Abstract
The purpose of this study aimed to investigate the clinicopathologic characteristics of breast cancer according to its cancer-associated fibroblast (CAF) phenotype. Immunohistochemistry staining of estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER-2), Ki-67, podoplanin, prolyl 4-hydroxylase, fibroblast activation protein alpha (FAPα), S100A4, platelet-derived growth factor receptor alpha (PDGFRα), PDGFRβ, and chondroitin sulfate proteoglycan (NG2) was performed on tissue microarray consisting of 642 breast cancer cases. Samples were categorized into luminal A, luminal B, HER-2, or triple-negative breast cancer (TNBC) according to immunohistochemical results, whereas tumor stroma was classified into desmoplastic, sclerotic, normal-like, or inflammatory type based on histological findings. Expression of CAF-related proteins in the stroma differed depending on breast cancer molecular subtypes. All CAF-related protein expression was high (p < 0.05) in HER-2 type, whereas in luminal A, the expression of FAPα, PDGFα, PDGFβ, and NG2 was low, and in TNBC, the expression of podoplanin, prolyl 4-hydroxylase, and S100A4 was low. In the stromal component, CAF-related protein expression differed according to stromal phenotype (p < 0.001). The desmoplastic type showed high expression of podoplanin, prolyl 4-hydroxylase, S100A4, PDGFRα, and PDGFRβ, whereas the sclerotic type exhibited low expression of FAPα, PDGFα, PDGFβ, and NG2. The inflammatory type had high expression of FAPα and NG2 with low podoplanin, while normal-like type showed low expression of prolyl 4-hydroxylase and S100A4. Our results suggested that differential CAF-related protein expression depended on the molecular subtypes and stromal histologic features of breast cancer, indicating that in the future, this system could potentially use these markers for prognosis prediction and targeted therapy of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of tumor microenvironment has become increasingly significant as cancer research progresses. Tumor microenvironment is the non-transformed element located in the tumor area, consisting of the immune system component (such as macrophages and lymphocytes), blood vessel cells, fibroblast, myofibroblast, mesenchymal stem cells, adipocytes, and extracellular matrix (ECM). The most important and best studied elements of tumor microenvironments are cancer-associated fibroblasts (CAF) [1–3], which surround cancer cells and are related to tumor initiation [4], tumor-stimulatory inflammation [5], metabolism [6], metastasis [7], drug response [8], and immune surveillance [9]. Despite their important effects on cancer, no specific cell origin of CAFs has been identified, and the accurate definition of CAF remains controversial [1, 10]. Many possible CAF markers have been suggested, such as α-smooth muscle actin [11], tenascin-C [12], chondroitin sulfate proteoglycan (NG2) [13], platelet-derived growth factor receptor (PDGFR) α/β [14], fibroblast activation protein (FAP) [15], podoplanin [16], prolyl 4-hydroxylase [17], and fibroblast-specific protein (FSP) 1 [13]. Therefore, CAFs are thought to have various functional subtypes, and a recent study has divided them into four main subsets as follows: FAPα, FSP1, PDGFRα, and PDGFRβ types with different features [18]. It is reported that FAP-type CAFs are activated or reactive [19] and associated with modulation of ECM [20], tumor cell invasion [20], and immunomodulatory function, while FSP1-type CAFs are associated with metastatic colonization [21], macrophage infiltration [22], and protection from carcinogens [23]. Also, it is shown that PDGFRα-type CAFs have a relationship with paracrine signaling for tumor cell growth and angiogenesis [24], as well as macrophage recruitment [25].
The cross-talk between CAFs and breast cancer cells has been actively studied, suggesting that CAFs might exert significant effects on tumors as breast cancer is representative of malignancies with a diverse amount of tumor stroma. Furthermore, CAFs have been associated with tumor progression [26], invasion or metastasis [27, 28], therapeutic resistance [29], and prognosis in breast cancer [30]. However, the correlation between breast cancer and CAF phenotype has not been well defined. The purpose of this study was to investigate the relationship between clinicopathologic factors and different breast cancer CAF phenotypes.
