Abstract
In the present work, adiabatic copolymerization allowed us to synthesize two poly(AM-4VP) (s) copolymers with various macromolecular weights as determined by viscosity measurement. The 1H-NMR was used for copolymer’s structure verification. UV–Visible was also used to determine the percentages of acrylamide (AM) and 4-vinylpyridine (4VP) monomers in each copolymer. Synthesized copolymers were tested in the aim to eliminate turbidity from bentonite suspension. A first study was realized on a conventional jar-test in order to determine the optimum parameters, such as time and the speed of stirring of different flocculants, during the flocculation process. Optimized parameters were then used on a semi-industrial pilot of coagulation/flocculation. Flocculation efficiency of the synthesized copolymers was compared with a commercial cationic flocculant FO4910 obtained from Sigma-Aldrich (France). The effect of macromolecular weight and 4VP amounts were also studied. The flocculation experiments results showed that a good turbidity removal superior to 80% was recorded using low copolymers concentrations of <5 mg/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
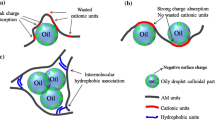
Polyacrylamides and their water soluble-derived copolymers became lately the most widely used materials in many fields, such as corrosion inhibition and water treatment by coagulation/flocculation [1, 2] as they offer the possibility to be charged. The routes of synthesis and modification of acrylamide (AM) to endow them with different functionalities were widely studied recently [3,4,5,6]. The cationic, anionic and zweterionic polyelectrolytes based on polyacrylamide were the most widely used polymers as coagulant/flocculant in the field of wastewater treatment because of their high macromolecular weight and charged sites, which are the most important parameters for the flocculation mechanism resulting from synergy phenomena of charge neutralization and adsorption [7,8,9]. Copolymerization of acrylamide and 4-vinylpyridine propylsulfobetaine using potassium persulfate (K2S2O8) as an initiator was studied by Gui et al. [10]. This study reported that the obtained copolymer showed better results in the elimination of both anionic kaolin and cationic hematite suspensions by flocculation compared to pure polyacrylamide. Another polyacrylamide-derived copolymer, poly(acrylamide-co-(4-vinilpyridine)) modified by quaternization was studied by Baojiao et al. [11], who observed good results in flocculation and corrosion inhibition. Flocculation of montmorillonite, bentonite and kaolinite suspensions using cationic biopolymers have also been reported by Huang and Chen [12] and several other groups [13,14,15]. In the light of these previous studies, we could conclude that the coagulation step was based on the opposite charges neutralization mechanism giving place to the flocculation step which led to the aggregation of colloidal particles forming flocs and eliminated by settlement [16, 17].
In the present work, we prepared two poly(acrylamide-co-(4-vinylpyridine)) (s) with different macromolecular weights and 4VP amounts. UV–Visible, viscosimetric measurement and 1H-NMR were used to characterize these copolymers. A comparative study was performed with a commercially available cationic FO4910 in jar-test and semi industrial pilot of flocculation in the aim to remove bentonite suspension turbidity.
Experimental
Materials and Reagents
For the synthesis of different copolymers, monomers, a solvent and a polymerization initiator were used. Acrylamide (AM) obtained from Merck (France) was used without further purification. 4-Vinylpyridine (4VP), purchased from Sigma-Aldrich (France), was purified by fractional distillation under reduced pressure. Ammonium persulfate (APS) acquired from Sigma-Aldrich (France) was used as an initiator. Bi-distilled water was used in all synthesis steps and aqueous copolymer solutions preparation. 1 M Hydrochloric acid (HCl) and 1 M sodium hydroxide (NaOH) solutions from Sigma-Aldrich (France) were used for the adjustment of pH in all the steps of the present work. Bentonite, which was used as a turbidity agent, was obtained from VWR (France). It is generally a mineral powder consisting of montmorillonite from the phyllosilicates family [18]. In addition, stable water suspension could be formed by bentonite giving turbidity, which is one of the major problems encountered with water [19]. In the aim to compare the flocculation efficiency of synthesized copolymers, a cationic hydrosoluble polymer FO4910 in the form of a white powder commercialized by SNF S.A.S (France) was used in this study as a flocculant of reference. The FO4910 aqueous solution was used at a concentration of 5 g/L and a pH = 2.5–4.5.
