Abstract
Cationic hyperbranched oligomer poly(N-acryloyl-1,2-diaminoethane hydrochloride) (HADE) was firstly synthesized by Michael addition reaction. And then, a series of cationic flocculants poly(acrylamide/N-acryloyl-1,2-diaminoethane hydrochlorides) (PAM-HADEs) with hyperbranched structure was prepared from HADE as macro-monomer and acrylamide (AM). The structures of PAM-HADEs were characterized by Fourier transform infrared spectrometry, 1H and 13C nuclear magnetic resonance spectroscopy, gel permeation chromatography (GPC) and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF). And the properties were systematically evaluated by intrinsic viscosity, zeta potential and hydrodynamic radius. The mechanism of the cationic hyperbranched copolymer used in water treatment was extensively studied via a jar test in which the transmittance of the supernatant, settling time, and average floc size were used to evaluate the flocculability. Compared with the linear flocculant poly (acrylamide/liner-N-acryloyl-1,2-diaminoethane hydrochloride) (PAM-LADE), the novel hyperbranched polymeric flocculants exhibited outstanding flocculability which were reflected by shorter settlement time, high transmittance and large floc size. The primary cause that PAM-HADEs owned excellent flocculability is the more stretching configuration and less chains entanglement of PAM-HADEs in waste-water due to their hyperbranched structure compared with that of the linear PAM-LADE which exhibited curly coil configuration. On the other hand, abundant and exposed cationic terminal groups of PAM-HADEs originated from their hyperbranched structure also hint higher flocculation capacity. At optimum dosages of the polymer, the transmittance of the supernatant is less at low and high pH values, indicating that the natural pH (pH 7.29) of the suspension is the most appropriate pH for the flocculation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, much attention has been paid to waste-water treatment, since water pollution has become a serious problem that is threatening the survival of human beings, plants, and animals [1, 2]. Flocculation is one of the most important industrial processes for water treatment, which is widely used due to its facile operation, high efficiency, and economic advantages [3,4,5]. However, most suspensions have negatively charged surfaces, and flocculants with cationic groups are more favorable to aggregation and settle down these colloids in water due to the effect of charge neutralization flocculation. Especially, cationic polyacrylamide (CPAMs) one of the most widely applied polymer flocculants has exhibited high flocculating efficiency in water treatment [6,7,8,9]. Although linear CPAMs show good application performance in water treatment, the further improvement in their flocculating efficiency is still anticipated because of their coil confirmation, intra- and intermolecular entanglement impeding the interaction between pollutants and them.
Differing from conventional linear polymers, dendrimers own unique properties, including highly branched architectures, many inner cavities, good solubility, low solution and bulk viscosity and a large amount of terminal functional groups, due to their three dimensional torispherical irregular macromolecules [10,11,12,13]. Among some researches, dendrimers have been used as flocculants in order to overcome the drawbacks of linear polymeric flocculants and they exhibited intriguing advantage [14, 15]. However, they cannot be broadly applied in water-treatment because of their expensive cost and complex preparation process. In contrast to the dendrimers, hyperbranched polymers (HBPs) have irregular branched structures, low degree of branching and still inherit the desirable properties similar to those of dendrimers. Furthermore, they can be prepared conveniently and cost-effectively on a large scale in a one-pot procedure. Therefore, hyperbranched polymers are considered to be alternatives to dendrimers for emerging application in wide fields [16,17,18]. Especially, various synthesis methodologies have been developed during the past two decades, which can provide the promising chance for their application [19, 20].
Based on practical point of view, HBPs can be utilized as novel organic polymeric flocculants in that their inner cavities and terminal functional groups of HBPs can accommodate metal ions, organic compounds and the original suspended matter in waste-water via adsorption bridging and charge neutralization. Recently, temperature-responsive amine-based hyperbranched polymers were synthesized for solid–liquid separation. The suspension treated with hyperbranched copolymers exhibited the remarkable separation of the fine particles at a low polymer dosage. Compared to linear polymers, the hyperbranched copolymers exhibited better particle flocculation at 40 °C [21]. Arts et al. reported that hyperbranched polyester amide, flocculant component for flocculating material, dispersed in aqueous medium could be used to paper production, detergents, etc. [22,23,24].
