Abstract
Azohydromonas lata DSM 1122 was utilized to synthesize short-chain-length (scl-) polyhydroxyalkanoate (PHA) terpolymers containing 3-hydroxybutyric (3HB) acid, 3-hydroxyvaleric (3HV) acid, and 4-hydroxyvaleric (4HV) acid from mixtures of glucose (GLC; 1 wt%) and levulinic acid (LevA; 0–0.4 wt%). LevA media concentrations greater than 0.4% completely inhibited cellular growth. At LevA concentrations ≤ 0.4%, the 3HV polymer content remained constant (3–5 mol%). The 4HV content was two-fold higher in the polymers derived from the 0.2% LevA-containing cultures reaching a maximum of 9 mol% (vs. 4 mol% in the 0.4% LevA-containing cultures). Polymer molecular weights (based on number-average molecular weight, Mn) were smallest (Mn = 240,000 g/mol) when synthesized in the presence of 0.2% LevA. At 0.4% LevA and at 1% GLC the average Mn values were 43% and 87% larger than the polymers synthesized in the presence of 0.2% LevA, respectively. Mixed-cultures containing A. lata and Burkholderia sacchari DSM 17165, a known poly-3-hydroxybutyrate-block-3-hydroxyvalerate (P3HB-block-3HV) producer, using LevA media concentrations ≤ 0.4% and staggered inoculations resulted in scl-PHA polymer mixtures with improved tensile properties. The results of this study show that LevA can be utilized in combination with simple sugars to produce unique scl-PHA terpolyesters and scl-PHA mixtures with enhanced properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are quickly becoming a major concern around the world due to problems associated with their disposal. Recycling is one approach for plastic disposal but it is estimated that only about 9% of all plastics end up being effectively reprocessed [1]. That means that greater than 90% of the world’s plastic is disposed of by some other means. The sad fact is that much of these potentially reusable plastics end up in landfills and in the world’s waterways causing immense pollution problems. In fact, projections indicate that approximately 8 million metric tons of plastic waste enters the oceans annually [2]. One needs to look no further than the so-called ‘Great Pacific Garbage Patch’ that is currently swirling in the Pacific Ocean to see the ecological consequences of indiscriminant plastic disposal.
There are three potential approaches to reduce the amount of plastic waste in the biosphere. The first is to reduce or eliminate the use of recalcitrant plastics, particularly single-use plastics. In fact, many cities and countries around the world have initiated steps to control the use of single-use plastic by either instituting taxes or levies on them or by outright banning their use [3]. The second approach is through the scientific discovery of unique enzymes capable of efficiently breaking down commonly-used plastics, particularly poly(ethylene terephthalate) (PET) which is the most abundant petroleum-based polymer used in single-use plastics. In 2016 it was reported that the bacterium Ideonella sakaiensis 201-F6 produces an enzyme (PETase) that catalyzes the breakdown of PET [4]. A subsequent study characterized the 3-dimensional structure of the PETase, engineered the enzyme for improved PET degradation, and revealed that the enzyme could also degrade the PET replacement polyethylene-2,5-furandicarboxylate [5]. Unfortunately, the large-scale use of enzymes in plastic degradation (particularly in aqueous/marine environments) is hindered because of their low rates of reaction, salt-tolerance- and dilution- issues. A third approach is through the use of ‘enviromentally-benign’ plastic substitutes. Many of these materials can be produced through living systems either by whole-cell or enzymatic catalysis and are generally considered biocompatible, biodegradable, and biorenewable. The problem with this approach is that historically bio-based plastics have been prohibitively more expensive to produce than petroleum-based plastics, but faced with the choice of higher price vs. complete elimination of potential applications, bio-based plastic alternatives may be a favorable option provided their properties can be controlled to mimic those of petroleum-based plastics.
Polyhydroxyalkanoates (PHA) are one class of possible ecofriendly plastic substitutes. They are bacterial polyesters that are produced as carbon and energy reserve materials by a variety of bacterial species. These biopolymers are known to biodegrade to carbon dioxide and water in both terrestrial and aqueous/marine environments [6] and depending on the metabolic capabilities of the producing bacterial strain, they can be synthesized with either thermoplastic (short-chain-length PHA; scl-PHA) or elastomeric (medium-chain-length PHA; mcl-PHA) properties [7].
Typically, PHA synthesis occurs when surplus utilizable carbon is available but cellular metabolism is stunted by the lack of other vital nutrients. Mcl-PHA is typically synthesized by members of the genus Pseudomonas and is generally composed of 3-hydroxyalkanoic (3HA) acids ranging in length from 6 (3-hydroxyhexanoate; 3HHx) to 14 (3-hydroxytetradecanoate; 3HTD) carbons and whose side chains may be unsaturated and/or substituted with a host of chemical groups including but not limited to methyl, short-chain esters, phenoxy, cyanophenoxy, nitrophenoxy, epoxy among others [8]. Unfortunately, the elastomeric qualities in mcl-PHA come at a cost as the tensile strength and modulus are normally reduced in these polymers which can be problematic depending on the intended application. In contrast, scl-PHA is generally composed of 3HA acids with chain lengths ranging from 3 (3-hydroxypropionate; 3HP) to 5 (3-hydroxyvalerate; 3HV) carbons. Poly-3-hydroxybutyrate (P3HB) is the most well-known of the scl-PHA polymers but is stiff, brittle and challenging to process making it a less favorable option for widespread use. In order to improve the properties of scl-PHA, copolymers composed of 3-hydroxybutyric (3HB) acid, and 3-hydroxyvaleric (3HV) acid [9], 4-hydroxybutyric (4HB) acid [10] or 4-hydroxyvaleric (4HV) acid [11, 12] have been synthesized. These copolymers have markedly improved mechanical properties over P3HB but the precursor carbon substrates for these syntheses are comparatively more expensive and the yields lower than for petroleum-based polymers, thus hindering the cost differential between petroleum-based plastics and PHA plastics.
