Abstract
A sustained drug delivery system is developed by using nonionic polymer to formulate drug release rate from silica capsules. To serve this purpose, silica capsules filled with poly(ethylene glycol) (PEG) were incorporated with a veterinary antibiotic drug enrofloxacin (ENF); as a model hydrophobic drug by using a general and facile sol–gel route. The physicochemical properties of the prepared drug-loaded composites were investigated by scanning electron microscope (SEM), nitrogen adsorption, Fourier transform infrared spectroscopy and thermal analysis (TGA). The impact of the media’s ionic strength on the drug release was evaluated over a range of 0–0.4 M to simulate the gastrointestinal feed in two physiological pH conditions. Sodium chloride was applied for ionic concentration adjustment due to its ability to salt out polymers in the midrange of the lyotropic series. Simultaneously, the drug release kinetics was evaluated by fitting experimental data to common empirical (zero-order, first order and Higuchi) and semi-empirical (Ritger–Peppas and Sahlin–Peppas) models. The drug release kinetics from capsules revealed a non-Fickian diffusion and pure relaxation-controlled release. Of these models, Sahlin–Peppas equation best fit the release data of ENF. To determine the best model, non-linear regressions were carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbitrary drug delivery systems prepared with organic or inorganic structures have certain problems such as uncontrolled release, biocompatibility, cytotoxicity, etc. Recent advances in drug delivery applications require more suitable drug carriers which are biodegradable and stimuli-responsive with considerable cytotoxicity to regulate drug release. Hence, the resulting rapid development of organic–inorganic hybrid materials produced nanoscopic matter with an excess of new features [1,2,3,4]. Encapsulation technology of poor water-soluble compounds by polymer-based materials, such as polymer particles and polymer based micelles have attracted significant interest [5,6,7]. Furthermore, mesoporous silica and other forms of silica have been identified as a drastic agent to increase the solubility of active hydrophobic compounds and modulate drug release because of their unique properties for in vivo applications [8,9,10], such as hydrophilic surface suitable to adsorb some functional groups, high surface area, tuneable size (60 nm–10 µm) [11], versatile silane chemistry, ease of surface functionalization and low cost of nanoparticles production [12,13,14]. Polymer micelles and silica capsules have been proven successful as simple carriers for hydrophobic drugs [15,16,17]. Although, there have been no reports of silica capsules enclosing PEG and drug molecules, they may provide significant storage for hydrophobic drugs with biocompatibility and lower toxicity properties. Poly(ethylene glycol) (PEG) is a well-known hydrophilic and non-ionic polymer widely used for drug delivery systems and biomedical devices due to its proven biocompatibility.
The focus of this research is to evaluate the applicability of silica-based encapsulation to biodegradable micelles such as poly(ethylene glycol) (PEG) which are commonly used in drug delivery devices. Enrofloxacin, a well-known veterinary antibiotic drug, was encapsulated into silica-based polymer capsule. Enrofloxacin (ENF), a fluoroquinolones antibiotic with a broad spectrum of activity, has been proven effective in the treatment of the main bacterial processes affecting farm animals [18]. Enrofloxacin was employed as a hydrophobic model drug due to it’s antibacterial activity against a broad spectrum of Gram-negative and Gram-positive bacteria. Various techniques have been used in the preparation of enrofloxacin carrier including capsules, coating with water-insoluble polymers [19, 20], microencapsulation [21], cyclodextrins [22], surface modifications [23], alginate microspheres [24], solid-lipid beads [25], polyvinylpyrrolidone films [26], and silica sol [27]. In this process, silica NPs are formed using a sol–gel method, which is a two step inorganic polycondensation reaction consisting of hydrolysis and condensation. Adding the enrofloxacin molecule, as a hydrophobic drug model during oxide backbone formation assists in it’s encapsulation within the oxide matrix. This results in the production of a composite containing enrofloxacin trapped inside the silica matrix. This combination is a simple route benefitting form the advantages of both systems with their enhanced properties and better design. The physicochemical properties of the drug-loaded capsules are evaluated by scanning electron microscope (SEM), nitrogen adsorption, Fourier transform infrared spectroscopy, and thermogravimetric analysis (TGA). The released data were evaluated by five kinetic models. To identify the best model, error analysis equations were used.