In this study, a series of cationic flocculants PAM-HADEs with hyperbranched structure was synthesized and characterized. Meanwhile, the effects of polymer dosage, charge density and molecular weight on flocculating performance were studied and compared with those of linear flocculant in simulating waste-water. Moreover, PAM-HADEs was utilized to treat oil-field fracturing waste-water to investigate the application value in practical production.
Materials and Methods
Materials
Di-tert-butyl pyrocarbonate (BOC anhydride, Aladdin), acryloyl chloride (AC, Adamas), ethylenediamine (Aladdin), were used as received. Acrylamide (Shandong Shouguang Fine Chemicals Co.,Ltd.) was twice recrystallized from acetone and vacuum dried at 30 °C. 2,2-Azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044, from Wako Pure Chemicals, Japan) was used as the initiator without further purification. Triethylamine, hydrochloric acid (37%), dichloromethane, ethyl acetate, acetone, trichloromethane, tetrahydrofuran and ethyl ether were of analytical grade that were purchased from Beijing Chemical Works, used as received.
Preparation of N-Acryloyl-1,2-diaminoethane Hydrochloride (ADE)
ADE was prepared according to improved method previously reported in literature [25], as follows.
N-t-Butoxycarbonyl-1,2-diaminoethane (N-Boc-DEA)
A stock solution of ethylenediamine (45 g) in dichloromethane (400 mL) was added into a 2-L flange flask equipped with a condenser, a pressure equalizing dropping funnel, a mechanical stirrer and a nitrogen inlet, and then was cooled in an ice/salt bath (− 10 to 0 °C). A solution of BOC anhydride (40 g) in dichloromethane (200 mL) was added dropwise to the solution over 2 h with stirring. The mixture was allowed to warm to room temperature and stirred overnight (16 h). The solvent was removed by rotary evaporation and water (200 mL) was added to the residue, resulting in the precipitation. The filtrate was saturated with sodium chloride, extracted with ethyl acetate (3 × 100 mL) and the combined organic fraction was concentrated under reduced pressure to give pale oil. Residual sodium chloride in the oil was removed by dissolving it in chloroform (100 mL) and filtering it through porosity 3 sinter. The solvent was removed under reduced pressure and the product was dried under vacuum to yield N-Boc-DEA (27 g, 93%).
N-t-Butoxycarbonyl-N′-acryloyl-1,2-diaminoethane(N-Boc-DEA-AC)
A stock solution of acryloyl chloride (13 g) in chloroform (300 mL) was firstly cooled in an ice/salt bath (− 10 to 0 °C), and then was slowly added dropwise into a solution of triethylamine (12.65 mL) and N-Boc-DEA (20 g) in chloroform (150 mL) for at least 1 h. After addition, the reaction was allowed to equilibrate to room temperature and stirred for 1 h before the solvent was removed under reduced pressure. The residue was washed with water and extracted with chloroform (3 × 150 mL). The combined organic fraction was concentrated under reduced pressure and the resultant white solid was dried under vacuum to give N-Boc-DEA-AC (23 g, 89%).
N-Acryloyl-1,2-diaminoethane hydrochloride(ADE)
A stirred solution of N-Boc-DEA-AC (10 g) in HCl/tetrahydrofuran (50 mL) was added in a 100-mL round-bottom flask. After 6 h at room temperature the solvent was removed under reduced pressure and the residue was triturated with diethyl ether and dried under vacuum to afford ADE (6 g, 88%).
Preparation of Hyperbranched Poly(N-acryloyl-1,2-diaminoethane hydrochloride) (HADE)
After ADE (3 g) was added to a 100-mL three-necked round-bottom flask equipped with a mechanical stirrer, a nitrogen inlet, and a thermometer, the flask was purged with nitrogen. And then, the temperature of oil bath was heated to 210 °C. As the Michael addition reaction had been conducted continuously at 210 °C for 1 h, the reaction mixture was stirred with an efficient mixer at a constant rate of 125 rpm under a constant nitrogen flux. Having been stirred for 1 h, the reaction mixture was cooled to room temperature, and a clear brown sticky solid was obtained which was removed from vessel by dissolution in deionized water. Having been dialysed against pure water, the solid reaction product was recovered by freeze-drying under vacuum.
