Abstract
To better understand insect learning ability, demonstration of learning through conditioning is an effective research tool. Conditioning in social bees takes advantage of the proboscis extension reflex (PER) as a response to an unconditioned stimulus (US) (e.g., sucrose solution). The US is then associated with a conditioned stimulus (CS) (e.g., an odor) to elicit PER. However, solitary bees do not readily exhibit a PER when restrained and then prodded with an offering of sucrose. Managed solitary bees can be maintained in laboratory benchtop cages where they discover and feed from wicks soaked with aqueous sugar or honey. We sought to devise a protocol that demonstrates learning through simple conditioning for two managed solitary bees, Osmia lignaria and Megachile rotundata by exploiting their ability to locate a small feeder and reflexively extend the proboscis to retrieve a sucrose reward (US). In this study, the rewarded feeder was paired with a floral odor (CS) during training bouts. Newly emerged adult bees began training on the day of their emergence, continued training for another day, and were tested on the next day using a choice bioassay that revealed whether bees had learned the CS. The conditioning assay was effective for both bee species and sexes as revealed by proboscis extension towards material dosed with the reward-associated training odor. The ability to condition solitary bees could support important studies that address questions related to bee preferences for nesting and floral resources and for effects of pesticides or other stressors on learning and memory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For over half of a century, scientists have performed conditioning experiments with honey bees, Apis mellifera (Hymenoptera: Apidae), to explore their sensory abilities (e.g., von Frisch 1950; Reisenman and Giurfa 2008) and cognitive processes, such as learning and memory (e.g., Bitterman et al. 1983; Sandoz et al. 2001; Giurfa 2007; Matsumoto et al. 2012; Giurfa and Sandoz 2012; Villar et al. 2020; Giurfa et al. 2021; Sezen et al. 2021). Protocols that study learning processes afford the ability to discover how insects perceive, assess, and interpret a stimulus, and how learning begets or mediates behavioral responses (Smith et al. 2006; Guerrieri and d’Ettorre 2008). Much of the current information for bee cognition has been obtained from honey bee studies (Giurfa 2007; Giurfa and Sandoz 2012; Raine and Chittka 2012; Paoli and Galizia 2021) and, to a lesser extent, for bumble bees and stingless bees (e.g., Heinrich et al. 1977; Abramson et al. 1999; Dyer and Chittka 2004; McCabe et al. 2007; Dyer et al. 2008; Wertlen et al. 2008; Ings et al. 2009; Muth et al. 2016; Amaya-Márquez et al. 2019). However, social bee learning capacity and responses may not be representative of all bee species, especially for solitary bees whose diversity is vast in morphology, nesting behavior, foraging preference, and social structure (e.g.,Campan and Lehrer 2002; Bar-Shai et al. 2011). Understanding solitary bee learning ability using sucrose solution as the unconditioned stimulus and odors as conditioned stimuli may offer a means for studying their ability to detect, learn, and respond to relevant cues.
A few studies have investigated solitary bee learning and memory centered around foraging or nesting. These studies have exposed bees to different flower colors, shapes, odors, abundance, and amounts of reward (e.g.,Dukas and Real 1991; Campan and Lehrer 2002; Amaya-Márquez et al. 2008, 2019; Howard 2021) using conditioning methods for open foraging and sometimes comparing solitary bees to each other or to social bees. These studies have been performed under controlled field conditions, such as in field cages, but they have not been performed in the laboratory using a training protocol in the absence of environmental cues. Additionally, these previous studies have either tested females only or have tested females and males together, without differentiating responses by sex (e.g.,Dukas and Real 1991; Amaya-Márquez et al. 2008; Howard 2021). The focus on female bees in most studies is likely due to the precedence of testing worker honey bees (females) in social colonies or the perception that learning is most important for females that invest much time and energy in finding resources for building nests and feeding brood. Therefore, females may be predicted to have a greater need and capacity for learning than males. Males may be assumed to innately feed themselves and find mates with little need for learning, although some learning may be required for species like orchid bees whose males form leks or must find sources of scents for attracting and competing for females (e.g.,Kimsey 1980; Pokorny et al. 2017).