Materials and Methods
Materials
Cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS, ≥ 98%) as a silylation agent, poly (ethylene glycol) (PEG) (Mw = 2000, 10,000), monobasic sodium phosphate (NaH2PO4) and dibasic sodium phosphate (Na2HPO4) were obtained from Sigma Aldrich. Ammonia solution (25%, Scharlau) and ethanol (≥ 99.9%) were obtained from Merck. Enrofloxacin (purity 99.7%) was obtained from Alborzdarou Pharmaceutical Company, Iran. Dissolution buffers were prepared by using sodium phosphate monobasic, two basic crystals, and phosphoric acid for pH 2.8 and 6.4 media.
Preparation of Drug Loading Polymer–Silica Capsule
Enrofloxacin-PEG SiOH (EPS) capsules were fabricated using the modified Stöber method [28]. 100 mg enrofloxacin was dissolved in 100 mL of a 10% (w/v) PEG solution and 0.028 mol/L ammonia solution. Then, 1.5 mL of TEOS was added dropwise to the solution. The solution was stirred at room temperature under ultrasonic agitation to form a clear hydrolyzed sol. After intensive stirring for 30 min, 100 mg of CTAB was added to the sol and agitation continued until colloidal particles appeared. The obtained sol was aged under stationary conditions for 12 h. Following the gelation, the sample was centrifuged and washed by ethanol and deionized water several times to remove impurities. Solid powder was obtained after drying at ambient temperature. For the purpose of comparison, polymer–silica capsule (PS) was synthesized using the same procedure in absence of enrofloxacin.
Characterization Techniques
The morphologies of particles and structure of the prepared capsules were characterized through scanning electron microscopy (SEM). FT-IR spectroscopy was recorded with a Perkin-Elmer Spectrum GX FT-IR spectrometer between 550 and 3900 cm−1 using a KBr wafer.
The actual drug loading was determined with a thermal gravimetric analyzer (TGA). The samples were heated at a rate of 10 °C/min in a stream of nitrogen gas. The calculation of weight loss amount is described as follows. The surface area, pore volume, and pore diameter were characterized using surface area analyzer (Instrument ID BELSORP-mini Ver 2.5.7) and the nitrogen adsorption isotherms. The obtained capsule sample was degassed in a nitrogen atmosphere at 100 °C for 2 h, while the calcined PS sample was degassed at 120 °C for 2 h. According to the (BET) method and experimental data at relative pressure of P/P0, the surface areas were determined and the adsorption peak of N2 isotherms by the Barrett–Joyner–Halenda (BJH) method was applied to calculate pore size distributions.
Enrofloxacin Release Experiments
Drug release studies have been carried out through in vitro experiments under batch conditions. All dissolution studies have been performed using two different dissolution media: simulated gastric fluid (SGF, pH 2.8) and simulated intestinal fluid (SIF, pH 6.4) by adding 10 mg of dried silica capsules containing enrofloxacin in 10 mL of pH 2.8, 6.4 (10 mM) phosphate buffer (PBS) at 37 °C in a test tube. Afterward, the mixtures were placed in a shaking thermostat (N-BIOTEK, NB-300) with a shaking speed of 160 rpm stirring at a predetermined temperature. In order to evaluate the effect of temperature on the drug release rate, a kinetic experiment was performed in simulated intestinal fluid (pH 6.4) at 25 °C. At given time intervals, the samples were centrifuged at 11,000 rpm for 2 min. The amount of enrofloxacin released into the solution was determined using UV–Vis spectrophotometer (Perkin Elmer) at a maximum wavelength of 270 nm with the help of calibration curve.