Preparation of Poly(acrylamide/hyperbranched N-acryloyl-1,2-diaminoethane hydrochloride) (PAM-HADE) by Conventional Free Radical Polymerization
A 100-mL four-necked round-bottom flask equipped with a mechanical stirrer, a condenser, a nitrogen inlet, and a thermometer was charged with AM and HADE at a desired ratio and the total monomer concentration was fixed at 20%. The reactive mixture was heated to 30 °C under bubbling nitrogen for at least 30 min, and then VA-044 (0.5 wt%) was added to it with a syringe. Polymerization was conducted continuously at 30 °C for 24 h. The harvest mixture prepared was precipitated by a large quantity of acetone and washed three times to remove all traces of water, initiator, and residual monomers. Then the precipitate was sliced and dried to constant height under vacuum at 50 °C. Finally, a pale yellow powder was obtained by shattering the slices dried. The molar ratios of AM to HADE: 99:1, 98:2, 97:3 and 96:4, and four final products named PAM-HADE-1, PAM-HADE-2, PAM-HADE-3 and PAM-HADE-4, respectively. The more HADE content was not employed in that the intrinsic viscosity of PAM-HADEs would become very low when the HADE content exceeded 4 mol%.
As a reference sample, poly (acrylamide/liner-N-acryloyl-1,2-diaminoethane hydrochloride) (PAM-LADE) was synthesized via the same method and the total monomer concentration was also fixed at 20%. However, the feed ratio of AM to ADE was 85:15 mol/mol in order that PAM-LADE owned similar molecular weight and Zeta potential.
Characterization
Fourier transform infrared spectra (FT-IR) were recorded with a Bruker 70 spectrometer, KBr pellets were used and the wavelength was ranged from 4000 to 500 cm−1.
1H NMR and 13C NMR spectra were obtained by a Bruker AV 600 NMR spectrometer (Bruker Group Company, Germany). The structures of monomers were characterized by 1H NMR in deuterochloroform (CDCl3; sample: N-Boc-DEA, N-Boc-DEA-AC) or deuterium oxide (D2O; sample: ADE, HADE, and PAM-HADEs).
The molecular weights of the polymers were determined by gel permeation chromatography (GPC) (Waters Corporation, USA). The GPC analysis was performed in 0.1 M NaNO3 aqueous solution, at a flow rate of 0.5 mL/min of eluent. All the analyses were conducted at 30 °C.
The particles were determined with a Winner 2000 laser particle size analyzer (Zhuhai OMEC Particle Technology Co., Ltd., Zhuhai, China) at room temperature.
Dynamic light scattering (DLS) and zeta potential studies were conducted by virtue of Malvern Zetasizer 1000 HSA (Malvern, Worcestershire, UK) at room temperature.
Intrinsic viscosity of polymer solution was determined by means of a dilution-type Ubbelohde viscometer with 1.0 mol/L NaCl as solvent at a concentration of 0.05 g/dL of the polymer. The measurement was carried out at 30.0 ± 0.1 °C. The relation of reduced viscosity and polymer concentration was extrapolated to c 0 and intrinsic viscosity and Huggins constant were obtained by intercept and slope. The reduced viscosity was calculated by dividing flow time of polymer solution by flow time of solvent obtained by the dilution method.
Apparent viscosities of dilute solutions were measured via a Brookfield DV III Ultra rheometer (Brookfield Engineering Laboratories, Inc., USA) equipped with ultra-low viscosity taker and ULA spindle. The measurement was conducted at a temperature of 30 °C in a temperature-control water bath. The concentration of the polymer was 0.5 g/dL.
Elemental analyses were carried out on an Elemental Analyser Vario EL (Elementar, Germany).