In order to help the economics of PHA biosynthesis, less expensive feedstocks have been the focus over the past few years. Carbon sources such as soy molasses [13], wheat hydrolysate [14,15,16] and crude glycerine [17,18,19] have all been tested as primary feedstocks for both mcl- and scl-PHA production, but in those instances where scl-PHA was the goal, a viable secondary carbon source was required to produce scl-PHA copolymers.
Levulinic acid (LevA; 4-oxovaleric acid) is an inexpensive molecule. In 2000, Bozell et al. predicted that the production costs of LevA could go as low as between $0.04 and $0.10/lb [20] depending on the scale of the operation. LevA can be produced by acid-catalysis from both 5- and 6-carbon sugars [21] and since it is a structural analogue to pentanoic acid and can be easily synthesized from lignocellulosic biomass, it has been assessed and successfully applied as a supplemental substrate in PHA biosynthesis. In 2011, Jaremko and Yu suggested a metabolic pathway for LevA in Cupriavidus necator [22]. This pathway demonstrated the possible enzymatic reactions responsible for the production of 3-hydroxybutyryl-CoA (3HB-CoA) and 3-hydroxyvaleryl-CoA (3HV-CoA), precursors for scl-PHA polymers containing 3HB and 3HV monomers, respectively. Evidence from previous studies has suggested that certain bacterial strains can, in fact, only synthesize 3HB and 3HV monomers from LevA. In one report, Keenan et al. reported that Burkholderia cepacia ATCC 17,759 could only synthesize P3HB-co-3HV when grown on xylose and LevA [23], while in a second study, Koller et al. reported that the same thing occurred when Hydrogenophaga pseudoflava DSM 1034 was grown on mixtures of whey permeate and LevA [24]. Interestingly, other bacterial strains have been reported to synthesize scl-PHA terpolyesters containing 3HB, 3HV, and 4HV. Valentin et al. reported the synthesis of scl-PHA terpolyesters in various strains of Alcaligenes eutrophus, Alcaligenes xylosoxidans ssp. denitrificans, and Pseudomonas oxalaticus when grown on 4-hydroxyvaleric acid or 4-valerolactone [11]. In that report, the 4HV content within the terpolyesters never exceeded 6.3 mol% which indicated that even when grown on closely related carbon substrates, these bacterial strains could only assimilate a small portion into the elongating polymer chains. The same phenomenon was reported by Gorenflo et al. for wildtype Ralstonia eutropha HF 39 (4HV content of 2.1 mol%) and Pseudomonas putida KT2440 (4HV content of 0.8 mol%). Recombinant strains of these same organisms gave increased 4HV polymer contents to as high as 30 mol% in recombinant P. putida [12]. Unfortunately, the metabolic pathway postulated by Jaremko and Ju [22] did not account for the synthesis of 4-hydroxyvaleryl-CoA (4HV-CoA), the precursor for 4HV production. A subsequent paper by Rand et al. did account for the formation of 4HV-CoA from the metabolism of LevA. In that paper it was demonstrated that in P. putida KT2440 the lvaRABCDEFG operon (9,323 bp) was responsible for the formation of both 3HV-CoA and 4HV-CoA intermediates making possible the synthesis of 3HV- and 4HV-containing scl-PHA polymers [25].
In this paper we demonstrated that under suitable growth conditions Azohydromonas lata DSM 1122 can synthesize terpolymers of 3HB, 3HV, and 4HV from mixtures of glucose and LevA. A. lata, when used in mixed-cultures with Burkholderia sacchari DSM 17165 (a known producer of poly-3-hydroxybutyrate-block-3-hydroxyvalerate (P3HB-block-3HV) from mixtures of glucose, xylose, and LevA, [26]), could produce scl-PHA polymer mixtures with enhanced tensile properties. The use of inexpensive carbon feedstocks like LevA to produce unique scl-PHA copolymers with enhanced mechanical properties may make these environmentally friendly alternatives more appealing for use as substitutes for petroleum-based plastics and reduce the amount of plastic waste present in the environment.
Materials and Methods
Materials
Azohydromonas lata (A. lata; formerly classified as Alcaligenes latus) DSM 1122 and Burkholderia sacchari (B. sacchari) DSM 17165 were obtained from the Leibniz-Institut DSMZ—Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). Stock cultures of both bacterial strains were prepared as glycerol stocks as described previously [27]. All simple salts were purchased from Sigma–Aldrich Chemical Company (St. Louis, MO), Fisher Scientific (Fair Lawn, NJ) or Mallinckrodt Baker Inc. (Phillipsburg, NJ). D-(+)-glucose (≥ 99.5%), D-(+)-xylose (≥ 99%), and chloroform-d (99.8 atom %, containing 0.03% (v/v) tetramethylsilane; TMS) were purchased from Sigma–Aldrich Chemical Company. Bacto™ tryptone and Bacto™ yeast extract were obtained from Becton Dickinson (Sparks, MD). Levulinic acid (LevA; 98+%) was purchased from Acros Organics (Geel, Belgium). All organic solvents were HPLC grade and purchased from Honeywell Burdick and Jackson (Muskegon, MI) or Fisher Scientific (Fair Lawn, NJ).