Error Analysis
To verify the goodness of fit of the most appropriate model with the experimental data of drug release, it is necessary to analyze the data using error analysis. The six error functions employed in this study are coefficient of determination (R2), sum of the square of the error (SSE), residual root mean square error (RMSE), sum of the absolute error (SAE), average relative error (ARE) and average relative standard error (ARS). The smaller the error function value, the better the fit of the curve fit. The expressions of error functions are as follows [29]:
-
(i)
Residual root mean square error (RMSE):
$$RMSE=\sqrt {\frac{1}{{n - 2}}\mathop \sum \limits_{{i=1}}^{n} {{\left( {{q_{\exp }} - {q_{cal}}} \right)}^2}}$$(1)where n is the number of experimental data points, \({q_{cal}}\) is the calculated data, \({q_{\exp }}\) is the experimental data, and q represents the ratio Ct/C0.
-
(ii)
Coefficient of determination (R2):
$${{\text{R}}^2}=\frac{{{{\left( {{q_{\exp }} - {{\bar {q}}_{cal}}} \right)}^2}}}{{\mathop \sum \nolimits_{{i=1}}^{n} {{\left( {{q_{\exp }} - {{\bar {q}}_{cal}}} \right)}^2}+~{{\left( {{q_{\exp }} - {q_{cal}}} \right)}^2}}}$$(2)where \({\bar {q}_{cal}}\) is the average of \({q_{cal}}\).
-
(iii)
Sum of the square of the error (SSE):
$${\text{SSE}}=\mathop \sum \limits_{{i=1}}^{n} {\left( {{q_{\exp }} - {q_{cal}}} \right)^2}$$(3) -
(iv)
Sum of the absolute error (SAE):
$${\text{SAE}}=\mathop \sum \limits_{{i=1}}^{n} {\left| {{q_{cal}} - {q_{\exp }}} \right|_i}$$(4) -
(v)
Average relative error (ARE):
$${\text{ARE}}=\frac{1}{n}\mathop \sum \limits_{{i=1}}^{n} \left| {\frac{{{Y_{cal}} - {Y_{\exp }}}}{{{Y_{\exp }}}}} \right|~$$(5) -
(vi)
Average relative standard error (ARS):
$${\text{ARS}}=100 \times \sqrt {\frac{{\mathop \sum \nolimits_{{i=1}}^{n} {{(({y_{cal}} - {y_{\exp }}){\text{/}}{y_{\exp }})}^2}}}{{n - 1}}}$$(6)
Results and Discussion
Characterization of Materials
SEM Analysis
The SEM images of the prepared PEG–CTAB–SiOH capsules and silica hollow nanoparticles [28] are compared in (Fig. 1a, b, respectively). Figure 1a shows ellipsoidal morphology with an average size of 300 nm–2 µm. The presence of polymer and enrofloxacin addition to CTAB micelle solution conduces an increase in micelle size.
FT-IR Analysis
Figure 2a–c display the FT-IR spectrum of pure enrofloxacin, the synthesized capsules, and PEG. The IR spectra of PEG–CTAB–SiOH depict three vibration bands at 1103, 806, and 969 cm−1 that were assigned to asymmetric stretching of Si–O–Si, symmetric stretching of Si–O–Si, and stretching of Si–OH groups. The three peaks at 1343, 1606, and 2887 cm−1 correspond to C–O of ether group, C=O (carboxylic acid), and O–H of PEG respectively. A broad O–H stretching together with C–H stretching peaks are displayed between 2824 and 3093 cm−1, the region related to carboxylic acid group. The bands at 1254, 1446, and 1503 cm−1 were assigned to aryl C–F stretching vibration and C=C vibration. Sharp peaks at 1738, 2963, and 2875 cm−1 show C=O vibration of carboxylic acid group [30]. According to these results and the similarity between three spectra, it is clear that there is interaction between drug and polymer in silica matrix.