Materials and methods
Patient selection and histologic evaluation
The present study included patients diagnosed with invasive ductal carcinoma, not otherwise specified, at Severance Hospital from January 2000 to December 2006. Patients receiving chemotherapy, radiotherapy, or hormone treatment before surgery were excluded. In total, there were 642 cases. This study was approved by the Institutional Review Board of Yonsei University Severance Hospital, which also exempted the requirement for patients’ informed consent. All cases were reviewed by a breast pathologist (Koo JS) using hematoxylin and eosin (H&E)-stained slides. Histological grades were assessed according to the Nottingham grading system [31]. Clinicopathologic parameters evaluated in each case included patients’ age at the initial diagnosis, lymph node metastasis, tumor recurrence, distant metastasis, and survival.
The tumor stroma of all breast cancer cases was classified based on microscopic observation as follows: desmoplastic type, when the tumor stroma was composed of cellular fibroblast or myofibroblast proliferation (the number of fibroblasts or myofibroblasts/high power field (HPF): >30); sclerotic type, when the cell component was low (the number of fibroblasts or myofibroblasts/HPF: ≤30) and the tumor stroma was composed of fibrotic collagenous tissue; normal-like type, when no stromal reaction near the tumor and normal breast stroma was observed (different from desmoplastic type in the lack of stromal reaction, and different from sclerotic type in the lack of sclerotic collagenous tissue); and inflammatory type, when the tumor stroma was mostly composed of inflammatory cells such as lymphocytes (the number of inflammatory cells/HPF: >200).
Tissue microarray
A representative area of tumor and tumor stroma was selected on an H&E-stained slide, and a corresponding spot was marked on the surface of the paraffin block. The selected area was obtained using a biopsy needle, and a 3-mm tissue core was transferred to a 6 × 5 recipient block. Two such tissue cores were extracted from invasive tumors to minimize extraction bias. Each tissue core was assigned a unique tissue microarray location number that was linked to a database containing other clinicopathologic data.
Immunohistochemistry
All antibodies used for immunohistochemistry are listed in Table 1. Immunohistochemical studies were performed using formalin-fixed, paraffin-embedded tissue sections. Briefly, 5-μm-thick sections were obtained using a microtome, transferred onto adhesive slides, and dried at 62 °C for 30 min. After incubation with primary antibodies, immunodetection was performed with biotinylated anti-mouse or anti-rabbit immunoglobulin, followed by peroxidase-labeled streptavidin using a labeled streptavidin biotin kit with 3,3′-diaminobenzidine chromogen as the substrate. The previous antibody incubation stem was omitted in negative controls. Tissue samples for positive controls were obtained as per the manufacturer’s recommendation. Slides were counterstained with Harris hematoxylin.
Interpretation of immunohistochemical staining results
All immunohistochemical staining was evaluated by light microscopy. A cut-off value of 1 % or more positively stained nuclei was used to define estrogen receptor (ER) and progesterone receptor (PR) positivity [32]. Human epidermal growth factor receptor 2 (HER-2) staining was analyzed according to the American Society of Clinical Oncology/College of American Pathologists guidelines using the following categories: 0, no immunostaining; 1+, weak incomplete membranous staining, less than 10 % of tumor cells; 2+, complete membranous staining, either uniform or weak in at least 10 % of tumor cells; and 3+, uniform intense membranous staining in at least 30 % of tumor cells [33]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases with 0 to 1+ staining were regarded as negative, and those showing 2+ expression were further evaluated for HER-2 amplification by fluorescent in situ hybridization (FISH). The HER-2 gene copy number on the slides was evaluated using an epifluorescence microscope (Olympus, Tokyo, Japan). At least 60 tumor cell nuclei in three separate regions were investigated for HER-2 and chromosome 17 signals. HER-2 gene amplification was determined using the ASCO/CAP guidelines [33]. An absolute HER-2 gene copy number lower than 4 or an HER-2 gene-to-chromosome 17 ratio (HER-2/Chr17 ratio) less than 1.8 was considered HER-2 negative. An absolute HER-2 copy number between 4 and 6 or an HER-2/Chr17 ratio between 1.8 and 2.2 was considered HER-2 equivocal. Also, an absolute HER-2 copy number greater than 6 or an HER-2/Chr17 ratio higher than 2.2 was considered HER-2 positive.
