Abstract
In this work, poly(methacrylatoethyl trimethyl ammonium chloride -co-acrylamide)/diatomite composite flocculant was synthesized via in situ polymerization in aqueous solution and applied in waste water treatment. The structure of composite flocculants was characterized by FT-IR, 1HNMR and XRD, TGA and viscometer. Herein, the apparent viscosity of composite flocculants was employed as comparison standard of their performance to evaluate the influence of the reaction parameters, such as monomer feeding ratio, diatomite mass fraction and polymerization temperature, etc. on their flocculation performance. And based on the above investigations, the optimum synthesis condition could be found. By comparing flocculation properties of composite flocculants with that of the conventional cationic flocculant, the dosage of composite flocculant that could make the transmittance of treated waste water exceed 95 % was only 7.5 ppm which was far lower than that of conventional flocculant (60–90 ppm). Meanwhile, the settling time was lower than 5 s which was similarly to that of conventional flocculant. Finally, the conclusion was that the composite flocculant owned higher absorption capacity and larger chain extending space than those of conventional linear flocculant due to the introduction of diatomite as backbone, which could make linear polymer chains free from entanglement and improve the flocculation capacity notably.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world’s current crisis of domestic water necessitates the treatment of municipal wastewater and industrial effluents under water recycling paradigm, for there is a good deal of waste water comes into being in dyeing, paper and cement manufacturing industry annually [1, 2]. So it calls for the widely use of water treatment agent.
Polyelectrolyte flocculants are water-soluble polymers carrying ionic charge along the polymer chain as one of the water treatment agents [3, 4]. These polymers are distinguished into three groups depending on the charge, which are anionic, cationic or amphoteric polymers. The polyelectrolyte flocculants endowed with several distinct characteristic are being increasingly applied for the treatment of municipal and industrial wastewater through flocculating [5, 6]. Actually, flocculation is a process of bringing the smaller particles together to form a larger one which is settled more easily and ready for liquid–solid separation effectively [7, 8]. It cannot be denied that flocculants take an important role in wastewater treatment, especially the polyacrylamide-based synthetic polymers [9]. Due to the high polymerization activity of acrylamide monomer, it can copolymerize with a great range of monomers. Hence appropriate flocculants with high efficiency can be designed based on the type of the sewage. Such as the cationic polyacrylamide-based flocculant which is synthesized and widely applied to treat dyeing and paper manufacturing waste water containing a number of ions.
However, it has to be admitted that the polyacrylamide-based flocculant still has some flaws, and one of the primary points is the entanglement of polymer chains. The flexible chains of conventional flocculant tangle easily when they extend in aqueous solution, which inevitably reduces the flocculation efficiency. In order to improve their flocculation efficiency many work have been done for years. Gao et al. synthesized a flocculant with higher efficiency through composting polyaluminum chloride (PAC) and poly(dimethyl diallyl ammonium chloride) (PDMDAAC) [10]. Zou, and Zhu have prepared a new inorganic–organic composite flocculant starch (St), acrylamide (AM) and SiO2 sol, and this composite flocculant also showed better flocculation properties [11]. And it is reasonable that the composite flocculant performs better than traditional flocculants, the stereo composite can make its organic chains escape tangling to some extent for its relatively large volume structure. Therefore, to choose a suitable inorganic material comes to be very important.
Diatomite is a natural biogenetic mineral, which is commonly found from diatom shells, a diverse array of microscopic single-cell algae which have the capability of extracting silica from water to produce their skeletal structure when they were alive [12], and natural diatomite is shaped into macro-porous structure and comprised of 85 % silica the balance being other inert oxides. The chemical composition and the physical structure of diatomite make it of great conventional value for a broad spectrum of applications such as beer filter aids, removal of textile dyes from waste water, and sorption of heavy metal ions [13, 14]. Diatoms can also serve as a starting material for the production of nanostructure particles of different compositions such as shape-preserving and thermal storage materials [15, 16]. In recent years, more and more researchers are focusing on it because of its low-cost, high-quality, resourceful, and with high adsorption performance in water treatment. In 2003, Al-Ghouti et al. [17] found that diatomite performed well in removing the problematic reactive dyes from textile wastewater. Then Al-Ghouti and Al-Degs [18] modified raw local diatomite with micro-emulsion and finally got a new adsorbent with high absorption capacity in treating inorganic pollutants. And many results indicated that diatomite played an important role in wastewater treatment, and also be a well starting material for composite.