TGA Analysis
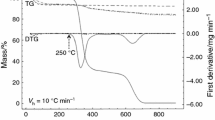
The thermogravimetric (TGA) and the differential thermal analysis (DTA) curves of the PEG2000–CTAB–SiOH and PEG10,000–CTAB–SiOH capsules are shown in Fig. 3. Each DTA curve presents one endothermic peak situated between 50 and 100 °C and assigned to evaporation of H2O (physically adsorbed) [31]. In the TGA curve, this peak conforms to the first loss of mass (2–10%). The drug-loaded PEG10,000–CTAB–SiOH composite exhibits lower mass losses in the first stage in comparison to the another sample. This effect is explained by the higher hydrophobicity of this sample. In other words, it adsorbs less H2O. The second endothermic DTA peak among 190–250 °C relates to the decomposition of the ethoxy groups (-OEt). The polymerization of the remaining SiOH groups is matched by this peak to loss of mass (10–15%) in the TGA curve [32]. According to Fig. 3b, the DTA data present a remarkable incident at 308 °C assigned to the decomposition of the CTAB. As well, the TGA curve confirms the decomposition of CTAB that occurs at temperatures ranging from 140 to 381 °C. An exothermic incident between 250 and 470 °C in the TGA and DTA curves occurs because of the polymer decomposition [33]. Most significantly, in the DTA curve no signal at 225 °C was observed relating to the melting point of enrofloxacin. Therefore, this lack of signal confirms that the drug in our samples converts to an amorphous state. The mass loss of PEG2000–CTAB–SiOH and PEG10,000–CTAB–SiOH capsules resulting from drug uptake was approximately (49.7 ± 3%) and (23 ± 5%), respectively. This result confirms that the FT-IR results are due to the presence of hydrogen bonds in the capsules. The DTA signal at temperatures higher than 257 °C is associated with the degradation of drug and other organic components in samples.
The actual enrofloxacin loading of PEG2000–CTAB–SiOH and PEG10,000–CTAB–SiOH evaluated by extracting 10 mg of composites with ethanol was (7.5 ± 0.2 µg/mL) and (4.4 ± 1.5 µg/mL) respectively.
In Fig. 4a, b, the nitrogen adsorption/desorption isotherms of PS, EPS (inset in Fig. 4), and pore size distribution are presented. It is clear that the isotherms of PS illustrated typical IV properties of IUPAC classification with H1 hysteresis loops, which corresponds to well defined cylindrical pore channels and independent pore model. The sharp liberate, potent hysteresis at P/P0 = 0.8–0.9, and pore volume = 1.35 cm3 g−1 are characteristic of mesopores (pore diameter = 9.8 nm) that are loaded via capillary condensation only at high relative pressure. The BJH analysis of nitrogen adsorption in Fig. 4b indicates two pore diameter peaks (2.40 and 9.21 nm) and one sharp peak (22.86 nm) for PS sample, however, the reduced pore size peak at 22.86 in the EPS sample confirmed the interaction between drug and PEG. Two other peaks at 40 and 53.82 nm are related to the encapsulated drug. When drug molecules were encapsulated, the total pore volume was reduced from 1.35 cm3 g−1 in PS to 0.0046 cm3 g−1 in EPS capsule.
In Vitro Solubility and Drug Release Studies
The solubility of pure enrofloxacin drug at pH 6.4 in PBS is partial, whereas the alkaline solution of PEG and silica in preparation of EPS capsules increases the solubility of enrofloxacin significantly. In other words, the increment of drug solubility might be due to the increase of hydrophilicity and wettability properties of the drug in polymer and silica of media [34]. In vitro drug release evaluations were performed in simulated gastric fluid (pH 2.8) and intestinal fluid (pH 6.4) dissolution medium. The enrofloxacin release profile from drug-loaded capsules considering the effect of temperature and PEG molecular weight on the drug release rate is displayed in Figs. 5 and 6. As shown in Fig. 5, the release rate increases with the decrease in the pH value of the release medium. A fast release of enrofloxacin from EPS capsules occurred in the simulated gastric fluid in the initial 30 min to almost 77%. The amount of ENF dissolving from EPS capsules until 60 min reached 79% followed by a sustained release of ENF from capsules within 30 h. However, in the simulated intestinal fluid, a slow release rate of ENF was observed. In Fig. 6, the slower drug release from the prepared sample of PEG2000 is clearly observed.