Bacterial Cultivation Conditions
Azohydromonas lata Pure-Culture Experiments
All PHA-production experiments were conducted in triplicate in shake-flask cultures (1-L volumes contained in 2-L Erlenmeyer flasks) using simple salts media. The basal salts media was comprised of the following in g/L: Na2HPO4, 3.7; KH2PO4, 1.7; (NH4)2SO4, 1.1; MgSO4 × 7 H2O, 0.2; ferric ammonium citrate, 0.07; CaCl2 × 2 H2O, 0.01 and 1.1 mL of microelement solution consisting of the following in 1 L of 0.1 N HCl: H3BO3, 0.3 g; CoCl2, 0.1 g, ZnSO4 × 7 H2O, 0.2 g, MnCl2 × 4 H2O, 0.03 g; Na2MoO4 × 2 H2O, 0.4 g; NiCl2 × 6 H2O, 0.02 g, CuSO4 × 5 H2O, 0.01 g. Simple salts basal media was initially prepared in 4-L volumes and 950 mL of this media was transferred into four separate 2-L Erlenmeyer flasks containing either 0, 2 g, 4 g, or 6 g of LevA. The pH of each production flask was adjusted to between 6.9 and 7.0 using sodium hydroxide pellets and each flask was autoclaved at 121 °C for 15 min to sterilize. Once each flask had cooled to room temperature, 50 mL of a 20% filter sterile stock solution of glucose (GLC) was aseptically added to obtain 1 L final media volumes with 0, 0.2, 0.4, or 0.6 wt% LevA contents.
The inoculum for each production flask (described above) was prepared by aseptically transferring 1.5 mL of A. lata DSM 1122 from a frozen glycerol stock culture into 50 mL of sterile Luria–Bertani (LB; 1% tryptone, 0.5% NaCl, 0.5% yeast extract) broth (in a 125-mL Erlenmeyer flask) and incubating the culture at 30 °C and 200 rpm in a shake incubator. After 24 h, the entire 50-mL volume was aseptically poured into a new sterile 200-mL LB broth culture (total 250 mL; in a 500-mL Erlenmeyer flask) and incubated as described above. After an additional 24 h, four separate 40-mL aliquots were removed from the LB culture and aseptically placed into separate sterile centrifuge tubes. The tubes were centrifuged (10,400 × g; 15 min; 4 °C) and the supernatants were discarded. Each cell pellet was aseptically suspended in 10 mL of sterile medium from one of the production flasks and each suspension was used as the inoculum for the respective 1-L production flasks. The production flasks were placed into the shake incubator at 30 °C and 200 rpm. 100-mL aliquots were aseptically harvested from each flask at 24 h intervals (up to 96 h) by centrifugation (conditions described above).
Azohydromonas lata/Burkholderia sacchari Mixed-Culture Experiments
The mixed-culture experiments were also conducted in triplicate using the same basal production media and culture conditions as described in the previous section with minor exceptions. It was previously reported that B. sacchari DSM 17165 was capable of utilizing xylose as a co-substrate for scl-PHA biosynthesis [26]. As such, the mixed-culture experiments were conducted utilizing a carbon source ratio of 1 wt% GLC:1 wt% xylose (XYL):0-0.4 wt% LevA. The basal simple salts medium was identically prepared in 2 separate 6-L volumes as described in the previous section and 900 mL of that bulk media was portioned into 12 separate 2-L Erlenmeyer flasks containing 0 (4 flasks), 2 g (4 flasks), or 4 g (4 flasks) of LevA (total 12 production flasks). The pH in each flask was adjusted to between 6.9 and 7.0 as previously described. Once sterilized and cooled, 50 mL of 20% filter-sterile stock solutions of GLC and XYL were aseptically added to each production flask to obtain 1-L final media volumes with either 0, 0.2, or 0.4 wt% LevA contents.
Staggered inoculation/growth patterns were used to induce variation in the recovered scl-PHA blends. All production cultures were grown for 72 h. A. lata was inoculated at the onset of each shake-flask culture and B. sacchari was inoculated at 24 h intervals resulting in A. lata:B. sacchari time/growth ratios of 72 h:72 h, 72 h:48 h, 72 h:24 h, and 72 h: 0 h (Table 1). The inocula for each production flask were prepared by initially transferring 1.5 mL of A. lata and 1.5 mL of B. sacchari from frozen cryovials into separate 125-mL Erlenmeyer flasks containing 50 mL of LB broth with incubation as described previously. At 24 h, all 50 mL from each overnight LB culture was aseptically poured into separate 1-L Erlenmeyer flasks containing 500 mL of fresh LB media (total volume = 550 mL) and the newly-inoculated flasks were incubated under the same conditions. After another 24 h, 12 separate 40 mL aliquots of overnight A. lata cells and 3 separate 40 mL aliquots of overnight B. sacchari cells were aseptically transferred to separate sterile centrifuge tubes and centrifuged (10,400 × g; 15 min; 4 °C). The supernatants were discarded and the pelleted cells were suspended in production media (A. lata cells were separately suspended in 10 mL of production media from all 12 production flasks while the B. sacchari cells (3 tubes) were suspended in 10 mL of production media from Set 1 (72 h:72 h time/growth ratio) of the production flasks including 0, 0.2, and 0.4 wt% LevA). The cells were then reintroduced back into the respective PHA production flasks and incubated as described previously. At the same time, 50 mL of overnight B. sacchari LB culture was aseptically added to a new 1-L Erlenmeyer flask containing 500 mL of fresh LB broth and incubated at 30 °C, 200 rpm. After an additional 24 h, the same procedure was carried out from the overnight culture and B. sacchari was inoculated into the second set (72 h:48 h time/growth ratio) of production flasks. This procedure was performed once more and the B. sacchari cells were inoculated into the third set (72 h:24 h time/growth ratio) of production flasks. The fourth set of production flasks (72 h:0 h time/growth ratio) were not inoculated with B. sacchari. At 72 h the entire 1-L volumes were harvested by centrifugation as described previously. At the conclusion of all the shake-flask experiments the cultures were processed to determine cell dry weight CDW), polymer concentration, carbon source utilization, and polymer characterization.