Based on that, diatomite was adopted as a start material to be bonded/absorbed with poly (AAm-co-DMC) copolymer under the suitable condition. And the composite flocculants (PDAD) were synthesized with a variety of diatomite and DMC contents and used to treat the wastewater simulated with bentonite, whilst the flocculation performance was compared with conventional cationic flocculant.
Materials and Methods
Materials
Methacrylatoethyl trimethyl ammonium chloride (DMC) was procured from Sangyo Co., Japan. Acrylamide (AM) was supplied by Fine Chemicals Co., Shandong. Diatomite was used as received which was from Linjiang HengTai filtration aid Co. Ltd. Complexing agent EDTA-2Na which aimed to purify diatomite through complexation was from Sinopharm Chemical Reagent Co., Ltd. The water soluble initiator 2, 2′-azobis[2-(2-imidazolin-2-yl)propane]dihydro chloride (VA-044) provided by Wako Pure Chemicals, Japan was used without further purification. And the bentonite which was used to simulate wastewater was from Jiutai nine battalion bentonite processing co., LTD. The solutions throughout entire experiment were prepared with deionized water.
Synthesis of Composite
The typical synthesis proceed of composite flocculant was described as follow: the diatomite (29.74 g) was dispersed into the monomers aqueous solution containing DMC(37.39 g, 0.18 mol) and AM(51.84 g, 0.72 mol), and the monomer content was fixed at 30 wt%. Straight after, the solution was poured into a 500 mL three-neck separable flask, and heated to 40 °C in water bath at agitation of 150 rounds per minute throughout the experiments. After purging with nitrogen for 30 min, other substances were introduced; including EDTA-2Na (0.015 g, 0.40 × 10−4 mol), which disposes the metal ions in diatomite, and VA-044 (0.28 g, 0.86 × 10−3 mol). Then, the system was kept at constant temperature for 24 h to escalate the conversion of monomers, and protected from air by nitrogen throughout the polymerization process. It should be noted that the more diatomite was employed, the slower reaction rate was due to the chain transfer effect of diatomite. Although the conversion exceeded 98 % within 6–8 h when the content of diatomite was 25 wt%, it could achieve 96 % within 24 h when the content of diatomite was 45 wt%. Thus, the time of synthesis was fixed at 24 h to ensure the consistency of all synthesis. The final product was dissolved to be concentrated solution in water, and then the copolymer was precipitated by pouring the polymer solution into a great deal of acetone and washed several times to remove the traces of water, the residual monomers and initiator. Afterwards, the sample was dried under vacuum at 40 °C to constant weight. The ready dried sample was used to determine the structure and properties.
Characterization Methods
The samples of FTIR spectra were crushed with KBr to make pellets and recorded with a FTIR spectrophotometer (Bruker, Group Company, Germany) between 400 and 4000 cm−1.
1HNMR spectra of the dried resulting copolymer was obtained on Bruker AVANCE Model using deuterium oxide (D2O) polymer solution in a 5 mm tube at ambient temperature.
The wide-angle XRD patterns were recorded on a BRUKER X-ray diffractometer, operating at a voltage of 40 kV and a current of 30 mA using a Cu Kα (λ = 0.154 nm).
Thermogravimetry analysis (TGA) tests of the prepared samples were analyzed with purging nitrogen and heated from ambient temperature to 800 °C at the rate of 10 °C per minute.
Flocculation Characteristics
The simulative wastewater with bentonite (the concentration of bentonite is 1 wt%) was settled in 100 mL measuring cylinder, then, it was flocculated by adding prescribed amount of flocculants (the optimal amount of flocculant is only 7.5 ppm). Large amount of flocs were forming visibly with the cylinder shocked up and down strongly, and the small flocs would not stop aggregating until it was large enough to subside. After ended shocking the cylinder, suspensions were kept still for a certain time to ensure the flocculation process reached steady state. Then the supernatant would be extracted for transmittance test.