Immunohistochemical staining for podoplanin, prolyl 4-hydroxylase, FAPα, S100A4, PDGFα, PDGFβ, and NG2 was accessed via light microscopy. Semi-quantitative evaluation was performed on stained slides using previously reported methods [34]. Tumor and stromal cell staining was assessed as follows: 0, negative, or weak immunostaining in <1 % of the tumor/stroma; 1, focal expression in 1–10 % of the tumor/stroma; 2, positive in 11–50 % of the tumor/stroma, and 3, positive in 51–100 % of the tumor/stroma. The whole area of the tumor was evaluated and defined as positive when the score exceeded 2. Immunostaining only in the ECM was discounted.
Tumor phenotype classification
In this study, we classified breast cancer phenotypes according to the immunohistochemistry results for ER, PR, HER-2, and Ki-67 as well as FISH results for HER-2 as follows [35]: luminal A type, ER or/and PR positive, HER-2 negative and Ki-67 labeling index (LI) <14 %; luminal B type (HER-2 negative), ER or/and PR positive, HER-2 negative, and Ki-67 LI ≥14 %; luminal B type (HER-2 positive), ER or/and PR positive, and overexpressed or/and amplified HER-2; HER-2 overexpression type, ER and PR negative, and overexpressed or/and amplified HER-2; and triple-negative breast cancer (TNBC) type, ER, PR, and HER-2 negative.
Statistical analysis
Data were analyzed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). For determination of statistical significance, Student’s t and Chi-square tests were used for continuous and categorical variables, respectively. In cases of multiple comparisons, a corrected p-value with the application of the Bonferroni multiple comparison procedure was used. Statistical significance was set to p < 0.05. Kaplan–Meier survival curves and log-rank statistics were employed to evaluate time to tumor recurrence and overall survival. Multivariate regression analysis was performed using the Cox proportional hazards model.
Results
Characteristics of breast cancer cases
In this study of 642 cases, 275 were of luminal A type (42.8 %), 152 of luminal B type (23.7 %), 58 of HER-2 type (9.0 %), and 157 were TNBC (24.5 %). TNBC patients had a higher histologic grade (p < 0.001), T stage (p = 0.004), Ki-67 LI (p < 0.001), tumor recurrence (p = 0.013), and death rate (p = 0.008) than other subtypes, whereas those with HER-2 type had a higher rate of older age (p = 0.014) (Table 2).
Clinicopathologic features according to stromal phenotype
When the studied cases were categorized based on the tumor stromal histology, 181 were of desmoplastic type (28.1 %), 62 of inflammatory type (9.7 %), 25 of normal-like type (3.9 %), and 374 of sclerotic type (58.3 %). There were significant differences in histologic grade (p = 0.006), ER status (p < 0.001), PR status (p < 0.001), HER-2 status (p = 0.020), Ki-67 LI (p < 0.001), and molecular subtype (p < 0.001) according to tumor stromal phenotype. The inflammatory type exhibited higher histologic grade, ER negativity, PR negativity, HER-2 positivity, and higher Ki-67 LI, whereas the sclerotic type showed lower histologic grade, ER positivity, PR positivity, and lower Ki-67 LI. Moreover, TNBC was more frequently observed among tumors of inflammatory and normal-like types, whereas luminal A was prominent in desmoplastic and sclerotic types (Table 3).