Carbon Source Utilization, Cell Growth, and Polymer Yield
Carbon source (GLC, XYL, LevA) utilization from both the pure-culture and mixed-culture experiments was determined by High Performance Liquid Chromatography (HPLC) of culture supernatants while cell growth and PHA concentrations were measured gravimetrically. Upon harvesting, cells were centrifuged as previously described and 3-mL volumes of the supernatants were removed and sequentially filtered through separate 0.45-µm and 0.22-µm nylon filters into separate, clean auto-sampler vials. An Agilent 1200 series HPLC instrument equipped with an Aminex HPX-87H ion exclusion column (Bio-Rad, Hercules, CA) and a refractive index detector was used for all analyses. The column was maintained at 60 °C. A 5-µL injection volume was used and eluted with 5 mM sulfuric acid (prepared in HPLC-grade water) at a flow rate of 0.6 mL/min. Data were processed using the Agilent ChemStation software. The instrument was calibrated with GLC, XYL, and LevA standards. Peaks from the unknowns were identified by comparative retention times. Each sample (n = 3) was injected twice (total injections per growth condition = 6) and the results were averaged to obtain the final residual carbon source concentrations in each flask.
Dry cell weights were determined from the cell pellets derived from each shake-flask culture after centrifugation. In all cases, cells were washed twice with deionized water, re-centrifuged and the water fractions discarded. The cell pellets were frozen, lyophilized to dryness and accurately weighed. Polymer concentrations were determined from the chloroform-extracted cell masses. The lyophilized cells were extracted for at least 24 h in excess chloroform at 30 °C and shaking at 250 rpm. Cellular material was removed by vacuum filtration through Whatman #2 filter paper. The chloroform was evaporated under vacuum to give the crude polymers. Each crude polymer was dissolved in a small volume of chloroform and precipitated (3×) by dropwise addition into cold methanol. The polymers were placed into separate tared vials, dried in vacuo for at least 24 h and weighed. Polymer concentration was determined by weight difference. Volumetric productivities for both cell growth (Qx) and PHA concentration (Qp) were calculated as described in a previous publication using an initial dry cell mass of 0.30 ± 0.05 g/L [28].
Polymer Characterization
Repeat unit compositions were determined for each polymer isolate by Proton—Nuclear Magnetic Resonance Spectroscopy (1H-NMR) as described in a previous publication [26]. In short, solution-state NMR spectra were recorded at 14.1 T on an Agilent DD2 NMR Spectrometer using a 5-mm OneNMR probe with Z-axis pulsed field gradients operating at 25 °C. All samples were dissolved in deuterated chloroform (CDCl3) with tetramethylsilane (TMS) added as an internal reference. The proton spectra, at 600 MHz, had a sweep-width of 5387 Hz and were acquired with a 45-degree pulse angle and a 1 s relaxation delay. Compositions from replicate polymers derived from identical culture conditions (n = 3) were averaged and reported.
Thermal properties were determined for each polymer isolate (n = 3) using a Perkin Elmer Pyris 1 (Norwalk, CT) differential scanning calorimeter (DSC) that was calibrated using indium (Tm = 156 °C) and cyclohexane (transition temperatures at − 87 °C and 6 °C). Each PHA polymer sample was accurately weighed (~ 5 mg) and sealed in separate aluminum pans. The heating profile was as follows: (1) equilibration at 25 °C for 2 min, (2) heating to 200 °C at 10°C/min to eliminate thermal history, (3) isothermal step at 200 °C for 2 min, (4) cooling to − 50 °C at 100°C/min, (5) isothermal step at − 50 °C for 2 min, (6) reheat to 200 °C at 10°C/min. All thermal transitions were determined based on the second heat and the averages from replicate polymers were reported. The glass transition temperatures (Tg) were measured as midpoint temperatures, while the melting temperatures (Tm) and crystallization temperatures (Tc) were measured as peak temperatures from the melting endotherms and crystallization exotherms, respectively.
The molecular weights of the polymers were measured by gel permeation chromatography (GPC) using an Agilent Technologies (Santa Clara, CA) modular GPC system. A PLgel 10 µm MIXED-B (300 × 7.5 mm) column and a PLgel 5 µm MIXED-C (300 × 7.5 mm; Agilent Technologies) column were arranged in series and a calibration curve was generated using polystyrene standards (Polymer Laboratories, Amherst, MA) ranging in molecular weight (Mp) from 580 to 3.05 × 106 g/mol and narrow (≤ 1.13) polydispersities. Replicate PHA samples (n = 3) derived from identical culture conditions were dissolved in HPLC grade chloroform at a concentration of 0.5 mg/mL. The samples were filtered through a 0.45 µm nylon syringe filter and injected into the system with an injection volume of 100 µL. The column oven was kept at 40 °C and the flow rate was 1 mL/min. All data was processed using the Cirrus™ GPC/Multidetector Software (Agilent Technologies). Averages were determined from the replicate samples and reported.
Tensile properties were measured using solution cast films that were cast in aluminum dishes (dia. = 7.5 cm) from chloroform (250 mg PHA/10 mL CHCl3) solutions. Five individual measurements were made from each polymer film cast from the polymers derived from each culture condition performed in triplicate (total = 15 measurements/polymer). Once cast, the films were allowed to air-dry for at least 24 h and were then carefully cut into strips (width = 7 mm). After incubation in an environmental chamber with relative humidity of 50 ± 5% for 24–48 h, an upgraded Instron mechanical property tester, model 1122 (Instron, Norwood, MA), and Testworks-4 data acquisition software (MTS Systems Corp., Minneapolis, MN) were used to measure tensile strength (MPa), elongation at break (%), Young’s Modulus (MPa), and fracture energy (J/cm3). All samples were measured with a sample length of 2 cm between the grips and a crosshead speed of 5 cm/min. All measurements were made in a conditioning room set at 23 ± 2 °C and 50 ± 5% relative humidity.