Flocculating Characteristics
Simulating waste-water was prepared by dispersing 1 wt% kaolinite in deionized water (Zeta potential:-30 mv, the average particle size = 12 μm). The original pH value of the kaolin suspension was 7.29, and it was adjusted by using 0.1 M HCl or 0.1 M NaOH, as needed. And the oil-field guar gum fracturing waste-water was provided by Daqing Oil-field Company (Transmittance: 10.3%, Oil content: 315.8 mg/L, Suspended solids: 3800.0). The flocculating performances tests of flocculants PAM-HADEs were carried out in a jar-test apparatus (ZR4-6, Zhongrun Water Industry Technology Development Co., China) at room temperature. The waste-water (1 L) was transferred to beakers, and a predetermined dosage of flocculant (1 g/L aqueous solution) was added to the system. Rapid agitation was conducted at 250 rpm for 1 min to mix the flocculant with the waste-water completely. After that, the beakers were left for a certain time until the flocs settled down completely. This period of time was measured as the settling time.
At the end of the settling time, clean supernatant liquid was drawn from a depth of 10 cm and the light transmittance(%) of the supernatant liquid was measured with an ultraviolet spectrophotometer U-3900 (Hitachi, Tokyo, Japan). The tests were carried out at 460 nm wave-number. The slit width was at E = 2.0 nm and scanning time was 60 s. The light transmittance of the supernatant liquid of each sample was the average value of 5 test results under the same test conditions.
Results and Discussion
Synthesis of PAM-HADEs
The monomer ADE was synthesized as synthetic route shown in Scheme S1, and the structures of intermediate products (N-Boc-DEA and N-Boc-DEA-Ac) were characterized by FTIR (Fig. S1) and NMR (Fig. S2). Moreover, hyperbranched polymer HADE was synthesized by Michael addition reaction of the amino (or ammonium) groups of ADE added to the vinyl double bond of another monomer molecule, which was described in literatures [25, 26]. The zeta potential of HADE was 18.6 mv. The structures of HADEs could be controlled by regulating the reaction time. Based on the results of the MALDI-TOF analysis of HADEs (Fig. S3), the hyperbranched polymer HADEs were the mixtures of different molecular weights. Moreover, the HADEs were utilized as macro-monomer to copolymerize with AM, and a series of cationic flocculants with hyperbranched structure was obtained. The synthetic route is shown in Scheme 1.
The FT-IR spectra of ADE, HADE and PAM-HADE-3 are shown in Fig. 1. Seen from Fig. 1a, the peaks at 1665 and 1620 cm−1 belong to C=O and C=C stretching vibration for ADE. The peaks located at 1545 and 1170 cm−1 are attributed to N–H stretching vibration for amine group and C–N stretching vibration respectively. In Fig. 1b, compared with the characteristic signals of ADE (Fig. 1a), the absorption peak of alkene at 1620 cm−1 became weak. The strong absorption peaks for C–N at 1170 and 1390 cm−1 are clearly observed in the spectrum of HADE, manifest that HADE was indeed prepared by the Michael addition reaction of ADE. The FT-IR spectrum of PAM-HADE-3 is shown in Fig. 1c, the absorption bands at 3420 and 1670 cm−1 are assigned to the N–H and C=O stretching vibration for acrylamide. The peaks at 2925 and 2905 cm−1 are ascribed to the C–H absorption of the methyl and methylene groups of PAM-HADE-3. The absorption peak of alkene at 1620 cm−1 almost completely disappeared. The above analysis indicates that PAM-HADE-3 was synthesized successfully.
In this work, the 1H NMR analyses were also utilized to investigate the structures of ADE, HADE and PAM-HADE-3. From Fig. 2a, the peaks at 5.6 and 6.2 ppm should be attributed to the unsaturated carbon resonance of ADE. After the completion of Michael addition reaction, the vinyl signals at 5.6 and 6.2 ppm decreased significantly in strength, as shown in Fig. 2b. This observation confirms that most of the vinyl groups was consumed by the Michael addition reaction and new methylene hydrogens (proton c, d, e) could be observed in the region between 2.40 and 3.80 ppm, which indicates the HADE was prepared. From Fig. 2c, the relative peaks of the vinyl protons in the region between 5.6 and 6.2 ppm almost completely disappeared. The result elucidates that PAM-HADE-3 was synthesized.
