysis on heterogeneous Fe(III)/alginate mediated photo-Fenton process. Chem Eng J 318:272–280. https://doi.org/10.1016/j.cej.2016.09.014
Rostamizadeh M, Jalali H, Naeimzadeh F, Gharibian S (2019) Efficient removal of diclofenac from pharmaceutical wastewater using impregnated zeolite catalyst in heterogeneous fenton process. Phys Chem Res 7(1): 37–52 https://doi.org/10.22036/pcr.2018.144779.1524
Zhang Y, Chen S, Wang H, Luo M (2019) Separation of polyvinylchloride and acrylonitrile-butadiene-styrene combining advanced oxidation by S2O8(2-)/Fe(2+) system and flotation. Waste Manag 91:80–88. https://doi.org/10.1016/j.wasman.2019.04.048
Wang JC, Wang H, Huang LL, Wang CQ (2017) Surface treatment with Fenton for separation of acrylonitrile-butadiene-styrene and polyvinylchloride waste plastics by flotation. Waste Manag 67:20–26. https://doi.org/10.1016/j.wasman.2017.05.009
Qu YH, Li YP, Zou XT, Xu KW, Xue YT (2020) Microwave treatment combined with wetting agent for an efficient flotation separation of acrylonitrile butadiene styrene (ABS) from plastic mixtures. J Mater Cycles Waste Manag 23(1):96–106. https://doi.org/10.1007/s10163-020-01099-y
Liu S-T, Huang J, Ye Y, Zhang A-B, Pan L, Chen X-G (2013) Microwave enhanced Fenton process for the removal of methylene blue from aqueous solution. Chem Eng J 215–216:586–590. https://doi.org/10.1016/j.cej.2012.11.003
Zhang Y, Jiang H, Wang H, Wang C, Du Y, Wang L (2020) Flotation separation of polystyrene and polyvinyl chloride based on heterogeneous catalytic Fenton and green synthesis of nanoscale zero valent iron (GnZVI). J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.122116
Zhao Y, Li Y-P, Huang J, Liu J, Wang W-K (2015) Rebound and attachment involving single bubble and particle in the separation of plastics by froth flotation. Sep Purif Technol 144:123–132. https://doi.org/10.1016/j.seppur.2015.02.016
Zhang Y, Jiang H, Wang H, Wang C (2020) Flotation separation of acrylonitrile-butadiene-styrene and polystyrene in WEEE based on oxidation of active sites. Miner Eng. https://doi.org/10.1016/j.mineng.2019.106131
Cui Y, Li Y, Wang W, Wang X, Lin J, Mai X, Song G, Naik N, Guo Z (2021) Flotation separation of acrylonitrile-butadienestyrene (ABS) and high impact polystyrene (HIPS) from waste electrical and electronic equipment (WEEE) by potassium permanganate surface modification. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.118767
Wang CQ, Wang H, Huang LL (2017) A novel process for separation of polycarbonate, polyvinyl chloride and polymethyl methacrylate waste plastics by froth flotation. Waste Manag 65:3–10. https://doi.org/10.1016/j.wasman.2017.04.006
Wang C, Wang H, Liu Y, Huang L (2016) Optimization of surface treatment for flotation separation of polyvinyl chloride and polyethylene terephthalate waste plastics using response surface methodology. J Clean Prod 139:866–872. https://doi.org/10.1016/j.jclepro.2016.08.111
Acknowledgements
The authors would like to thank the Natural Science Foundation of Shanxi Province (2020JM-236) and the Fund Project of Shanxi Key Laboratory of Land Consolidation (2018-ZD04) for funding this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Li, Y. Selective flotation separation of polycarbonate from plastic mixtures based on Fenton treatment combined with ultrasonic. J Mater Cycles Waste Manag 24, 917–926 (2022). https://doi.org/10.1007/s10163-022-01367-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01367-z