Results and Discussion
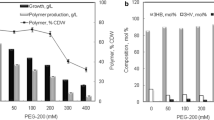
In an effort to reduce the amount of plastic pollution in the world, scientists have been actively engaged in the synthesis and characterization of carbon–neutral biopolymers that can be applied in various applications as petroleum-based plastic substitutes. Unfortunately, successes in this area of research have been thwarted to some extent by the high costs associated with bio-based syntheses. To overcome this obstacle, many inexpensive feedstocks have been tested for their applicability in biopolymer production. LevA is one such molecule. Relatively few past studies have documented the synthesis of scl-PHA polymers that contain 4HV [11, 12]. In this study we utilized LevA as a co-substrate for the synthesis of unique PHA biopolymers using a previously undocumented LevA-utilizing bacterial strain. Azohydromonas lata, when grown in pure-culture in the presence of 1% GLC and 0-0.4% LevA, was capable of cell growth and scl-PHA polymer synthesis (Table 2). In the absence of LevA, cell growth was initiated earlier in the growth cycle resulting in CDWs of 4.6 and 5.0 g/L at 48 and 72 h, respectively. In fact, under these growth conditions, the GLC was entirely exhausted by 72 h (Fig. 1). At the same time, higher PHA concentrations (1.8 and 1.7 g/L) were achieved from those cell masses resulting in PHA/cell content values of 39.1 and 34.0%, respectively. In contrast, when LevA was introduced into the media, the rate of cell growth and PHA production was reduced but higher maximal PHA concentrations were achieved albeit, later in the growth cycle. Specifically, a 0.2% LevA media concentration lead to a maximum CDW of 4.8 g/L and PHA concentration of 2.0 g/L after 96 h signifying a maximal PHA/cell content of 41.7% which was the highest value achieved under the growth conditions tested. At LevA media concentrations of 0.4 and 0.6% very little-to-no cell growth or polymer synthesis occurred (Table 2). These results are supported by the carbon utilization patterns (Fig. 1). At a carbon source ratio of 1% GLC:0.2% LevA, the GLC was completely used up by 96 h while only 59% (1.2 g) of the available LevA was consumed. In comparison, at a carbon source ratio of 1% GLC:0.4% LevA, only 55% of the GLC and 31% (1.3 g) of the LevA was used after 96 h. Interestingly, equal amounts of LevA were utilized on a weight-basis from both the 0.2 and 0.4% LevA-containing cultures which indicated that GLC metabolism was the limiting factor between relatively large cell growth and smaller cell growth results.
Carbon source utilization by pure-cultures of Azohydromonas lata DSM 1122 (n = 3) grown on mixtures of 1% GLC:0 LevA (GLC, Black filled circle; LevA, open circle), 1% GLC:0.2% LevA (GLC, Black inverted triangle; LevA, open triangle), and 1% GLC:0.4% LevA (GLC, Black filled square; LevA, open square). Error bars represent ± standard deviation
The 1H-NMR analyses of the PHA polymers produced from pure-cultures of A. lata are shown in Fig. 2. Integration of the resonances located between 4.9 and 5.3 ppm corresponding to the methine protons of 3HB (5.25 ppm), 3HV (5.16 ppm) and 4HV (4.91 ppm) showed that the polymers derived from GLC alone were composed solely of 3HB. As LevA was introduced into the cultures scl-PHA polymers were synthesized that contained low levels of both 3HV and 4HV components. Interestingly, the highest average content for non-3HB components (13 mol%) was realized when 0.2% LevA was present in the culture media as opposed to 0.4% LevA which resulted in polymers containing only 8 mol% non-3HB components (Table 3). These compositional deviations caused variation within the polymers with respect to their thermal properties. The polymers produced in the presence of 0.2% LevA exhibited Tm values of 150 °C regardless of the culture times, but the energy required to melt the samples (melting enthalpies; ΔHm) decreased as the culture times prolonged. In addition, the crystallization temperatures (Tc), energies involved in crystallization (ΔHc), and glass transition temperatures (Tg) all varied based on culture times. This indicated that the crystal dynamics within the polymers were different and dependent upon the length of the cultures, even when the compositions were relatively consistent. Molecular weight data revealed that the scl-PHA polymers synthesized in the presence of 0.2 and 0.4% LevA were on average 46% and 23% smaller (based on Mn data) respectively, than the P3HB homopolymers synthesized in the absence of LevA (Table 3).
In a previous report we documented the ability of Burkholderia sacchari DSM 17165 to produce block copolymers of 3HB and 3HV from mixtures of GLC, XYL, and LevA [26]. In that study it was noted that the strain could grow and produce scl-PHA polymers at LevA concentrations as high as 0.8 wt% and that the LevA and carbohydrates present in the media were preferentially utilized (LevA > carbohydrate) resulting in controlled 3HB:3HV ratios and compositionally distinct block regions. Specifically, the 3HV content of the resulting copolymers was higher after 24 h than after 96 h, supporting the notion that LevA was preferred by the bacterial strain early in the culture. Once LevA was used, the strain could then metabolize the carbohydrate portion of the media. Since B. sacchari was proven to make these block copolymers from the same culture media and under the same growth conditions as A. lata DSM 1122, it was decided to utilize both of these bacterial strains in mixed-cultures to produce scl-PHA polymer blends from 1% GLC:1% XYL:0 to 0.4% LevA. Xylose was introduced into the media because B. sacchari was capable of metabolizing XYL to produce scl-PHA and although A. lata cannot use XYL, it was thought that incorporating XYL into the media would aid in the production of specific polymer blends and establish B. sacchari growth compared to A. lata growth within the mixed-cultures. The LevA media concentrations (0–0.4 wt%) were chosen because both bacterial strains were capable of growth at these LevA concentrations (B. sacchari demonstrated in the previous study and A. lata determined from the pure-culture experiments described in this paper; Table 2). In order to introduce compositional variation into the system, a staggered inoculation pattern was used to allow bacterial growth of each strain for different lengths of time. A. lata was inoculated into all of the cultures at the onset of bacterial growth while B. sacchari was introduced into separate cultures at 24 h intervals (see “Materials and Methods” section and Table 1).