Furthermore, the quantitative 13C NMR was employed to characterize the structure of PAM-HADEs and the peaks of NMR spectrum of the copolymer could be assigned to various carbon atoms of the copolymer structure as shown in the scheme inserted in Fig. 3. The intense signal at 179 ppm (a) is assigned to carbon atoms of –C=O. The peaks at 41.5 ppm (c) and 29.4 ppm (g) are attributed to the carbon atoms of AM. The peaks in the range of 54.5, 42.6, 35.2, 34.7 and 33.9 ppm (b, d, e, f, h) correspond to the carbon atoms of HADE. In addition, the molar percentages of AM and HADE in the PAM-HADE copolymer could be calculated by integrating the areas of a–h peaks. The results show that the compositions of the copolymers (shown in Table 1) are in accordance with the feed ratios.
The characteristics of the copolymers, including feed ratio, molecular weight, zeta potential and intrinsic viscosity, are summarized in Table 1. According to the results of Table 1, the zeta potentials of PAM-HADEs increase obviously with the increasing of the HADE content (from 0.61 mv for PAM-HADE-1 increased to 4.58 mv for PAM-HADE-4). However, the intrinsic viscosities of PAM-HADEs exhibited the opposite trend (from 625 mL/g for PAM-HADE-1 decreased to 260 mL/g for PAM-HADE-4). These results indicate that the bigger steric effect and lower reactivity of HADE led to the lower molar mass of PAM-HADE. Meanwhile, the cationic hyperbranched structure of HADE units could significantly elevate the positive charge capacity of PAM-HADEs. In addition, it was found that the intrinsic viscosities of PAM-HADEs would become too low to employ in practical application when the content of HADE in feed exceeded 4 molar percent.
In order to investigate the effect of hyperbranched structure on flocculating performance of copolymer, PAM-LADE was also synthesized as a reference sample and the feed ratio of AM and ADE was 85:15 mol/mol. The zeta potential, molecular weight and intrinsic viscosity of PAM-LADE were 2.88 mv, 5.18 × 10−5 and 462 mL/g respectively, which were similar to those of PAM-HADE-3 (2.85 mv, 5.21 × 10−5 and 458 mL/g respectively).
Impact of Hyperbranched Copolymer Dosage on Clay Particle Flocculation
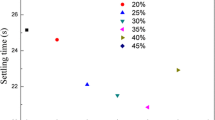
The dosage of flocculant is a very important factor for the flocculating properties. Hence, the dosage effects of HBP flocculant were investigated first. The mean settling time and transmittance of the supernatant were used to evaluate the flocculating efficiency of HBP flocculant. As shown in Fig. 4a, with the increase of flocculant dosage, the light transmittance of supernatant sharply rises and comes to an optimum value (about 90%). And then it descends slightly. Based on Fig. 4b, the settling time of floc seriously falls and then keeps nearly a constant. All the results indicate that HBP flocculant has pretty good flocculating performances in a range of dosage (from 40 to 60 mg/L), which are mainly embodied in fast settling time (below 15 s) and good transparency of the supernatant (above 80%). For the sake of contrast, the settling time and transmittance of the supernatant of linear PAM-LADE were also investigated. From Fig. 4c, it can be found that the light transmittance of supernatant was only 40.5% and the mean settling time was more than 45 s when the dosage of linear PAM-LADE was 40 mg/L.
Transmittance (a) and the settling time (b) of flocculating kaolinite clay suspensions with various dosages; the transmittance and settling time of flocculating kaolinite clay suspensions with the flocculant at 40 mg/L (c); the average floc size of flocculating kaolinite clay suspensions treated with various dosages of PAM-HADE-3 (d)
At the same time, a fact should be noted that the flocculability of HBP flocculant show obvious difference with diversity of HADE content and PAM-HADE-3 exhibits optimal flocculating efficiency. It is well known that the flocculating performances of polymer flocculant mainly depend on the molecular weight and charge density. Thus the increase of HADE content in HBP flocculant could improve the charge density of flocculant (from 0.61 mv for PAM-HADE-1 increased to 4.58 mv for PAM-HADE-4), and the molecular weight was decreased from 625 mL/g for PAM-HADE-1 to 260 mL/g for PAM-HADE-4 in the range of our investigation. Abundant charges on polymer flocculant could validly neutralize and flocculate the solid waste with counter sign in sewage due to the effect of charge neutralization flocculation. Meanwhile, appropriate molecular weight of polymer flocculant is crucial to bridge solid waste in sewage and completes flocculation. Thus, a subtle balance between charge density and molecular weight should be attained during the preparation of PAM-HADEs flocculant, which could endow it with excellent flocculability. According to Fig. 4d, the average floc size initially increased and attained the maximum value. And then it decreased slowly. The optimum dosage was 40 mg/L when the transmittance of waste-water supernatant was at the highest and the floc size was at the largest, which corresponded well with previous researches [27, 28]. The repulsion between the negatively charged kaolinite surfaces allowed small floc size at low flocculant content. With the increase of the cationic PAM-HADEs flocculant, the electrostatic attraction between positively charged PAM-HADEs chains and negatively charged kaolinite surface increases with increased surface charges. So the number of attachments points between cationic PAM-HADEs flocculant chain and kaolinite surface became higher, which led to the formation of larger flocs.