The transmittance of supernatant liquid was measured at room temperature by ultraviolet spectrophotometer U-3900 (Hitachi, Japan.). Wavenumber was adopted as 460 nm, the canning time was 60 s and slit width was at E = 2.0 nm. Each settling time and transmittance value was obtained by averaging the 5 datum that tested under the same conditions.
The zeta potential of the supernatant was measured by the Nano Zetasizer (ZEN3600, Malvern, Inc., UK) at room temperature.
Results and Discussion
Preparation of Flocculant
As known the molecular weight of flocculant has great influence on flocculation efficiency [19]. Therefore, series influencing factors of polymerization have been tested aiming to get the desirable formula, under which the composite has higher apparent viscosity and better flocculation property. The results were summarized from Tables 1, 2 and 3.
As shown in Table 1, the apparent viscosity of composite flocculant declined sharply from 685 to 125 mPa s with increasing the diatomite content from 15 to 45 %. It indicated that the diatomite content had strong influence on composite apparent viscosity. That was mainly because diatomite which was electronegative could absorb the cationic organic chains and made them shrink and attach to the surface of diatomite, and with diatomite content rising the adsorption area and absorbability would increase. Besides, what was worth mentioned is that diatomite also would lead to reduce of system polymerization reactivity to some extent for it was an inorganic material. These all brought about the falling of composite apparent viscosity with adding diatomite. The flocculation test was also conducted with the composite samples which were synthesized as Table 1. Figures 1 and 2 gave the information on the flocculation result. And seen from the figures, the composite synthesized with the diatomite content at 25 % (it was PDAD-3 in Table 1) was performed better flocculation properties, it mainly reflected in higher transmittance with short settling time by comparing with other samples at the same dosage.
Furthermore, the influence of polymerization temperature was tested. As presented in Table 2, the apparent viscosity rose then declined markedly with the polymerization temperature increasing. And the final apparent viscosity of composite reached the peak point value at 40 °C. At this temperature, initiator and monomer molecules moved at an appropriate rate, simultaneously the initiator (VA-044) could be decomposed at a matched rate to meet and initiate monomers spontaneously. Thus, the efficiency of the initiator was improved whilst the conversion rate of monomer was increased, and resulted in a higher molecule at micro level which was shown as higher apparent viscosity at macro level.
Table 3 gave the information about the impact of the monomer ratio on the apparent viscosity. From the Table 3, the apparent viscosity of composite stood at approximately 460 mPa s when the monomer feed ratio was 2/8, and it declined to 275 mPa s with the ratio rose to 8/2. This was primarily due to that the reactivity of DMC was weaker than that of AM under the same conditions, hence with the concentration of DMC increasing, reaction activity of polymerization decreased. And it resulted in lower apparent viscosity of composite at higher monomer feed ratio of DMC/AM. So it was reasonable to take suitable monomer feed ratio as 2/8, at which the synthesized composite had higher apparent viscosity than others.
Characterization of Composite
FT-IR Analysis
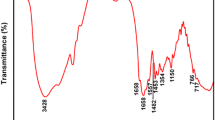
The FT-IR spectrum of diatomite was illustrated in Fig. 3a, the wave-numbers of 1099.54 cm−1 was the peak related to Si–O–Si anti-symmetric stretching vibration, and that was reconfirmed by the peaks at 792.65 and 469.95 cm−1 was due to the symmetric stretching of Si–O. Seen from Fig. 3b, the peaks at wavenumbers of 1452.32 and 955.87 cm−1 were assigned to the methyl groups of ammonium and quaternary ammonium in composite respectively, and the N–H and C=O stretching vibration of acrylamide severally belonged to the strong peaks at wavenumber of 3380.19 and 1666.69 cm−1. The peak at 2945.42 cm−1 came from the C–H stretching vibration of the backbone. And all these characteristic peaks could be clearly found in the spectrum of composite flocculant (Fig. 3c). Appearances of all these peaks indicated the composite had been produced assuredly.