Copolymers Synthesis and Characterization
Radical adiabatic copolymerization of acrylamide and 4-vinylpyridine using two different amounts of aqueous ammonium persulfate solution (NH4)2S2O8 (APS) as an initiator in aqueous medium was performed to synthesize poly(AM-4VP) with different macromolecular weights [20]. The obtained copolymers had the structure shown in Fig. 1, which was confirmed by 1H-NMR (400 MHz) analysis. The UV–Visible spectrophotometry (Optizen 2120) was used to determine the effective weight ratio of each co-monomer in the obtained copolymers. The macromolecular weight was determined by viscosity measurement using a standard Ubbelhode capillary viscometer at 22 °C using the Mark–Houwink law [21, 22]. The different copolymers have been noted as poly(AM-4VP) X%, where X% is the percentage of the initiator’s mass.
Turbidity Measurement
Bentonite suspensions were prepared by direct dispersion of bentonite particles in bi-distilled water under vigorous stirring to obtain a homogenous suspension. The initial and final turbidities were then measured using a calibrated nephelo-turbiditymeter (Hanna HI-93703C). The bentonite suspensions showed a good stability. The natural decantation experiment of a suspension (100 mg/L) exhibited a weak turbidity removal of 20% after 48 h of decantation [17].
Jar-Test Operations
Coagulation-Flocculation tests were carried out on a six-spindle jar-test with stainless steel paddles. Different volumes of flocculant solution were added to each suspension and the final optimum concentration of flocculant was then calculated. The mixture was stirred under rapid agitation of 100 rpm for 30 s and then under reduced agitation of 50 rpm for further 4 min. At the end of the coagulation/flocculation process, the suspension was allowed to settle and the final turbidity (final Tu) was measured after various settling times [23, 24]. The turbidity removal efficiency was estimated by the following equation:
All flocculation tests were done in duplicate at ambient temperature 20 °C.
Semi-Industrial Coagulation/Flocculation Pilot
The flocculation behaviour of the different polymers was also studied on a semi-industrial installation as shown in Fig. 2.
An initial concentration of bentonite suspension of 100 mg/L was used as polluted water. The suspension was kept under stirring condition in a tank of 300 L (Fig. 2A). The flocculant solution was continuously added from the flocculant Tank (Fig. 2E) into the reactor during all the flocculation process. In the decanter (Fig. 2C), the mixture was allowed to settle and the turbidity of the produced water was then measured each 10-min during the 2-h treatment period [1]. All experimental parameters were optimized from the jar-test results such as the flocculant dose, speed and time of stirring during the process.
Results and Discussion
In the present study, the flocculant behaviour of acrylamide-based copolymers was studied in the aim to eliminate turbidity from bentonite suspension. The first step of our work was the synthesis of two different copolymers by adiabatic radical polymerization using varying amounts of the initiator (APS%). As shown in Table 1, the obtained copolymers were purified by dissolution/precipitation using water/acetone (solvent/non-solvent), and then dried in a desiccator.
Characterization by 1H-NMR
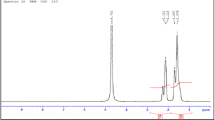
The structure of the synthesized copolymers was assessed by 1H-NMR for poly(AM-4VP) 0.1% using Bruker Advance 400 spectrometer in D2O/DCl used as a solvent. The spectrum obtained is shown in Fig. 3 while the attribution of signals is reported in Table 2 [10, 25, 26].
From Table 2, we could observe that all the characteristic protons of the different chained monomers as well as their functional groups were present on the spectrum. Similarly, all characteristic peaks of poly (AM-4VP) 0.5% in D2O were also noticeable on the NMR spectrum.
Characterization by UV–Visible
UV–Visible spectra depicted in Fig. 4 were recorded on UV–Visible Optizen 2120 for the aqueous copolymer solutions with the same concentration of 10−4 g/mL. We could note that these copolymers had two major transitions one at λmax = 200 nm, which could be attributed to the acrylamide (AM) groups, and another at λmax = 255 nm, which is specific to the 4-vinylpyridine (4VP) groups. The absorbance value (A) varies from one polymer to another. Using the law of Beer–Lambert (Eq. 2) shown below, one could calculate the mass percentage of 4VP unit in each copolymer and thereafter deduct the percentage of AM.
where A, ε, C and l are the absorbance, the molar attenuation coefficient ε = 1.66.103 (L/mole cm) for the poly(4-vinylpyridine); the copolymers solutions’ concentration C = 1.10−4 (g/mL) and the path length l = 1 (cm), respectively.
The effective weight composition of the copolymer was estimated by UV–Visible [27]. The percentage of each co-monomer was calculated (AM% and 4VP%) and findings were reported in Table 1.