Impact of pH Value on Clay Particle Flocculation
The effect of pH on the flocculating performance of hyperbranched flocculant PAM-HADE-3 was examined in a range of pH 3–11 at a dosage of 40 mg/L. The pH values of kaolin suspensions were adjusted by either NaOH or HCl. The influences of different pH on light transmittance of supernatant are shown in Fig. 5. As shown in Fig. 5, the pH value of the suspension plays a significant role in the flocculation process.
The light transmittance of supernatant ascended with the increase of pH, and at natural pH (pH 7.29) the transmittance of supernatant reached the maximum. And then, the flocculating performance started to decline rapidly in high pH ranges (pH > 8). The pH of suspension directly affected the protonation of amino group on PAM-HADE chain and determined the sign of the surface charges of the particles. The cationic –NH3 + groups were dominant and the polymer chains were easily open because of the mutual repulsion of cationic groups of cationic PAM-HADE in low pH ranges (pH < 8). Under neutral conditions, the particle surfaces charges were neutralized more effectively and extend the molecular chains were extended thus further flocculating. In acidic media, the H+ adsorbed on the negative kaolin particles led to weakened electrostatic attraction of the negatively charged particles. Since the zeta potential of original sample was about − 15 mV at pH 3, at natural pH (pH 7.29) the zeta potential was about − 30 mv. As a result, the flocculating effect decreased. At the high pH values, the amide groups (–CONH2) of polymer molecules started hydrolysis. At the same time, cationic –NH3 + of PAM-HADE-3 was neutralized in the alkaline medium to change into non-ionic flocculant, thus leading to low flocculating efficiency.
Impact of Polymer Architecture on Clay Particle Flocculation
The flocculating performance of hyperbranched PAM-HADE-3 was compared with that of linear PAM-LADE (similar charge density and molecular weight). Figure 4c indicates that PAM-HADE-3 owned much better flocculability than PAM-LADE. In order to clearly elucidate the influence of polymer flocculant architecture on its flocculating performance, a series of investigations on solution property and architecture of PAM-HADE-3 and PAM-LADE was carried out.
At first, the solution properties of PAM-HADE-3 and PAM-LADE in salt solution were investigated. The maximum settling rates of flocs produced by polyacrylamides increased with increased apparent viscosity [29]. As shown in Fig. 6, the apparent viscosity of PAM-HADE-3 (85 mPa.s) in distilled water was lower than that of PAM-LADE (115 mPa.s). The important feature of hyperbranched polymer was that its viscosity in solution was lower than those of their linear analogs. The plots of intrinsic viscosity vs. molecular weight of hyperbranched polymer showed unusual bell-shaped curves, indicating the decrease in the intrinsic viscosity of the hyperbranched polymer of higher molecular weight [30] and the hardly operative chain entanglement. The viscosity of hyperbranched polymer did not obey the well known Mark–Houwink–Sakurada equation, \(\left[ \eta \right]{\text{=K}}{\left[ M \right]^\alpha }\). The behavior is ascribed to the transition from an extended structure for lower molecular weight to globular shape at increased molecular weight, which has been well recognized via the studies of spectroscopic measurements and computer molecular modeling [31, 32]. With the increase of salt concentration, the apparent viscosities of the two samples sharply descended firstly and then kept a constant nearly due to typical polyelectrolyte behavior. However, the residual apparent viscosity of PAM-HADE-3 (60 mPa.s) was far better than that of PAM-LADE (20 mPa.s). These results suggest that PAM-HADE-3 own better salt-tolerance originated from its hyperbranched structural units.