1HNMR Analysis
Figure 4 gave the information on 1H NMR spectrum of composite. As shown in the spectrum, the peaks at (a) corresponded to proton-resonance of –CH2– for AM. The signal at (b) was assigned to hydrogen of –CH– belonging to AM. And the peak at (c) was attributed to proton in –CH2– of DMC. The peaks at (e) and (f) belonged to –CH2– in the side chains of DMC. And the (d) and (g) peaks were assigned to hydrogen of –CH3 in the side chains of that also. By integrating the areas of the resonance peaks –CH2– (a) of AM and –CCH3 (d) of DMC in the composite spectrum, the approximate organic composition of composite was calculated. The result suggested that organic composition was the same as the monomer ratio in the feed.
XRD Analysis
Figure 5 showed the X-ray diffraction profiles of diatomite and composite flocculant. From the profiles, the spectra diffraction peaks at 22.4° and 27.76° were associated with the diatomite due to the crystalline phases of diatomite. Nevertheless, the wide diffuse peak of composite PDAD suggested that copolymer section of composite flocculant could affect the crystalline-amorphous of diatomite. This result was ascribed to the influence of P(AM-co-DMC) part which was bonded/absorbed on diatomite. Therefore, the XRD results showed again the exact structure of the composite.
TGA Analysis
Thermogravimetry was performed on the sample, with nitrogen protecting with a heating rate at 10 °C/min. Figure 6 gave information on degradation extent of copolymers with increasing heating temperature. Seen from the Fig. 9, with the temperature rose to roughly 225 °C, the amounts of both weights were decreased from 100 to 95 % and it was the process of water losing. The temperatures between approximately 225 and 300 °C witnessed a dramatic decline in weight of PDAD-3-3-1 and PDAD-0 reaching some 70 and 59 % of total respectively. After that, there was a significantly decrease in weights bottoming out at 35 % as opposed to 10 % with the temperature rose to near 430 °C. Then, there was no change in both weights. And the TGA curve of the composite PDAD-3-3-1 confirmed the remained ratio 25 % as diatomite, which was the energy-storing part that has high thermostability intrinsically [20, 21].
Flocculation Performances of Composite Flocculant
Effect of Dosage on Transmittance and Settling Time of Flocculation
The flocculation performances of the composite (PDAD-3-3-1), PDAD-0 and conventional cationic polyacrylamide were compared in 1 wt% bentonite simulating wastewater under the same conditions. Hereinto, PDAD-3-3-1 was prepared with 25 wt% diatomite and the mole ratio was 2/8 (DMC/AM). The transmittance results of PDAD-3-3-1, PDAD-0 and conventional cationic flocculant were illustrated in the Fig. 7. And the settling time of experiments was shown in Fig. 8.
As shown in Fig. 7, the transmittance of supernatant liquid, treated by composite flocculant PDAD-3-3-1, performed a sharp rise peaking at roughly 96 % with some 7.5 ppm. Then there was no change until beyond more or less 50 ppm where it fell down slightly. Seen from the curves of PDAD-0 and conventional cationic flocculant, the transmittance of supernatant liquid treated the tow flocculants increased gradually by adding the flocculant dosage, until reaching 90 ppm the transmittance arrived the top point almost 95 % in conventional cationic flocculant curve, as opposed to 100 ppm with the transmittance reached the same value in PDAD-0 curve. After that they both kept stable. In general, the transmittance in PDAD-3-3-1 curve was far higher than that in the other two curves, except exceeded 75 ppm where the conventional flocculant and PDAD-0 performed better. Specifically speaking, the transmittance of supernatant was 96 % after being flocculated with composite at a dosage of 7.5 ppm, whilst it was more less 0.20 % treated by conventional flocculant and PDAD-0 with the same dosage. When dosage was beyond 75 ppm, transmittance in PDAD-3-3-1 curve was lower than that in the others. Above all was attributed to diatomite which had macropore and large volume could boost absorption capacity and escape linear chains tangling to some extent, thereby improved flocculation efficiency. On the contrary, beyond 75 ppm, conventional flocculant and PDAD-0 showed better flocculation effect on the wastewater. That was mainly because the dosage was excess for PDAD-3-3-1 and the extra part played as stabilizer which made the flocculation performance variation, nevertheless, it was the optimal dosage for the other two flocculants.