Expression of CAF-related proteins according to molecular subtype
The expression of all CAF-related proteins, except PDGFRβ and NG2, was observed in not only the stromal component but also cancer cells. Cancer cell expression of podoplanin and S100A4 was more commonly observed in HER-2 type (p = 0.001 and p < 0.001, respectively), whereas expression of FAPα and PDGFRα was more commonly seen in TNBC (p < 0.001). On the other hand, luminal types less commonly expressed FAPα, S100A4, and PDGFRα (p < 0.001).
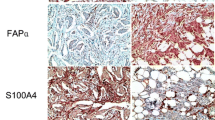
All CAF-related proteins were more commonly expressed in the stromal component of HER-2 type (p < 0.05), whereas luminal A type less commonly expressed FAPα, PDGFRα, PDGFRβ, and NG2. In addition, the expression of podoplanin, prolyl 4-hydroxylase, and S100A4 was less commonly observed in TNBC (Table 4; Fig. 1).
Expression of CAF-related proteins according to molecular subtype. Tumoral expression of podoplanin and S100A4 was more commonly seen in HER-2 type, whereas FAPα and PDGFRα expressions were more commonly seen in triple-negative breast cancer cells. The expression of CAF-related proteins in the stromal component was as high as their overall expression in HER-2 type. PDGFRβ and NG2 were not expressed in cancer cells
Expression of CAF-related proteins according to stromal phenotype
The expression of prolyl 4-hydroxylase and PDGFRα in cancer cells differed depending on stromal phenotype (p = 0.003 and p < 0.001, respectively). Tumoral expression of prolyl 4-hydroxylase was more commonly seen in the desmoplastic type yet observed less in the inflammatory type, whereas PDGFRα expression in tumor cells was more commonly seen in the normal-like type and less commonly seen in the sclerotic type.
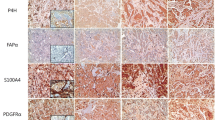
Furthermore, the expression of all CAF-related proteins in the stromal component also differed according to stromal phenotype (p < 0.001). The desmoplastic type more commonly expressed podoplanin, prolyl 4-hydroxylase, S100A4, PDGFRα, and PDGFRβ in the stromal component, whereas FAPα, PDGFRα, PDGFRβ, and NG2 expression in the sclerotic type was less commonly seen. The inflammatory type exhibited FAPα and NG2 expressions more commonly and podoplanin expression less commonly in the stromal component, whereas the normal-like type less commonly expressed prolyl 4-hydroxylase and S100A4 (Fig. 2; Table 5).
Expression of CAF-related proteins according to stromal histologic phenotype. Tumor cell expression of prolyl 4-hydroxylase was high in the desmoplastic type, whereas that of PDGFRα was high in the normal-like type. Desmoplastic type had high expression of podoplanin, prolyl 4-hydroxylase, S100A4, PDGFRα, and PDGFRβ in the stromal component, while expression of FAPα and NG2 was high in the inflammatory type. Both PDGFRβ and NG2 were not expressed in cancer cells
Correlation between the expression of CAF-related proteins and clinicopathologic factors
Stromal FAPα positivity (p < 0.001), tumor S100A4 positivity (p = 0.001), and tumor PDGFRα positivity (p < 0.001) were related to higher histologic grade. Stromal NG2 positivity (p < 0.001), stromal PDGFRβ positivity (p = 0.003), tumor FAPα positivity (p < 0.001), tumor S100A4 positivity (p < 0.001), and tumor PDGFRα positivity (p < 0.001) were associated with ER negativity. PR negativity was related to stromal NG2 positivity (p < 0.001), tumor FAPα positivity (p < 0.001), tumor S100A4 positivity (p < 0.001), and tumor PDGFRα positivity (p = 0.002). On the other hand, HER-2 positivity was associated with stromal PDGFβ positivity (p < 0.001), tumor podoplanin positivity (p = 0.001), stromal prolyl 4-hydroxylase positivity (p < 0.001), and stromal S100A4 positivity (p < 0.001). In addition, stromal NG2 positivity (p < 0.001), stromal PDGFRβ positivity (p < 0.001), tumor prolyl 4-hydroxylase negativity (p < 0.001), stromal FAPα positivity (p < 0.001), tumor S100A4 positivity (p = 0.001), tumor PDGFRα positivity (p < 0.001), and stromal PDGFRα positivity (p = 0.001) were related to higher Ki-67 LI (Fig. 3).