To further prove our results, the hydrodynamic radiuses of PAM-HADE-3 and PAM-LADE at various CaCl2 concentrations were measured via DLS with the results shown in Fig. 7. As is shown in Fig. 7, the hydrodynamic radius of PAM-HADE-3 exhibits negligible change and is ca. 84–88 nm at various CaCl2 concentrations. However, with the increasing of CaCl2 concentration, the hydrodynamic radius of PAM-LADE sharply reduced from 284 nm in 0 M CaCl2 solution to 70 nm in 0.1 M CaCl2 solution. The result suggests that the impact of small molecular salt on the configuration of PAM-HADE-3 is nearly negligible due to the existence of hyperbranched structural units in our investigation range, which could guarantee the excellent flocculation efficiency of PAM-HADE-3.
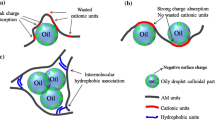
The flocculating mechanism of PAM-HADEs and PAM-LADE in waste-water is elucidated in Fig. 8. Seen from Fig. 8a, the linear PAM-LADE flocculants exhibit curly coil configuration due to electrostatic interaction between them and pollutants in waste-water. Thus, the more dosage and poorer flocculating efficiency could be found. However, as for hyperbranched PAM-HADEs (Fig. 8b), more stretched configuration and less chains entanglement in waste-water, due to steric hindrance effect of hyperbranched structure, indicate their better flocculating performances. This mechanism could also be proved by the aforementioned results of apparent viscosity and DLS of PAM-LADE and PAM-HADE-3 (Figs. 6, 7). Based on the view of practice, all the results hint hyperbranched flocculants own excellent salt-tolerance capacity and a wide prospects of development in waste-water treatment.
Treatment of Oil-Field Fracturing Water with Hyperbranched Copolymers
Large amounts of fracturing waste-water generated during hydraulic fracturing to recover oil from deep shale formations caused considerable environmental hazards if discharged without effective treatment. It is characterized by high suspended solids and oil, which becomes one of the main sources of water pollution in oilfields [33]. PAM-HADE-3 was utilized to treat oil-field fracturing waste-water to investigate the practical application value with the results shown in Fig. 9.
Form Fig. 9, it can be seen that the transmittance of waste-water supernatant treated by PAM-HADE-3 exceeded 90% when the dosage was around 60 mg/L. And the settling time firstly decreased with increasing flocculant dosage, and then increased slightly, the optimal settling time was only ca. 15 s. With the dosage increasing, more flocculants grabbed pollutants and bridged, which led to higher transmittance and shorter settling time. The oil content and suspended solids of raw water were 315.8 and 3800.0 mg/L, respectively. According to the results of Table 2, the oil content and suspended solids decreased to 10.2 and 210.0 mg/L, respectively after the raw water was treated by PAM-HADE-3. All the results indicate that hyperbranched flocculant PAM-HADE-3 owns better flocculability in the treatment of oil-field fracturing waste-water. Thus, it could be anticipated that novel hyperbranched flocculants AM-HADEs have a promising future due to their high-efficiency.
Conclusions
Hyperbranched oligomer HADE was synthesized and utilized as macro-monomer. A series of novel cationic flocculants with hyperbranched structure was prepared by free-radical copolymerization of AM and HADE in aqueous solution, and the flocculating performance was investigated. The influences of polymer dosage, charge density and architecture on transmittance of supernatant, settling time and floc size were evaluated. The cationic flocculants with hyperbranched structure show better flocculating efficiency in treating waste-water, which is reflected by shorter settlement time, lower dosages, higher transmittance and large floc size. With hyperbranched structure, PAM-HADEs has stretched configuration and less chains entanglement than conventional line-type PAM-LADE. PAM-HADEs flocculants could be used as a new type of organic polymer flocculants agent, which have a potential application in the treatment of oilfield waste-water.