And in Fig. 8, it gave the information on setting time of flocculation experiments. It was obvious seen from the results that in each curve that the settling time was decreasing firstly then kept steady with rising flocculants dosage. On the whole, the PDAD-3-3-1 spent less time than the other two flocculants with the same dosage during experiment. When dosage was added to 75 ppm, the flocs settled quickest by PDAD-3-3-1 at some 5 s, whereas the least settling time of conventional flocculant and PDAD-0 was around 8 s at almost 88 ppm. Furthermore the biggest difference in time between them was at 7.5 ppm, PDAD-3-3-1 spent only 23 s, conventional flocculant and PDAD-0 was at 87 and 90 s or so, respectively. According to Fig. 8, at this point, transmittance in composite curve was at some 96 % which was higher than that in the other two flocculants at 0.2 %.
Zeta Potential Test
Figure 9 gave the information on zeta potentials of the supernatant in the bentonite suspension flocculated with the composite flocculant and conventional flocculant. From the figure, zeta potential of the supernatant flocculated by composite flocculant rose considerably with increasing the dosage of flocculant. As the dosage added to 7.5 ppm, the zeta potential was arriving at almost −5 mV. And when zeta potentials reached neutral point, the dosage of composite flocculant was only 22.5 ppm, from where composite flocculant performed over dosage properties. As for the conventional flocculant, zeta potentials of the supernatant grew up gradually along with increasing its dosage. At some 42.5 ppm, zeta potential climbed to approximately −5 mV. And it was not until it arrived at roughly 60 ppm, the zeta potential almost reach the neutrality. This indicates that bentonite suspension particles which has a great deal of negative charges were aggregated to sedimentation due to the charge neutralization of the two flocculant, it also turned out that both charge neutrality played an important role in flocculation process. In addition, the composite flocculant performed superior flocculation properties with the help of diatomite, which exerted high absorption ability and made it easy for linear chains of composite flocculant to grab and neutralize with the bentonite suspension particles.
Flocculation Mechanism
Taking into account the effects of various factors on the flocculation performance, the flocculation mechanism in this work can be explained and described as Scheme 1: differing from conventional cationic linear flocculant, at the beginning of the flocculation process the cationic chains of composite flocculant were shrunk and attached to diatomite by electrostatic interactions between them for that diatomite shown a certain of electronegativity, just as presented in Table 1 that the apparent viscosity of PDAD-0 was far higher that of composite PDAD series. However, with the small electronegative pollutants particle moving and colliding the composite, it would replace diatomite and neutralized with the cationic groups of the organic chains because it was more agile and performed stronger electrostatic shielding effect, and owing to the repulsion between diatomite and pollutants the linear chains were forced to extend. Then, the flexible organic chains of the composite that had absorbed insoluble pollutants would aggregate and form super-large net-like flocs. This net-like flocs could further seize residual pollutants from water through sweeping effect [22,23,24,25,26], and then the compacted flocs were formed and settled down finally as shown in Scheme 1a. As to the conventional flocculant without diatomite, the curly polymer chains needed a bit longer time to extend [1]. Then, they absorbed pollutants by neutralization, whilst the pollutants were aggregated, and the relaxing supple chains bridged with each other nearby until this net aggregation settling down [27,28,29]. The specific flocculation process can be seen in Scheme 1b.
On the other hand, the entanglements of polymer chains including entanglement in the polymer chain and entanglement between different polymer chains are also key factor to impact the flocculation performance of flocculant. As for conventional cationic linear flocculant, the entanglement in the linear polymer chain more easily occurs due to their longer chain length which unavoidably attenuates their flocculation performance. However, there is relatively small amount of entanglement in the composite flocculant chain owing to their shorter chain length. After they absorbed sufficient pollutants by neutralization, the polymer chains of composite flocculant exhibits more extend confirmation and entanglement between different polymer chains occurs. Thus, the tangled polymer chains that had absorbed insoluble pollutants aggregate and form large net-like flocs, which can improve the flocculation performance of composite flocculant.