Impact of CAF-related protein expression on patient prognosis
Univariate analysis revealed that tumor S100A4 negativity and S100A4 positivity was related to shorter disease-free survival (DFS, p = 0.015) and overall survival (OS, p = 0.016), respectively (Table 6; Fig. 4). Furthermore, analysis by molecular subtype showed that tumor podoplanin positivity was associated with shorter OS in luminal A (p = 0.002), while tumor FAPα positivity with shorter OS in luminal B (p = 0.018). Additionally, stromal phenotype was associated with varying DFS in TNBC, with normal-like type having shorter DFS (p = 0.008) (Fig. 5).
Discussion
Our results from the present study indicated differential expression of CAF-related proteins in the stromal component of breast cancer according to the molecular subtype. Different molecular subtypes of breast cancer exhibit various clinical, histological, molecular, and therapeutic features, possibly owing to different cancer cell characteristics [36–39]. Consequently, the cross-talk between breast cancer cells and the tumor stroma might differ, affecting tumor microenvironment [40]. In the present study, we tested the hypothesis that the tumor stroma exhibited different characteristics depending on breast cancer subtype. Differential CAF gene expression according to breast cancer molecular subtype has been reported previously [41], with the expression of genes associated with cancer cell migration being higher in CAFs from HER-2 type than those from TNBC and ER-positive type. Our results also showed high expression of all CAF-related proteins in HER-2 type (p < 0.05). Since CAF-related proteins are reportedly associated with tumor invasiveness [18], such results were in agreement with those from prior studies.
In this study, the expression of all CAF-related proteins in the stromal component also differed according to stromal histologic phenotype, with the desmoplastic type showing high expression of podoplanin, prolyl 4-hydroxylase, S100A4, PDGFRα, and PDGFRβ, and the inflammatory type having high FAPα and NG2 expressions. Although the tumor stroma of breast cancer has been histologically categorized in previous studies [42], the differences in CAF-related protein expression according to the tumor stroma histology has not been investigated. Nonetheless, CAF markers are known to possess distinct features, despite difficult comparison. Our results were in agreement with previous reports that the desmoplastic responses of PDGFRα-type CAFs occurred via PDGF-AA signal in breast cancer [43]. Similarly, we observed high FAPα expression in the inflammatory type, which was in agreement with reports that FAPα was associated with immunomodulatory function [18]. Therefore, our findings suggested a relationship between CAF features and tumor stromal histology, which warrants further investigation.
Furthermore, we found that tumor expression of CAF-related proteins, but not their expression in the stromal component was related to patient prognosis, and that tumor S100A4 positivity was associated with poor prognosis in breast cancer. Previous studies have demonstrated such a relationship between tumor expression of S100A4 and poor prognosis in hepatocellular carcinoma [44], endometrial carcinoma [45], lung cancer [46], and pancreatic cancer [47]. In addition, our results on the association between poor prognosis and tumor podoplanin positivity in luminal A type were also in agreement with other reports on podoplanin expression in esophageal cancer [48] and oropharyngeal cancer [49]. Similar results on FAPα were also described in osteosarcoma [50] and pancreatic cancer [51]. Therefore, CAF-related proteins could be used as prognostic markers in breast cancer. This study shows that normal-like stroma are associated with shorter DFS in TNBC; however, the previous study suggested that stroma-high-type TNBC tumors (those with a high rate of stroma) have a poor prognosis compared to stroma-low-type tumors, which indicates that stroma rate is associated with prognosis in TNBC [52]. However, this study did not include stroma rates and is only categorized within a histological view; therefore, further study is required.