In the laboratory, appetitive olfactory conditioning of social bees often employs the proboscis extension response (PER), with honey bees having the strongest and most consistent responses among bees (honey bees: Takeda 1961; Bitterman et al. 1983; Farooqui et al. 2003; Smith et al. 2006; Giurfa 2007; bumble bees: Laloi et al. 1999; Laloi and Pham-Delègue 2004; Riveros and Gronenberg 2009; stingless bees: Abramson et al. 1999; McCabe et al. 2007; Giurfa and Sandoz 2012; Amaya-Márquez et al. 2019; Adam et al. 2022). The unconditioned response, PER, is induced by touching a bee’s antennae or mouthparts with a sugar solution (an unconditioned stimulus), which can be paired with an odor (a conditioned stimulus). After a few training bouts, honey bees are successfully conditioned to express PER with the odor alone and remember the association for several days (Smith et al. 2006). During conditioning, individual bees may be completely restrained (honey bees and bumble bees) or free-moving but isolated (stingless bees). However, attempts to perform conditioning experiments on two species of managed solitary bees via the elicitation of PER completely failed, despite those bees being restrained in similar manners as for social bees and touching their antennae with a droplet of sucrose solution (Vorel and Pitts-Singer 2010). Thus, without a standard, simple PER bioassay for the conditioning of solitary bees, a different laboratory approach is needed to assess their learning ability in a controlled environment.
For this project, we sought to develop a method for olfactory conditioning of two species of managed solitary, cavity-nesting bees. The blue orchard bee, Osmia lignaria, and the alfalfa leafcutting bee, Megachile rotundata (Hymenoptera: Megachilidae), are used for commercial pollination (Richards 1984; Bosch and Kemp 2001; Pitts-Singer and Cane 2011; Peterson and Artz 2014) and can be purchased and easily maintained in the laboratory. Both species build nests in appropriately sized, tunnel-shaped cavities by gathering and mixing pollen and nectar to form provision masses. The masses are prepared inside individual brood cells that are partitioned with collected soil and leaf pieces by O. lignaria and M. rotundata, respectively. As a spring-flying bee, O. lignaria is used to pollinate orchard crops. Megachile rotundata flies in the summer and, although native to Eurasia, has been widely used for several decades to pollinate alfalfa for seed production and more recently for hybrid canola seed production in western North America.
Our objective was to develop an experimental system with which we can condition solitary bees to demonstrate a clear ability to learn by expressing an appetitive response to stimuli, in this case to choose reward-associated odors in the absence of the actual reward. We hypothesized that responses to selected odors would not be innate and that conditioning of unrestrained O. lignaria and M. rotundata with reward-associated odors would reveal learning ability in a choice bioassay.
Methods
All experiments were conducted at the Pollinating Insects Research Unit (PIRU) in Logan, Utah. Osmia lignaria originated from wild-trapped northern Utah populations (3 B Sales and Service, North Logan, Utah- no longer in business). Megachile rotundata were purchased from a Canada bee supplier who flies bees in alfalfa seed fields to increase their populations (JWM Leafcutters, Inc., Saskatchewan, Canada). Although the M. rotundata were from one supplier, a recent study has shown that North American populations maintain high levels of genetic diversity (Strange et al. 2017). Both species were purchased in the fall and maintained at PIRU following standard protocols (Bosch and Kemp 2001 for O. lignaria; Richards 1984 for M. rotundata) throughout the winter (i.e., winter storage at 4–5 °C). Petri dishes (10 cm dia.), each containing five cocoons, were removed from cold storage, and then placed in an incubator (22 °C for O. lignaria, 29 °C for M. rotundata) so that a manageable number of adult bees would emerge over time for performing a series of conditioning trials. Osmia lignaria require only a few days of incubation before adult emergence (Bosch and Kemp 2001; Peterson and Artz 2014), while M. rotundata adult emergence begins after 17–19 days of incubation (Richards 1984; Pitts-Singer and James 2005). Males and females of each species were tested.
Odors used for conditioning trails were geraniol (a monoterpene; composition = 97%; C10H18O) (ThermoFisher Scientific, Waltham, MA, USA) and phenylacetaldehyde (PAA; an aldehyde; composition = ≥ 95% C8H8O) (Sigma-Aldrich, St. Louis, MO, USA). These floral odors have been used frequently in honey bee conditioning studies (e.g.,Bitterman et al. 1983; Smith 1991; Sandoz et al. 2001; Roussel et al. 2011) and are known components of floral aromas, for which there may be innate bee attraction (Knudsen et al. 1993). Flowers of Rosaceae and Fabaceae are known to be preferred by O. lignaria and M. rotundata, respectively (Horne 1995; Bosch and Kemp 2001). Geraniol is a component of Rosa spp. (Rosaceae), but a literature search could not verify the presence of geraniol as an odor in Fabaceae nor of PAA in either of the bee-preferred plant families (Knudsen et al. 1993).