It could be observed that the amount of the initiator used is directly related to the final percentage of the two monomers. Thus, it could be concluded that the reactivity of 4VP increased with the percentage of the initiator (APS). Therefore, the investigation of the effect of the copolymers’ chemical composition on the efficiency of flocculation process was performed [20, 28].
Viscosimetric Macromolecular Weight Mv Determination
The viscosimetric measurements were carried out using a capillary viscometer Ubbelhode. The temperature was maintained constant by using a thermostat at 22 ± 1 °C. Several concentrations were prepared for both copolymers in the presence or absence of 0.1 M NaCl. As depicted in Figs. 5 and 6, the variation of reduced viscosity of poly(AM-4VP) copolymers was a function of their concentration in the presence or absence of NaCl salt for different percentages of the initiator (0.5 and 0.1%) at 22 °C and a weakly acidic aqueous medium (pH ≈ 6). The obtained values of reduced viscosity for the two copolymers were in agreement with the results published by Mansri et al. [28] and other previous studies.
The values of the intrinsic viscosities [η] were determined by extrapolating the linear part of the curves and the values of the viscosimetric macromolecular weight (MV) were calculated using the Mark–Houwink relation [29]. The results obtained were reported in the Table 3.
Application in Turbidity Removal by Flocculation
Jar-Test Flocculation Experiments
In order to optimize the experimental parameters of the bentonite suspensions flocculation, a study was carried out on the jar-test, which allowed us to determine the optimum concentration of the various flocculants in addition to the stirring speed and time.
An initial turbid water 23 NTU was prepared by dispersion of 100 mg/L of bentonite powder in bi-distilled water. An aqueous stock solution of each flocculant was prepared at the concentration of 10−3 g/mL. 1 M HCl solution was used to adjust the pH of the poly(AM-4VP) flocculant solution and set it at 2.5. After adding the flocculant solution, the mixture was stirred at 100 rpm for 30 s and then at a reduced speed of 50 rpm for further 4 min to allow flocs to aggregate. The mixture was finally allowed to settle and the final turbidity was measured after different settling times. The results obtained were summarized in Table 4.
Figure 7a–c showed the efficiency of bentonite turbidity removal as a function of the concentration of each flocculant after different settling times. All the curves of turbidity removal showed an optimum elimination with the different flocculants. The poly(AM-VP) 0.5% led to 89% of turbidity removal with a concentration of 3 mg/L, while the poly(AM-VP) 0.1% recorded 87% of elimination with 1 mg/L. This result could be explained by the high macromolecular weight of poly(AM-VP) 0.1% and the small amount of 4-VP in the copolymer compared to the poly(AM-VP) 0.5% as mentioned in Table 1. The flocculant FO4910 had 90% of elimination with a concentration of 6 mg/L. The new synthesized copolymers showed also a good flocculant behaviour in acid pH medium. The treated water had a weak turbidity level of <3 NTU at the optimum concentration of each flocculant. The decantation step showed a slight improvement as a function of time, which meant that it was almost complete after the first 5 min of decantation as shown in Fig. 8.
After determining the optimum concentration of each flocculant, flocculation tests were carried out after keeping the optimum dose constant and varying the stirring speed of the mixture for 4 min. The results obtained were depicted in Fig. 9.
The turbidity removal from bentonite suspension 100 mg/L as a function of the stirring speed using the optimum concentration of each flocculant, previously determined, was evaluated. From the findings shown in Fig. 9, it could be concluded that the elimination efficiency increased with increasing stirring speed up to 50 rpm, beyond which a stability was noticed with all flocculants tested. The value of 50 rpm could therefore be considered as the optimum stirring speed for flocculation tests. Finally, to optimize stirring time, a set of flocculation tests was performed by varying stirring time and keeping the optimum concentration of the flocculant constant and the stirring speed set at 50 rpm.
Results of the different experiments were plotted in Fig. 10. The efficiency of turbidity removal was given as a function of stirring time under 50 rpm and the optimum concentration of each flocculant. It could be concluded from the results observed that the flocculation efficiency increased with the stirring time for all three flocculants. However, a slight stability was also observed beyond 5 min of stirring.