Influence of Ionic Strength
Ionic strength as an external stimulus has a significant role in controlling the pharmacological effect of a drug as it can influence the pharmacokinetic profile and the rate of release. The pH-responsive polymeric carriers are composed of a polymeric matrix consisting of ionic pendant groups. In this work, the silica species in the prepared silica capsules could be ionized with considerable pH leading to deprotonated silanol groups (Si–O−) or protonated silanol groups (Si–OH2+). In an aqueous medium with appropriate pH and ionic strength, the pendant groups ionize and increase fixed charges on the polymer network by generating electrostatic repulsive forces responsible for pH-dependent swelling or deswelling of the polymeric carriers. To study the effect of ionic strength on the release rate of ENF from capsules, sodium chloride was used to adjust the ionic concentration of buffer solutions due to its ability to salt out polymers in the midrange of lyotropic series. The ionic strength of the solution with pH 2.8 and 6.4 varied over a range of 0.1–0.4 M to simulate the gastrointestinal conditions [35, 36]. Figures 7 and 8 and Table 1 show the ionic strength effects on the release rate of the drug. It is clear that ionic concentration had a significant effect on drug release rate from EPS capsules, especially at pH 6.4. Determining the drug release rate was performed by using both of the prepared EPS capsules with polyethylene glycol (PEG) of 2000 and 10,000 molecular weights in pH 2.8 and 6.4 dissolution medium.
Kinetic Modeling
Mathematical modeling of the release process plays a primary role in the growth of controlled-release systems as it appoints the profile of drug release and determines more details for preparation of suitable carriers. A general method is based on the mechanism of transport in these systems such as diffusion-controlled, swelling-controlled, chemically controlled, and the structural characteristics of the polymer that can obtain the above objectives. The model equations can be used to prepare new systems by applying suitable geometry, route of synthesis, and size.
As presented in Table 2, the release of ENF from EPS capsules in this study was analyzed with the mathematical models of zero-order and first-order kinetics [37, 38], Higuchi [39], Baker–Lonsdale [40], Hixson–Crowell [41], Ritger–Peppas [42], and Sahlin–Peppas [43] equations. Linear and non-linear least-squares regression was employed for calculating the correlation coefficient (R2) and other parameters. To determine the quality of fit and predict the profile of drug release, the correlation coefficient (R2) and error analysis functions were applied as outlined in Table 2. According to the results, the zero order, first order, Higuchi, Hixson–Crowell, and Baker–Lonsdale models all produce poor curve fittings with relatively low (R2) values. The non-linear fitted curves with two models are shown in Figs. 9 and 10. Sahlin–Peppas and Ritger–Peppas models describe our experimental data and drug release kinetic better than other models. It appears that the release of ENF from EPS capsules is a two-step process consisting of diffusion and relaxation portions requiring a more suitable kinetic model with the capacity for use in the analysis of the experimental data in two sections.
Conclusion
Encapsulation of a veterinary antibiotic drug with poor water solubility was performed with based-polymeric silica capsules using single-step, self-assembly of surfactant, and polymer micelles route. Such experiment was conducted to examine the feasibility of using biocompatible polyethylene glycol micelles in silica matrix to create a significant environment for the drug and to regulate the drug release from (PEG–SiOH). These capsules combine polymer and silica nanodispersion advantages with increased drug solubility and stimuli-responsive properties. The experimental data show that the ionic strength of dissolution media can be employed to regulate the drug release rate, especially at low pH. At high pH, the release rate is favoured owing to the acid dissociation of carboxylic acid group of the drug molecule. The release of ENF from capsule (PEG10,000–SiOH) was completed in 8–9 h. However, the release of ENF from capsule (PEG2000–SiOH) was sustained for up to 24 h. We believe that this novel drug carrier and associated technique has strong potential application in biology as an active molecules carrier. In addition, that, this carrier enables the use of hybrid polymer and inorganic materials in the fields of biology and medicine.