Two groups of bees were designated in each experiment: an odor conditioned (OC) group and a no-odor conditioned (NOC) group. OC bees were conditioned to associate sucrose solution (reward) with an odor delivered via an odor-impregnated paper attached to a feeder and, during another time period, to associate water (no reward) in a second feeder whose attached paper was unscented (Table 1). The NOC bees served as controls because they were treated and tested exactly as were the OC bees, but an odor was never associated with any rewarded or unrewarded feeder during conditioning. The NOC bees only encountered an odor during the choice bioassay (see below). Therefore, results from NOC groups could be directly compared in statistical analyses to results from the OC groups. Treating the NOC groups in the same manner as the OC groups served to 1) rule out an innate odor attraction and 2) avoid disparity in bioassay outcomes by assuring bees (NOC and OC) were of similar age and had experience in encountering feeders in confinement.
Rewarded feeders contained 25% w/v sucrose solution, and unrewarded, unscented feeders contained purified (reverse osmosis, RO) water. This sucrose concentration is at the low end of average sugar concentrations of floral resources normally foraged upon by the solitary bees (Bitterman et al. 1983; Free 1993; Matsumoto et al. 2012) and is a sucrose concentration used at the PIRU for maintaining solitary bees in the laboratory.
Conditioning and testing occurred over several weeks for each species. Therefore, we mixed the trials for the different odors and for the OC and NOC bee groups across days and weeks to avoid any influence of when bees emerged from cocoons (i.e., how long they had been held in winter storage) and any differences in environmental conditions over time. Osmia lignaria were conditioned and tested April—June 2007. Megachile rotundata were conditioned and tested June—July 2007. Osmia lignaria were conditioned and tested in a windowless room at 22–23 °C with overhead lighting; artificial lighting was kept on an 11:13 day:night schedule. Because M. rotundata are mostly inactive at indoor temperature, it was imperative to use extra warmth to assure bee activity. Megachile rotundata were conditioned in a temperature-controlled greenhouse (~18–38 °C) under natural lighting (i.e., sunlight through glass), but were tested in a windowless room with overhead lighting. Because the testing room temperature was 22–23 °C, extra warmth was provided during the M. rotundata bioassays by placing testing arenas on heating pads (Kaz, Inc., Southborough, MA, USA; Sunbeam Products, Inc., Baton Rouge, FL, USA) with the dial set to “medium” (~27 °C as indicated on a thermometer placed directly on the pad).
All bees that emerged from their cocoons by 1200 h on Day 1 were placed in a ventilated Plexiglas cage (length × width × height: 26.2 cm × 26.2 cm × 30.5 cm) with a male:female ratio of approximately 1:1 (Pitts-Singer 2007). Each cage contained 2–10 bees at any one time. Two feeders were kept in each cage, introduced according to the schedule for adding either rewarded or unrewarded stimuli (with or without odors) (Table 1). Each feeder consisted of a small plastic 5.0 ml cup with a lid (ht. x dia.: 2.5 cm × 2.3 cm) (Nalgene, Rochester, NY, USA) (Fig. 1). Inserted into a hole in the lid was a wick (4.0 ± 0.5 cm) made from cotton cigarette filter. From our previous experience with maintaining bees in the laboratory, we knew that bees explore the cages and easily find wicks of the feeders. They extend the proboscis to imbibe liquid from the wicks. In this manner, bees learn to associate the feeders (a visual cue) with a liquid source (water or sucrose solution).
Feeder used to deliver conditioning odors for solitary bee condition trials. Feeder is a covered 5.0 ml plastic cup, with a hole drilled through the lid. Feeder and lid combined height is 2.5 cm; diameter is 2.3 cm. A wick (4.0 ± 0.5 cm) made of a segment of cigarette filter is inserted into the hole. A filter paper ring (2.3 cm diameter) surrounds the wick and is affixed to the lid with a small piece of white medical tape
For OC bee trials, a 2.3 cm ring of filter paper (Grade 1, Whatman International, Ltd., Maidstone, England) was placed around the wick and was secured from underneath using medical tape (Fisher Scientific, Pittsburgh, PA). If the filter paper was to deliver an odor for the rewarded feeder, then before affixing it to the feeder, either 0.50 μl geraniol or 0.25 μl PAA was dripped onto the filter paper ring using a Hamilton syringe and allowed to dry. Newly impregnated filter paper rings were made for each switching of feeders. These odor amounts were determined to be detectable to O. lignaria in preliminary assays. The filter paper inadvertently was moistened again upon contact with the wet wick.