Conclusions
The cationic composite flocculants were successfully synthesized via free radical polymerization in aqueous solution. Compared with conventional flocculants, this novel composite flocculant showed superior flocculation properties. It was reflected in shorter settlement time, higher transmittance at lower dosages in simulated wastewater because of the special structure that organic linear chains bonded/absorbed on diatomite, which effectively made molecule chains refrain from entanglement and aggregate to form supermolecular structure more pollutants were grabbed. Besides, assisted with high absorption capacity of diatomite, the flocculation efficiency was improved to a certain extent.
References
Ponou J, Ide T, Suzuki A, Tsuji H, Wang LP, Dodbiba G, Fujita T (2014) Water Sci Technol 69:1249–1258
Tripathy T (2006) Vidyasagar University. Midnapore, West-Bengal
Ríos HE, González-Navarrete J, Vargas V, Urzúa MD (2011) Colloid Surf A 384:262–267
Borkovec M, Papastavrou G (2008) Curr Opin Colloid Interface Sci 13:429–437
Granados MR, Acién FG, Gómez C, Fernández-Sevilla JM, Grima EM (2012) Bioresour Technol 118:102–110
Chen X, Chen G, Yue PL (2007) Sep Sci Technol 19:65–76
Wang JP, Chen YZ, Ge XW, Yu HQ (2007) Chemosphere 66:1752–1757
Wang LJ, Wang JP, Yuan SJ, Zhang SJ, Yong T, Yu HQ (2009) Chem Eng J 149:118–122
Ho YC, Norli I, Alkarkhi AFM, Morad N (2010) Bioresour Technol 101:1166–1174
Gao BY, Wang Y, Yue QY (2005) Acta Hydrochim Hydrobiol 33:365–371
Zou J, Zhu H, Wang F, Sui H, Fan J (2011) Chem Eng J 171:350–356
Lemons JF (1997) Am Ceram Soc Bull 76:92–98
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) Water Res 33:2469–2479
Al-Degs Y, Khraisheh MAM, Tutunji MF (2001) Water Res 35:3724–3728
Karaman S, Karaipekli A, Sarı A, Biçer A (2011) Sol Energy Mater Sol Cells 95:1647–1653
Xu B, Li Z (2013) Appl Energy 105:229–237
Al-Ghouti MA, Khraisheh MAM, Allen SJ, Ahmad MN (2003) J Environ Manag 69:229–238
Al-Ghouti MA, Al-Degs YS (2011) Chem Eng J 173:115–128
O’Shea JP, Qiao GG, Franks GV (2010) J Colloid Interface Sci 348:9–23
Sun Z, Zhang Y, Zheng S, Park Y, Frost RL (2013) Thermochim Acta 558:16–21
Shen WN, Feng LJ, Feng H (2012) Chem J Chin Univ 33:353–360
Barata-Rodrigues PM, Mays TJ, Moggridge GD (2003) Carbon 41:2231–2246
Ovenden C, Xiao H (2002) Colloid Surf A 197:225–234
Ji J, Qiu J, Wai N, Wong FS, Li Y (2009) Water Res 44:1627–1635
Biggs S, Habgood M, Jameson GJ, Yan YD (2000) Chem Eng J 80:13–22
Gregory J, Barany S (2011) Adv Colloid Interface Sci 169:1–12
Gao BY, Yan W, Yue QY, Wei JC, Qian L (2007) Sep Purif Technol 54:157–163
Bolto B, Gregory J (2007) Water Res 41:2301–2324
Wei J, Gao B, Yue Q, Wang Y, Li W, Zhu X (2009) Water Res 43:724–732
Acknowledgments
The authors are grateful for the financial support provided by Major Project of Jilin Province (Nos. 20140204083GX, 20160101306JC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Xu, K., Liu, Y., Wang, Y. et al. A Novel Wastewater Treating Material: Cationic Poly Acrylamide/Diatomite Composite Flocculant. J Polym Environ 26, 3051–3059 (2018). https://doi.org/10.1007/s10924-018-1176-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1176-9