The CDWs, PHA polymer concentrations and PHA/cell content results for the mixed-cultures are shown in Table 4. When both bacterial strains were present in the media for 72 h (72 h:72 h samples), the lowest CDWs and PHA concentrations occurred in the absence of LevA. This was somewhat surprising. LevA has been reported to be toxic to some bacterial strains [29] while others seem to be able to withstand the toxic effects of LevA at media concentrations greater than 20 g/L [30]. In this study, the LevA in the media seemed to stimulate cell growth and polymer synthesis. In these mixed-cultures maximum CDWs and PHA polymer concentrations were obtained from the 72 h:72 h and 72 h:48 h cultures containing media with 0.2% LevA. At 0.4% LevA the CDWs and polymer concentrations decreased in all of the mixed-cultures (Table 4), which was expected based on the reduced cell growth and polymer production results from the pure-cultures of A. lata at 0.4% LevA (Table 2). These results were supported by the specific carbon source utilization results shown in Table 5. Interestingly, in all of the 72 h:72 h, 72 h:48 h, and the 72 h:24 h cultures some utilization of XYL was evident. A. lata does not possess the metabolic traits for XYL utilization (see Table 5, 72 h:0 h cultures) and therefore, any XYL utilization would signal B. sacchari growth within the mixed cultures. In the LevA-containing 72 h:72 h cultures almost all of the LevA was used up after 72 h. Logically, if both strains were growing and producing scl-PHA polymer equally well under these culture conditions, one would expect some 4HV present in the resulting polymers. 1H-NMR analysis did not show any evidence of 4HV in these polymers (Table 6). In addition, the polymers produced from the 72 h:72 h cultures on 0.2 and 0.4% LevA both showed 2 Tg’s, one at approximately − 11 to − 15 °C (close to poly-3-hydroxyvalerate; P3HV) and one at 3 °C (same as P3HB). These results, taken together, indicate that when both bacterial strains were inoculated into the production flasks containing LevA at the onset of the cultures (72 h:72 h), B. sacchari outcompetes A. lata to produce primarily P3HB-block-3HV copolymers. In contrast, the results obtained from the 72 h:48 h and 72 h:24 h growth patterns showed relatively good cell growth and PHA concentrations but once again the maximum cell growth and polymer concentrations were realized at LevA media concentrations of 0.2% (Table 4). In these cases, the PHA/cell content values were calculated to between 39 and 53% indicating that under these culture conditions cell growth and polymer yields were near maximum. These results were once again supported by carbon source utilization data. In the 72 h:48 h cultures all of the GLC and LevA were consumed in the cultures containing 0 and 0.2% LevA. At 0.4% LevA, XYL utilization (0.54%) was more pronounced while some GLC was still present after 72 h. The 72 h:24 h cultures once again showed that the GLC media concentrations were essentially 0 upon harvesting the cultures containing 0 and 0.2% LevA. The polymers produced from the 72 h:48 h and 72 h:24 h cultures containing LevA exhibited polymer compositions containing small amounts of 4HV along with reduced Tm and ΔHm values. In addition, the 0.4% LevA cultures showed multiple Tg values (Fig. 3). These data taken together indicate the presence of PHA terpolymer (composed of 3HB, 3HV, and 4HV):P3HB-block-3HV polymer mixtures with reduced crystallinities. The last series of cultures (72 h:0 h) contained no B. sacchari. The CDWs, polymer concentrations and PHA/cell content values resembled those obtained in the A. lata pure-cultures described previously (Table 2). As expected, these cultures showed no XYL utilization and reduced LevA utilization. The polymers synthesized under these conditions were composed of 88–90% 3HB, 4–5% 3HV, and 6–7% 4HV, similar to the pure-culture results reported earlier in this study, and exhibited a reduced Tm and only a single Tg value of 1–2 °C. The lack of a B. sacchari inoculum indicates that the scl-PHA polymers obtained from these cultures were not mixtures but were in fact synthesized solely by A. lata.
One of the challenges in producing biopolymers with good tensile properties is maintaining tensile strength while reducing modulus and improving elongation. Typically, elastomeric polymers suffer from reduced tensile strength so finding polymers that can stretch yet maintain some physical strength can be a challenge. One way that these goals have been met is through the synthesis of co-polymeric PHA. The most well-known PHA copolymer is the P3HB-co-3HV copolymer which demonstrates isodimorphic behavior at various 3HV contents [31]. Another well-studied copolymer that has been particularly interesting based on its tensile properties is P3HB-co-4HB [32]. In both of these cases co-polymeric PHAs were synthesized that improved the crystallization behavior and hence the tensile properties. In an effort to further improve the tensile properties among scl-PHA polymers, we synthesized natural polymer blends using mixed-culture strategies. The blends contained both terpolyesters composed of 3HB, 3HV, and 4HV as well as block copolymers composed of 3HB and 3HV. The results of those tensile property studies are shown in Fig. 4. The polymers produced using the 72 h:72 h and 72 h:0 h growth patterns originated primarily from B. sacchari and A. lata, respectively and therefore were not apparent mixtures. The 1:1:0 culture and the 1:1:0.4 culture from the 72 h:72 h and the 72 h:0 h growth patterns, respectively did not result in sufficient polymer to test. The P3HB homopolymers produced in the absence of LevA showed increasing tensile strength (between 23 and 30 MPa; Fig. 4a) and modulus (between 1041 and 1480 MPa; Fig. 4b). These results showed that the P3HB homopolymers were stronger and more brittle as B. sacchari spent less time in the mixed-cultures. The PHA polymer mixtures obtained from the 72 h:48 h, and the 72 h:24 h cultures at 0.2 and 0.4% LevA showed slightly decreased tensile strengths and moduli when compared to the polymers obtained from the individual bacterial strains in the 72 h:72 h and 72 h:0 h cultures. These results show that while tensile strength may be slightly reduced, they still maintain sufficient strength to merit further study. A reduced modulus is favorable in that these results indicate that the polymers from the mixed-cultures are more malleable than the stiff, brittle P3HB homopolymers.