According to the designated schedule (Table 1), the rewarded feeders were offered at the start of the experiment (Day 1). Before the end of Day 1, the rewarded feeders were switched to unrewarded feeders. On Day 2, the rewarded and unrewarded feeders were switched out twice as scheduled. On Day 3, the bees were tested in a choice bioassay.
Bioassay arenas in which cues were presented for testing for the effect of conditioning were made from 1.4 L (48 fl. oz.) reusable plastic bowls (ht. × dia.: 8.5 cm × 16.5 cm) with lids (Western Family, Portland, OR, USA) (Fig. 2). For ventilation and to facilitate observation during testing, a center portion of each lid was excised and replaced with plastic window screen. Three holes (5.0 cm × 3.0 cm) also were cut in the sides of each bowl and covered with screen. Also, a circular hole was cut in one side of each bowl; the circular hole was large enough for a snug fit of the mouth of a 20.0 ml glass scintillation vial. At the onset of a bioassay, a vial was used to collect a bee from the holding cage. The bee was given a few minutes to settle down in the vial. The vial was then inserted into the hole, and the calm bee could crawl out into the arena on its own accord. Rather than use the same feeders that were used during conditioning, simplified mock feeders were created, which made bees’ choices quicker and easier to observe. Each mock feeder included a disk of filter paper (2.3 cm dia.) and a small length (1.0 – 2.0 cm) of cigarette filter wick, which was held in place by a thumbtack on the underside of the filter paper disk. Two mock feeders were placed on a rectangular piece of aluminum foil (l × w = 4.0 cm × 9.0 cm ± 0.5 cm) in the bottom of the bowl, approximately 2.5 cm apart and equidistant from the bee entry hole (Fig. 2). The foil allowed for rotating the mock feeders to exchange their positions between each individual test without touching feeders and, thus, eliminating the risk of fingers-to-feeder contamination. The wicks of both mock feeders (and consequently the affixed filter paper disks) were saturated with RO water. One mock feeder’s paper disk was scented with the same odor used for conditioning, either 0.50 µl geraniol or 0.25 µl PAA; the other mock feeder’s disk was unscented. Mock feeders were freshly made for each bioassay.
Side and top views of testing arena for solitary bee bioassays made from a 1.42 L plastic bowl (height x diameter: 8.5 cm × 16.5 cm) with three mesh windows (a) and a mesh lid (b). A bee enters the arena from a vial attached to the side (c). Once inside, the bee can choose between two feeders (d), which are filter paper disks with small segments of cigarette filter attached from below by thumbtacks. The feeders rest on a rectangular piece of aluminum foil (e)
On Day 3, a bee was removed from the Plexiglas holding box using a 20.0 ml glass scintillation vial. After the bee settled in the capped vial, the mouth of the vial was pressed into the entry hole of the arena (Fig. 2). As soon as the bee left the vial, a timer was started. The bee was given 10 min to choose between the two mock feeders. A feeder “choice” was recorded if the bee extended its proboscis to touch the disk or the wick of a feeder. Some bees tested (n < 10) never actually extended their proboscises, but they circled one of the feeders with their heads down, intensely probing the feeder with their antennae. If they continued this behavior for more than 10 s, it was recorded that the bee had chosen that specific feeder. We also recorded whether the bee failed to choose either feeder during the 10 min period.
Statistical Analyses
As a check whether choices in the bioassays were made according to the position of the mock feeder or due to innate odor preferences for each bee species, binomial tests (Zar 1999; Proc Freq, SAS Institute 2013) were used to answer 1) by sex, whether the choice of OC and NOC bees were affected by the location (right or left) of the mock feeders in the bioassay arena, and 2) by sex, whether NOC bees exhibited an odor preference during choice bioassays in which they experienced a test odor for the first time.
Chi-square tests (Proc Freq, SAS Institute 2013) then were used first to reveal whether experience with odors affected bioassay performance by answering 1) whether making any choice in the bioassay differed by bee species and by sex within species (for each OC and NOC bees) and 2) by species and sex, whether OC bees were more likely than NOC bees to make a choice during testing. Next, Chi-square tests were used to examine whether associative learning had occurred by species and sex by testing whether OC bees were more likely than NOC bees to choose the reward-associated feeder during testing.