Pilot Flocculation Experiments
Following the study of the efficiency of turbidity removal by coagulation/flocculation using various copolymers solutions on the jar-test, an optimization of the experimental parameters was done, where the optimum dose of every copolymer in addition to the optimal stirring speed and time were determined. In a second step, flocculation tests were conducted on a Semi-industrial pilot as shown in Fig. 2. To this end, we prepared a mother solution of each copolymer at the desired concentration and pH; i.e. (AM-VP 0.5%) 1 mg/L at pH = 2.5, (AM-4VP 0.1%) 0.5 mg/L at pH = 2.5 and (FO4910) 1 mg/L at pH = 4. The bentonite suspension of 100 mg/L, pH = 8 was continuously stirred and homogenized in a tank of 300 L and added to the reactor of 20 L with a flow rate of 80 L/h.
Copolymer solutions were added into the reactor at a flow rate of 210 mL/h for both poly(AM-co-4VP) 0.1% and poly(AM-co-4VP) 0.5%, and at a flow rate of 300 mL/h for FO4910. The flow rates were adjusted to achieve the optimum concentration of each flocculant inside the reactor. During the flocculation process, the mixture in the reactor was stirred under a speed of 100 rpm. When the reactor was completely filled, the treated suspension flowed into the decanter previously filled with clear water (Tu = 0 NTU). At the decanter outlet, the treated water turbidity was measured every 10 min for 2 h. The same experiments were repeated with and without lamella in the decanter in order to deduce the impact on the quality of the treated water. The flocculation process showed no significant effect on the value of initial pH = 8. The turbidity removal results using the three flocculants were reported in Table 5.
Figure 11a, b showed the turbidity removal using pilot with and without lamella as a function of decantation time with the three flocculants used. It could be concluded that the use of the lamella improved the quality of the treated water with all the three flocculants. The elimination efficiency went from 77 to 80% with poly(AM-co-4VP) 0.5%, from 62 to 79 with poly(AM-co-4VP) 0.1% and finally from 75 to 80% with FO4910 after 2 h of treatment. Synthesized copolymers showed results in alignment with previous findings reported by Mansri et al. [1] where a charged poly(acrylamide) polymer was used for turbidity removal from bentonite suspension by flocculation.
Conclusion
Radical adiabatic copolymerization led to the generation of high macromolecular weight copolymers with different amounts of AM and 4VP monomers. Several methods were used for the characterisation of the synthesized copolymers such as 1H-NMR for structural analysis and UV–Visible for determining the relative amounts of monomers in each copolymer. The macromolecular weights were calculated by viscosity. As an application in wastewater treatment, the flocculation of turbid water was studied firstly on a jar-test and then on a semi-industrial pilot of flocculation. Poly(AM-co-4VP) (s) showed good flocculant behaviour in comparison to a commercially available flocculant FO4910. At low optimum concentrations of poly(AM-co-4VP) (s), flocculation efficiency was superior to 80% when turbidity removal yield was recorded. Copolymer macromolecular weight, the amount of 4VP and the pH of flocculant solution were considered as the major parameters affecting flocculation efficiency. However, future studies are warranted to further investigate the effect of initial turbidity level on flocculation and the elimination of other complex pollutants or metal/bentonite.
References
Mansri A, Bendraoua A, Benmoussa A, Benhabib K (2015) New polyacrylamide [PAM] material formulations for the coagulation/flocculation/decantation process. J Polym Environ 23:580–587
Mansri A, Bouras B, Tennouga L, Medjehed K (2012) Effect of iodide ion on corrosion inhibition of mild steel in H2SO4 by polyacrylamide with different macromolecular weight and polyacrylamide poly4-vinylpyridine mixture. Der Pharm Chem 4(5):1803–1811
Yian R (1988) Water soluble polymer. Chemical Industry Press, Beijing
Bastiat G, Grassl B, Francois J (2002) The behaviour of poly(propylene oxide) in aqueous media, the synthesis of a thermo-associative and hydrosoluble copolymer based on PPO, and its properties in solution. Polym Int 51:958–965
Glass JE (2000) Associative polymers in aqueous media, ACS symposium series 765 Washington. American Chemical Society, Washington