Tensile property results (n = 5 measurements/polymer film derived from each culture condition using shake flask cultivation performed in triplicate; total = 15 measurements/polymer) for the PHA polymers derived from the 72 h:72 h, 72 h:48 h, 72 h:24 h, and 72 h 0 h mixed-cultures of A. lata and B. sacchari. Tensile strength (a), Modulus (b), Elongation (c), and Fracture energy (d) are shown for the polymers made in the presence of 1% GLC:1% XYL:0 LevA (Black filled square), 1% GLC:1% XYL:0.2% LevA (light grey filled square), 1% GLC:1% XYL:0.4% LevA (dark grey filled square). Error bars represent ± standard deviation. Different letters on bars within each graph indicate significant differences (P < 0.05)
Interestingly, the elongation and fracture energy from the polymers derived from all of the inoculation patterns showed comparable trends. Elongation values of the polymers derived from LevA-containing cultures were higher than the P3HB homopolymers but those copolymers/polymer mixtures derived from the 72 h:72 h, 72 h:48 h, and 72 h:0 h cultures showed maximum elongation values that were less than 50%. In contrast, the polymer mixtures derived from the 72 h:24 h cultures showed average elongations of 238% and 177% for the polymers produced in the presence of 0.2 and 0.4% LevA, respectively. These values were 349% and 378% higher than the polymer mixtures produced from the 72 h:48 h cultures containing 0.2 and 0.4% LevA (Fig. 4c). Fracture energy is defined as the energy required to open a unit area of crack surface and does not depend on the size of the structure (for a good review on fracture energy of elastomeric materials see reference [33]). In this study, the fracture energies of the polymers derived from LevA-containing cultures were greater than the P3HB homopolymers derived from the cultures in the absence of LevA. This was expected in that P3HB homopolymers are well-known to be relatively brittle materials. Once again, the polymers produced in the presence of LevA from the 72 h:72 h, 72 h:48 h, and 72 h:0 h cultures showed fracture energies that were between 3.3 and 9.9 J/cm3. The fracture energies from the polymer films derived from the 72 h:24 h cultures containing LevA were 276% and 297% greater than the polymers produced under identical culture conditions from the 72 h 48 h cultures (Fig. 4d).
In conclusion, wildtype A. lata was demonstrated to be capable of utilizing LevA as a co-substrate for the synthesis of bacterial terpolyesters composed of 3HB, 3HV, and 4HV. These terpolymers were unique in that the 4HV content (9%) was the highest so far reported for a wildtype strain grown on LevA. By utilizing A. lata along with B. sacchari, a known 3HB-block-3HV producer, in mixed-culture under staggered growth patterns, it was demonstrated that terpolyester/block copolymer mixtures could be obtained with enhanced tensile properties. As the scientific world explores new options for petroleum-based plastics, it is becoming imperative that the properties of the potential substitutes approach, if not mimic currently used materials and can be produced at competitive prices. Mixed-cultures are one means of controlling polymer properties. By using mixed-cultures polymer blends can be realized whose properties can be controlled based on the culture conditions and by using inexpensive carbon sources such as LevA, these mixtures can be produced more economically. The information presented in this paper demonstrates the feasibility of producing unique scl-PHA polymers and polymer blends and controlling their properties for targeted applications and reduced plastic waste.