The clinical implication of our findings was that CAFs might serve as a potential target for cancer therapy. However, a specified agent might be required for each CAF phenotype to achieve efficacy. Therefore, future research on such targeted agents depending on CAF phenotype is needed.
In conclusion, our results indicated differential expression of CAF-related proteins according to breast cancer molecular subtype and stromal histologic type. We also observed high expression of CAF-related proteins in the tumor stroma of HER-2 and desmoplastic types.
References
Franco OE, Shaw AK, Strand DW et al (2010) Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 21(1):33–39. doi:10.1016/j.semcdb.2009.10.010
Mueller MM, Fusenig NE (2004) Friends or foes–bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4(11):839–849. doi:10.1038/nrc1477
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401. doi:10.1038/nrc1877
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432(7015):332–337. doi:10.1038/nature03096
Mueller L, Goumas FA, Affeldt M et al (2007) Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol 171(5):1608–1618. doi:10.2353/ajpath.2007.060661
Pavlides S, Whitaker-Menezes D, Castello-Cros R et al (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8(23):3984–4001
Karnoub AE, Dash AB, Vo AP et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–563. doi:10.1038/nature06188
Muerkoster S, Wegehenkel K, Arlt A et al (2004) Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res 64(4):1331–1337
Fearon DT (2014) The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res 2(3):187–193. doi:10.1158/2326-6066.cir-14-0002
Ostman A (2014) Cancer-associated fibroblasts: recent developments and emerging challenges. Semin Cancer Biol 25:1–2. doi:10.1016/j.semcancer.2014.02.004
Desmouliere A, Guyot C, Gabbiani G (2004) The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 48(5–6):509–517. doi:10.1387/ijdb.041802ad
De Wever O, Nguyen QD, Van Hoorde L et al (2004) Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 18(9):1016–1018. doi:10.1096/fj.03-1110fje
Sugimoto H, Mundel TM, Kieran MW et al (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5(12):1640–1646
Pietras K, Sjoblom T, Rubin K et al (2003) PDGF receptors as cancer drug targets. Cancer Cell 3(5):439–443
Kraman M, Bambrough PJ, Arnold JN et al (2010) Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330(6005):827–830. doi:10.1126/science.1195300
Kawase A, Ishii G, Nagai K et al (2008) Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer 123(5):1053–1059. doi:10.1002/ijc.23611
Kojima Y, Acar A, Eaton EN et al (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 107(46):20009–20014. doi:10.1073/pnas.1013805107
Cortez E, Roswall P, Pietras K (2014) Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol 25:3–9. doi:10.1016/j.semcancer.2013.12.010
Scanlan MJ, Raj BK, Calvo B et al (1994) Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA 91(12):5657–5661
Lee HO, Mullins SR, Franco-Barraza J et al (2011) FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 11:245. doi:10.1186/1471-2407-11-245
O’Connell JT, Sugimoto H, Cooke VG et al (2011) VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108(38):16002–16007. doi:10.1073/pnas.1109493108
Zhang J, Chen L, Xiao M et al (2011) FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178(1):382–390. doi:10.1016/j.ajpath.2010.11.017
Zhang J, Chen L, Liu X et al (2013) Fibroblast-specific protein 1/S100A4-positive cells prevent carcinoma through collagen production and encapsulation of carcinogens. Cancer Res 73(9):2770–2781. doi:10.1158/0008-5472.can-12-3022
Crawford Y, Kasman I, Yu L et al (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15(1):21–34. doi:10.1016/j.ccr.2008.12.004
Erez N, Truitt M, Olson P et al (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147. doi:10.1016/j.ccr.2009.12.041
Gao MQ, Kim BG, Kang S et al (2013) Human breast cancer-associated fibroblasts enhance cancer cell proliferation through increased TGF-alpha cleavage by ADAM17. Cancer Lett 336(1):240–246. doi:10.1016/j.canlet.2013.05.011