Not all trials were included in statistical analyses due to elimination using rigid criteria as follows. If the number of bees that made a choice was <15 in a trial where binomial tests determined effects of the position of the mock feeder or an innate odor preference, then that trial was eliminated. For analyses using Fisher’s exact test, the choices made by both OC and NOC bees were required. Therefore, if the number of bees that made a choice was <15 for either OC or NOC bee groups (by sex) for a trial, then these pairings were excluded.
Results
By Sex, was the Choice of OC and NOC Bees Affected by the Location (Right or Left) of the Mock Feeders?
With one exception, the position of the mock feeder was not a significant factor in bee choice. There was one trial where OC M. rotundata females significantly selected the wick on the right side of the arena, which occurred during bioassays where PAA and blank were placed in the right-handed position an equal number of times (Table S1).
By Sex, Did NOC Bees Show any Odor Preferences During Choice Bioassays where Bees Experienced the Odors for the First Time?
No NOC bees showed a significant odor preference in the choice bioassay (Table S2), meaning there were no detected innate preferences for either floral odor used in the bioassay.
Conditioning Trials
Analyses first sought to understand overall bee response to the bioassay (regardless of choice made).
Were Conditioned and Unconditioned O. lignaria Adults as Likely as M. rotundata Adults to Make a Choice in the Bioassay?
By species, combining all trials and including female and male bees, we first examined the responsiveness of OC bees (n = 423; 69% O. lignaria made choice; 32% M. rotundata made choice) and NOC bees (n = 406; 48% O. lignaria made choice; 20% M. rotundata made choice). We found that O. lignaria were more likely to make a choice than M. rotundata (OC bees: χ2 = 59.38, P < 0.0001; NOC bees: χ2 = 34.96, P < 0.0001).
By Sex within Each Species, were OC and NOC Bees Equally Likely to Make a Choice in the Bioassays?
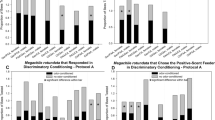
Within O. lignaria, OC females were significantly more likely than NOC females to make a choice (n = 249, χ2 = 20.05, P < 0.0001). The likelihood for OC versus NOC O. lignaria males to choose just failed to be statistically different (n = 178, χ2 = 3.73, P = 0.053) (Fig. 3). Similarly, within M. rotundata, OC females were significantly more likely than NOC females to choose (n = 197, χ2 4.70, P = 0.03); OC male choice rates did not differ from that of NOC males (n = 205, χ2 = 2.85, P = 0.09) (Fig. 3).
By Sex within Each Species, were OC and NOC Bees Equally Likely to Make a Choice in the Bioassays Due to the Odor Used in the Bioassay?
Next, we examined bioassays by each odor used in trials. OC O. lignaria females, but not males, were significantly more likely than NOC females to make a choice in bioassays using either geraniol or PAA as the conditioning odor (Table S3). OC M. rotundata females were more likely to make a choice than NOC females when geraniol was the bioassay odor, but in bioassays using PAA, OC and NOC M. rotundata females were equally likely to make a choice. The likelihood of OC males and NOC males was similar regardless of the odor used in the bioassay (Table S3).
Within Each Species (Pooled Sexes and by Each Sex), Were OC and NOC Bees Equally Likely to Choose the Reward-Associated Odor in the Bioassays?
Evidence of learning the association of an odor with a reward was found. Overall, OC O. lignaria females and males (by pooled sexes and by each sex) chose the feeder with the reward-associated odor significantly more often than did the NOC bees (sexes combined n = 254, χ2 = 57.19, P < 0.0001; females only n = 152, χ2 = 48.47, P < 0.0001; males only n = 102, χ2 = 9.80, P = 0.002) (Fig. 4). Both sexes of OC M. rotundata also chose the rewarded odor more often than did the NOC bees (sexes combined n = 106, χ2 = 17.73, P < 0.0001; females only n = 44, χ2 = 13.12, P = 0.0003; males only n = 62, χ2 = 6.45, P = 0.01) (Fig. 4).
For simple conditioning bioassays, the proportion of all Osmia lignaria and Megachile rotundata odor conditioned (OC) and no-odor conditioned (NOC) female and male bees that chose the scented mock feeder (regardless of which scent was used for training). See Table S3 for details of binomial tests
Analyses according to which odor was used in simple conditioning revealed that O. lignaria OC females conditioned with either geraniol or PAA were significantly more likely to choose the odor over no odor (93% and 85% for the respective odors) than were the NOC females (Fig. 5, Table S3). OC males chose the rewarded odor significantly more often than did NOC males when they were conditioned with PAA (84%), but not when conditioned with geraniol. Too few choices were made in the trials for each odor by M. rotundata females and males for statistical analyses, except for when OC males were trained with PAA, in which no significant difference was found between the OC and NOC males (Table S4).