References
http://www.economist.com/graphic-detail/2018/03/06/only-9-of-the-worlds-plastic-is-recycled. Accessed 6 Sept 2018
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste input from land into the ocean. Science 347:768–771
https://waste-management-world.com/a/blog-a-taxing-question-for-single-use-plastics-recycling. Accessed 8 Sept 2018
Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K (2016) A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351:1196–1199
Austin HP, Allen MD, Donohoe BS, Rorrer NA, Kearns FL, Silveira RL, Pollard BC, Dominick G, Duman R, El Omari K, Mylhaylyk V, Wagner A, Michener WE, Amore A, Skaf MS, Crowley MF, Thorne AW, Johnson CW, Woodcock HL, McGeehan JE, Beckham GE (2018) Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc Nat Acad Sci USA 115:E4350–E4357
Numata K, Abe H, Iwata T (2009) Biodegradability of poly(hydroxyalkanoate) materials. Materials 2:1104–1126
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128:219–228
Doi Y (1990) Microbial polyesters. VCH Publishers, New York
Kunioka M, Kawaguchi Y, Doi Y (1989) Production of biodegradable copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate by Alcaligenes eutrophus. Appl Microbiol Biotechnol 30:569–573
Valentin HE, Schönebaum A, Steinbüchel A (1992) Identification of 4-hydroxyvaleric acid as a constituent of biosynthetic polyhydroxyalkanoic acids from bacteria. Appl Microbiol Biotechnol 36:507–514
Gorenflo V, Schmack G, Vogel R, Steinbüchel A (2001) Development of a process for the biotechnological large-scale production of 4-hydroxyvalerate-containing polyesters and characterization of their physical and mechanical properties. Biomacromol 2:45–47
Solaiman DKY, Ashby RD, Hotchkiss AT, Foglia TA (2006) Biosynthesis of medium-chain-length poly(hydroxyalkanoates) from soy molasses. Biotechnol Lett 28:157–162
Koutinas AA, Xu YX, Wang R, Webb C (2007) Polyhydroxybutyrate production from a novel feedstock derived from a wheat-based biorefinery. Enz Microb Technol 40:1035–1104
Xu Y, Wang R-H, Koutinas AA, Webb C (2010) Microbial biodegradable plastic production from a wheat-based biorefining strategy. Proc Biochem 45:153–163
Cesario MT, Raposo RS, de Almeida MCMD, van Keulen F, Ferreira BS, Telo JP, da Fonseca MMR (2014) Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Burkholderia sacchari using wheat straw hydrolysates and gamma-butyrolactone. Int J Biol Macromol 71:59–67
Ashby RD, Solaiman DKY, Foglia TA (2004) Bacterial poly(hydroxyalkanoate) polymer production from the biodiesel co-product stream. J Polym Environ 12:105–112
Cavalheiro JMBT, de Almeida MCMD, Grandfils C, da Fonseca MMR (2009) Poly(3-hydroxybutyrate production by Cupriavidus necator using waste glycerol. Proc Biochem 44:509–515
Zhu C, Nomura CT, Perrota J, Stipanovic AJ, Nakas JP (2010) Production and characterization of poly-3-hydroxybutyrate from biodiesel-glycerol by Burkholderia cepacia ATCC 17759. Biotechnol Prog 26:424–430
Bozell JJ, Moens L, Elliot DC, Wang Y, Neuenscwander GG, Fitzpatrick SW, Bilski RJ, Jarnefeld JL (2000) Production of levulinic acid and use as a platform chemical for derived products. Res Conserv Recycl 28:227–239
Antonetti C, Licursi D, Fulignati S, Valentini G, Galletti AMR (2016) New frontiers in the catalytic synthesis of levulinic acid: sugars to raw and waste biomass as starting feedstock. Catalysts 6:196
Jaremko M, Yu J (2011) The initial metabolic conversion of levulinic acid in Cupriavidus necator. J Biotechnol 155:293–298
Keenan TM, Tanenbaum SW, Stipanovic AJ, Nakas JP (2004) Production and characterization of poly-β-hydroxyalkanoate copolymers from Burkholderia cepacia utilizing xylose and levulinic acid. Biotechnol Prog 20:1697–1704
Koller M, Hesse P, Fasl H, Stelzer F, Braunegg G (2017) Study on the effect of levulinic acid on whey-based biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Hydrogenophaga pseudoflava. Appl Food Biotechnol 4:65–78
Rand JM, Pisithkul T, Clark RL, Thiede JM, Mehrer CR, Agnew DE, Campbell CE, Markley AL, Price MN, Ray J, Wetmore KM, Suh Y, Arkin AP, Deutschbauer AM, Amador-Noguez D, Pfleger BF (2017) A metabolic pathway for catabolizing levulinic acid in bacteria. Nat Microbiol 2:1624–1634
Ashby RD, Solaiman DKY, Nuñez A, Strahan GD, Johnston DB (2018) Burkholderia sacchari DSM 17165: a source of compositionally-tunable block-copolymeric short-chain poly(hydroxyalkanoates) from xylose and levulinic acid. Bioresour Technol 253:333–342
Ashby RD, Solaiman DKY, Foglia TA, Liu C-K (2001) Glucose/lipid mixed substrates as a means of controlling the properties of medium chain length poly(hydroxyalkanoates). Biomacromol 2:211–216
Ashby RD, Solaiman DKY, Strahan GD, Zhu C, Tappel RC, Nomura CT (2012) Glycerine and levulinic acid: renewable co-substrates for the fermentative synthesis of short-chain poly(hydroxyalkanoate) biopolymers. Bioresour Technol 118:272–280
Park J-H, Kim S-H, Park H-D, Lim DJ, Yoon J-J (2013) Feasibility of anaerobic digestion from bioethanol fermentation residue. Bioresour Technol 141:177–183
Habe H, Sato S. Morita T, Fukuoka T, Kirimura K, Kitamoto D (2015) Isolation and characterization of bacterial strains with the ability to utilize high concentrations of levulinic acid, a platform chemical from inedible biomass. Biosci Biotechnol Biochem 79:1552–1555
Bluhm TL, Hamer GK, Marchessault RH, Fyfe CA, Veregin RP (1986) Isodimorphism in bacterial poly(β-hydroxybutyrate-co-β-hydroxyvalerate). Macromolecules 19:2871–2876
Saito Y, Doi Y (1994) Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int J Biol Macromol 16:99–104
Ahagon A, Gent AN (1975) Threshold fracture energies for elastomers. J Polym Sci 13:1903–1911
Acknowledgements
The authors gratefully acknowledge Nicole Crocker, Jennifer Thomas, and Nick Latona for their technical assistance throughout the study.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashby, R.D., Solaiman, D.K.Y. & Strahan, G.D. The Use of Azohydromonas lata DSM 1122 to Produce 4-hydroxyvalerate-Containing Polyhydroxyalkanoate Terpolymers, and Unique Polymer Blends from Mixed-Cultures with Burkholderia sacchari DSM 17165. J Polym Environ 27, 198–209 (2019). https://doi.org/10.1007/s10924-018-1332-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1332-2