Hu M, Yao J, Carroll DK et al (2008) Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13(5):394–406. doi:10.1016/j.ccr.2008.03.007
Qian BZ, Li J, Zhang H et al (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475(7355):222–225. doi:10.1038/nature10138
Mueller KL, Madden JM, Zoratti GL et al (2012) Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancers through paracrine activation of Met. Breast Cancer Res 14(4):R104. doi:10.1186/bcr3224
Finak G, Bertos N, Pepin F et al (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14(5):518–527. doi:10.1038/nm1764
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. doi:10.1200/jco.2009.25.6529
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. doi:10.1200/jco.2006.09.2775
Henry LR, Lee HO, Lee JS et al (2007) Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 13(6):1736–1741. doi:10.1158/1078-0432.ccr-06-1746
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747. doi:10.1093/annonc/mdr304
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. doi:10.1073/pnas.191367098
van ‘t Veer LJ, Dai H, van de Vijver MJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871):530–536. doi:10.1038/415530a
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100(14):8418–8423. doi:10.1073/pnas.0932692100
De Wever O, Mareel M (2003) Role of tissue stroma in cancer cell invasion. J Pathol 200(4):429–447. doi:10.1002/path.1398
Tchou J, Kossenkov AV, Chang L et al (2012) Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med Genomics 5:39. doi:10.1186/1755-8794-5-39
Ahn S, Cho J, Sung J et al (2012) The prognostic significance of tumor-associated stroma in invasive breast carcinoma. Tumour Biol 33(5):1573–1580. doi:10.1007/s13277-012-0411-6
Shao ZM, Nguyen M, Barsky SH (2000) Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 19(38):4337–4345. doi:10.1038/sj.onc.1203785
Zhai X, Zhu H, Wang W et al (2014) Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol 31(6):970. doi:10.1007/s12032-014-0970-z
Chong HI, Lee JH, Yoon MS et al (2014) Prognostic value of cytoplasmic expression of S100A4 protein in endometrial carcinoma. Oncol Rep 31(6):2701–2707. doi:10.3892/or.2014.3149
Bai H, Qian JL, Han BH (2014) S100A4 is an independent prognostic factor for patients with lung cancer: a meta-analysis. Genet Test Mol Biomark 18(5):371–374. doi:10.1089/gtmb.2013.0471
Tsukamoto N, Egawa S, Akada M et al (2013) The expression of S100A4 in human pancreatic cancer is associated with invasion. Pancreas 42(6):1027–1033. doi:10.1097/MPA.0b013e31828804e7
Chuang WY, Yeh CJ, Chao YK et al (2014) Concordant podoplanin expression in cancer-associated fibroblasts and tumor cells is an adverse prognostic factor in esophageal squamous cell carcinoma. Int J Clin Exp Pathol 7(8):4847–4856
Preuss SF, Anagiotos A, Seuthe IM et al (2014) Expression of podoplanin and prognosis in oropharyngeal cancer. Eur Arch Otorhinolaryngol. doi:10.1007/s00405-014-3105-4
Yuan D, Liu B, Liu K et al (2013) Overexpression of fibroblast activation protein and its clinical implications in patients with osteosarcoma. J Surg Oncol 108(3):157–162. doi:10.1002/jso.23368
Shi M, Yu DH, Chen Y et al (2012) Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol 18(8):840–846. doi:10.3748/wjg.v18.i8.840
Moorman AM, Vink R, Heijmans HJ et al (2012) The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol 38(4):307–313. doi:10.1016/j.ejso.2012.01.002
Acknowledgments
This study was supported by a grant from National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080). This study was supported by a faculty research grant from Yonsei University College of Medicine for 2013 (6-2014-0131).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S.Y., Kim, H.M. & Koo, J.S. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat 149, 727–741 (2015). https://doi.org/10.1007/s10549-015-3291-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3291-9