El-Hamshary H, El-Garawany M, Assubaie F, Al-Eed M (2003) Synthesis of poly(acrylamide-co-4-vinylpyridine) hydrogels and their application in heavy metal removal. J Polym Sci 89:2522–2526
Wan XF, Li YM, Wang XJ, Chen SL, Gu XY (2007) Synthesis of cationic guar gum-graft-polyacrylamide at low temperature and its flocculating properties. J Eur Polym 43:3655–3661
Zheng BQ, Qian JW, Li XK, Zhu ZH (2007) effect of the parent solution concentration on the flocculation performance of PAAm flocculants and the relation between the optimal parent solution concentration and critical concentrations. J Appl Polym Sci 103:1588–1592
Qian JW, Xiang XJ, Yang WY, Wang M, Zheng BQ (2004) Flocculation performance of different polyacrylamide and the relation between optimal dose and critical concentration. J Eur Polym 40:1699–1704
Gui Z, Qian J, An Q, Xu H, Zhao Q (2009) Synthesis, characterization and flocculation performance of zwitterionic copolymer of acrylamide and 4-vinylpyridine propylsulfobetaine. J Eur Polym 45:1403–1411
Baojiao G, Yuexian L, Hongfang J (2003) Synthesis and properties of cationic polyacrylamide containing pyridine quaternary salt. J Poly Int 52:1468–1473
Huang CP, Chen Y (1996) Coagulation of colloidal particles in water by chitosan. J Chem Technol Biotechnol 66:227–232
Divakaran R, Sivasankara pillai VN (2001) Flocculation of kaolinite suspensions in water by chitosan. Water Res 35:3904–3908
Liang C, Donghui C, Chongliang W (2003) A new approach for the flocculation mechanism of chitosan. J Polym Environ 11:87–92
Haradhan K, Dinabandhu S, Tridib T (2016) Novel biodegradable flocculating agents based on grafted starch family for the industrial effluent treatment. J Polym Environ. doi:10.1007/s10924-016-0825-0
Lee KE, Norhashimah M, Tjoon T, Beng Teik P (2012) Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater. Chem Eng J 203:370–386
Labanda J, Lorens J (2008) The optimization of the process of the coagulation/flocculation. Chem Eng Process 47:1061–1068
Shen YH (2005) Treatment of low turbidity water by sweep coagulation using bentonite. J Chem Technol Biotechnol 80:581–586
Mansri A, Ramdani N (2015) In situ polymerization of 4-vinylpyridine/bentonite composites and their application for toluene removal. Res Chem Intermed 41:1765–1776
Bouras B, Mansri A, Tennouga L, Grassl B (2015) Influence parameters in controlled adiabatic copolymerization of acrylamide/4-vinylpyridine (AM/4VP) system in aqueous media. Res Chem Intermed 41:5839–5858
Mansri A, Tennouga L, Bouras B (2014) Additive effect on the behavior of water-soluble hydrolyzed polyacrylamide copolymer. J Mater Environ Sci 5:37–42
Mansri A, Tennouga L, Desbrieres J (2007) Viscosimetric behaviour of hydrolyzed polyacrylamide-poly(4-vinylpyridine) [AD37-P4VP] mixture in aqueous solution. J Eur Polym 43:540–549
Nasser MS, James AE (2006) The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions. Sep Purif Technol 52:241–252
Zhen Y, Hu Y, Ziwen J, Tao C, Haijianj L, Aimin L, Rongshi C (2013) Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J Hazard Mater 254:36–45
Mansri A, Bouras B, Hammouti B, Warad I, Chetouani A (2014) Synergistic effect of AM-4VP-9 copolymer and iodide ion on corrosion inhibition of mild steel in 1 M H2SO4. Res Chem Intermed 39:1753–1770
Belkaid S, Tebbji K, Mansri A, Chetouani A (2012) Poly(4-vinylpyridine-hexadecyl bromide) as corrosion inhibitor for mild steel in acid chloride solution. Res Chem Intermed 38:2309–2325
Bernard Y, Coleman D, Fuoss RM (1955) Quaternization kinetics. I. Some pyridine derivatives in tetramethylene sulfone. J Am Chem Soc 77:5472–5478
Mansri A, Bendraoua A, Bouras B (2015) Physicochemical behaviour of poly(acrylamide-co-(4-vinylpyridine)). Mor J Chem 3:47–57
Brandrup J, Immergut EH, Grulke EA (1999) Polymer hand book, 4th edn. Wiley Interscience, New York
Acknowledgements
This work was supported by funds from “Direction Générale de la Recherche Scientifique et du Développement Technologique” (DGRSDT), Algeria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hocine, T., Benhabib, K., Bouras, B. et al. Comparative Study Between New Polyacrylamide Based Copolymer Poly(AM-4VP) and a Cationic Commercial Flocculant: Application in Turbidity Removal on Semi-Industrial Pilot. J Polym Environ 26, 1550–1558 (2018). https://doi.org/10.1007/s10924-017-1049-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1049-7