References
Jimenez A, Zaikov GE (2009) Recent advances in research on biodegradable polymers and sustainable composites. Nova Science Publishers, New York
Aich N, Plazas-Tuttle J, Lead JR, Saleh NB (2014) Environ Chem 11(6):609–623
de Sousa A, Maria DA, de Sousa RG et al (2010) J Mater Sci 45:1478–1486
Catauro M, Papale F, Bollino F et al (2014) Mater Sci Eng C 40:253–259
Kokkarachedu V, Vimala K, Sakey R et al (2012) J Polym Environ 20:573–582
Ravindra S, Varaprasad K, Narayana RN et al (2011) J Polym Environ 19:413–418
Khan NU, Bharathi NP, Shreaz S et al (2011) J Polym Environ 19:607–614
Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY (2008) Adv Drug Deliv Rev 60:1278–1288
Barbe BC, Bartlett J, Kong L et al (2004) Adv Mater 16:1959–1966
Chen Y, Wang YJ, Yang LM, Luo GS (2008) AIChE J 54:298–309
Trewyn BG, Slowing II, Giri S, Chen HT, Lin VSY (2007) Acc Chem Res 40:846–853
Persad CV, Swamy BY, Reddy CLN et al (2012) J Polym Environ 20:344–352
Vialpando M, Aerts A, Persoons J et al (2011) J Pharm Sci 100(8):3411–3420
Speybroeck MV, Barillaro V, Thi TD et al (2009) J Pharm Sci 98(8):2648–2658
Utech S, Boccaccini AR (2016) J Mater Sci 51:271–310
Catauro M, Renella RA, Papale F, Ciprioti SV (2016) Mater Sci Eng C 61:51–55
Kerkhofs S, Saidi F, Vandarvoort N et al (2015) J Mater Chem B 3:3054–3061
Haritova A, Lashev L, Pashov D (2003) Res Vet Sci 74:241–245
Nakagawa H, Keshikawa T, Matsumura M, Tsukamoto H (1991) Chem Pharm Bull 39:1837–1842
Baral SS, Das N, Ramulu TS, Sahoo SK et al (2009) J Hazard Mater 161:1427–1435
Friend DR (1992) J Microencapsul 9:469–480
Takahasi Y, Tsukuda T, Izum C et al (1988) Chem Pharm Bull 36:2708–2710
Alexander J, Fromtling RA, Bland JA et al (1991) J Med Chem 34(1):78–81
Suzuki S, Lim JK (1994) J Microencapsul 11:197–203
Kim EH, Choi HK (2004) Drug Deliv 11:365–370
Kumar GP, Phani AR, Prasad RGSV. et al (2014) Int J Pharm 471:146–152
Song M, Song J, Ning A et al (2010) Mater Sci Eng C 30:58–61
Ebadi A, Rafati AA (2015) J Mol Liq 209:239–245
Baral SS, Das N, Ramulu TS et al (2009) J Hazard Mater 161:1427–1435
Martinez YN, Pinuel L, Castro GR, Breccia JD (2012) Appl Biochem Biotechnol 167:1421–1429
Yang HS, Choi SY (1999) Thin Solid Films 348:69–73
Brinker CJ, Scherer GW (1990) Structural changes during heating: amorphous systems. Academic Press, New York, pp 547–615
Fonseca LC, Paulab AJ, Martinezc DST, Alves OL (2016) New J Chem 40:8060–8067
Dressman JB, Reppas C (2000) Eur J Pharm Sci 11:S73–S80
Johnson JL, Holinej J, Williams MD (1993) Int J Pharm 90:151–159
Lindahl WD, Ungell AL, Knutson L, Lennernas H (1997) Pharm Res 14:497–502
Colombo P, Catellani PL, Peppas NA et al (1992) Int J Pharm 88:99–109
Bravo SA, Lamas MC, Salomon CJ (2002) J Pharm Pharm Sci 5:213–219
Higuchi T (1963) J Pharm Sci 52:1145–1149
Baker RW, Lonsdale HS (1974) Controlled release of biologically active agents. Plenum Press, New York
Hixson AW, Crowell JH (1931) Ind Eng Chem 23:923–931
Ritger PL, Peppas NA (1987) J Control Release 5:23–36
Peppas NA, Sahlin JJ (1989) Int J Pharm 57:169–172
Acknowledgements
The authors gratefully acknowledge Bu-Ali Sina University for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ebadi, A., Rafati, A.A. Development of Novel Biodegradable Enrofloxacin–Silica Composite for In Vitro Drug Release Kinetic Studies. J Polym Environ 26, 3404–3411 (2018). https://doi.org/10.1007/s10924-018-1228-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1228-1