References
Tang B, Liu H, Cui Z, Zhang S (2014) J Appl Polym Sci 131:1–15
Razali MAA, Ahmad Z, Ahmad MSB, Ariffin A (2011) Chem Eng J 166:529–535
Song L, Johnson PR, Elimelech M (1994) Environ Sci Technol 28:1164–1171
Fan HK (2011) J Chem Technol Biotechnol 86:246–250
Wang Y, Chen K, Mo L, Li J (2013) J Appl Polym Sci 130:1092–1097
Lu Y, Shang Y, Huang X, Chen A, Yang Z, Jiang Y, Cai J, Gu W, Qian X, Yang H (2011) Ind Eng Chem Res 50:7141–7149
Sun Y, Zheng H, Tan M, Wang Y, Tang X, Feng L, Xiang X (2014) J Appl Polym Sci 131:2113–2124
Piazza GJ, Lora JH, Garcia RA (2015) J Chem Techn Biotechnol 90:1419–1425
Zou C, Liang M, Chen X, Yan X (2013) J Appl Polym Sci 131:93–98
Dykes GM (2001) J Chem Techn Biotechnol 76:903–918
Hawker CJ, Wooley KL (2005) Science 309:1200–1205
Fréchet JM (2003) J Polym Sci A 41:3713–3725
Sadeghi-Kiakhani M, Arami M, Gharanjig K (2013) J Appl Polym Sci 127:2607–2619
Peng X, Peng X, Liu S, Zhao J (2009) Express Polym Lett 3:510–517
Goino M, Esumi K (1998) J Colloid Interface Sci 203:214–217
Gao C, Yan D (2004) Prog Polym Sci 29:183–275
Lin Y, Gao J-W, Liu H-W, Li Y-S (2009) Macromolecules 42:3237–3246
Magnusson CD, Kelland MA (2015) Energy Fuel 29:1336–2341
Zhang Y, Peng H, Huang W, Zhou Y, Zhang X, Yan D (2008) J Phy Chem C 112:2330–2336
Tu C, Li N, Zhu L, Zhou L, Su Y, Li P, Zhu X (2013) Polym Chem 4:393–401
Wang Y, Kotsuchibashi Y, Liu Y, Narain R (2014) Langmuir 30:2360–2368
Arts HJ, Derks FJM, Hyett W, Witters S, US2015021274-A1
Arts HJ, Derks FJM, Hyett W, Witters S, Arts H, Derks F, WO2013092800-A1
Arts HJ, Dikken T, Dinkelberg R, WO2014202756-A1
Hobson LJ, Feast WJ (1999) Polymer 40:1279–1297
Lois J, Kenwright AM (1997) Chem Commun 1877–1879
Guan Q, Zheng H, Zhai J, Zhao C, Zheng X, Tang X, Chen W, Sun Y (2014) Ind Eng Chem Res 53:5624–5635
Zhang X, Yang Z, Wang Y, Gao BY, Yue Q (2012) Chem Eng J 211–212:186–194
Nasser MS, James AE (2006) Sep Purif Techno 52:241–252
Mourey TH, Turner S, Rubinstein M, Fréchet J, Hawker C, Wooley K (1992) Macromolecules 25:2401–2406
Hawker CJ, Farrington PJ, Mackay ME, Wooley KL, Frechet JMJ (1995) J Am Chem Soc 117:4409–4410
Farrington PJ, Hawker CJ, Fréchet JMJ, Mackay ME (1998) Macromolecules 31:5043–5050
Hickenbottom KL, Hancock NT, Hutchings NR, Appleton EW, Beaudry EG, Pei X, Cath TY (2013) Desalination 312:60–66
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 51673191 and 51321062) and Jilin Province Science and Technology Development Project Foundation (Grant No. 20160101306JC). And we also grateful thank Tao Wang, Yuan Tao and Chao Chen for their help in supplying the oil-field wastewater to investigate the properties of flocculants and useful discussion.
Funding
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, K., Wang, H., Liang, X. et al. A Novel Hyperbranched Polymeric Flocculant for Waste-Water Treatment. J Polym Environ 26, 2782–2792 (2018). https://doi.org/10.1007/s10924-017-1120-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1120-4