According to each odor used for simple conditioning bioassays, the proportion of all Osmia lignaria odor conditioned (OC) and no-odor conditioned (NOC) female and male bees that chose the scented mock feeder. Ger = geraniol; PAA = phenylacetaldehyde. Asterisks indicate significant difference between OC and NOC bees that chose the scented feeder. See Table S4 for details of Fisher’s exact tests
Discussion
In this study, we developed a protocol and then demonstrated associative learning ability through simple conditioning for O. lignaria and somewhat for M. rotundata, both of which are solitary bees that are commercially available and can be maintained in the laboratory. We employed a very simple protocol that can easily be set up on benchtops at ambient or in slightly warmed environments. Bees roaming freely in small rearing cages were conditioned using two floral odors as conditioned stimuli and a sucrose solution as an unconditioned reward. After using our simple conditioning protocol, the bees exhibited learning ability by responding to floral odors to indicate the expectation of the reward.
For the adult bees that made a choice and, thereby, demonstrated a learning ability, O. lignaria choose the mock feeder with the conditioning odor 86% of the time, which was more consistent than M. rotundata adults that chose the rewarded odor 75% of the time. We speculate that O. lignaria showed a higher learning ability in these odor conditioning trials because of certain life history traits. They tend to have a more polyfloral diet than M. rotundata and a shorter window of time for reproduction. The spring-flying O. lignaria experience shorter durations of foraging time than summer-flying M. rotundata due to daylength and periods of favorable weather conditions, so quick and efficient learning of which flowers are rewarding is important. Boyle et al. (2020) found that O. lignaria females readily visited and provisioned nests with non-Rosaceous spring flowers planted near almond orchards, although they mainly visited almond flowers while the trees were in bloom. Contrarily, McCabe et al. (unpublished) discovered that M. rotundata highly preferred Fabaceous flowers for nesting and collected legume pollen for larval provisions in landscapes where such plants were not locally abundant. Furthermore, because O. lignaria overwinter as adults and M. rotundata as prepupae, O. lignaria had been adults for 8–9 months and likely had advanced brain and ovary development at the time these trials were performed, compared to M. rotundata that had become adults just days before they emerged from cocoons. Such differences in the lives of these two species may result in differences in the mechanisms by which they learn and in their learning needs, speed, and abilities.
Bees with such clear learning responses, especially as those of O. lignaria, may afford a means to test solitary bees for which a conditioning method using restrained bees and eliciting PER (as for social bees) is not possible. Although these commercially available cavity-nesting, solitary bee species may serve as surrogates for testing learning capacity in many other solitary species that nest above and below ground (e.g., M. rotundata shape discrimination in Campan and Lehrer 2002; Lasioglossum lanarium colour discrimination in Howard 2021), they may fail to represent other bees that differ in innate capabilities, physiological (e.g., neural, developmental) adaptions (bee and other insect examples: Page et al. 1998; Brembs 2003; Ings et al. 2009; Adam et al. 2022), and propensity for being maintained in a controlled environment for rearing and use of a standard technique (Vorel and Pitts-Singer 2010). In some cases, conditioning experiments may help to elucidate aspects of bee foraging preferences (e.g., floral odors and sugar contents) and to determine sublethal impacts of pesticides and adjuvants (e.g., inability to learn, or memory loss after learning) (Ciarlo et al. 2012; Mullin et al. 2015; Farina et al. 2019; Ko et al. 2022). This training technique and further modification of its methods may be used to reveal learning and memory in other solitary species or to show more complex learning capacity, such as differentiating between two odors.
The use of common floral odors in our bioassay raises concern that conditioning could be influenced by innate biases, although a pre-existing preference would not necessarily preclude future behavioral modification as a result of experience or conditioning (Heinrich et al. 1977; Heinrich 1984; Dobson 1987; Amaya-Márquez et al. 2008). It was important to compare bioassay performance of NOC bees to performance of OC bees to resolve whether making a choice was due to the conditioning experience, innate preferences, or other influences unrelated to learning ability. If the NOC bees had learned that unscented feeders were sometimes rewarding, then they may have been more likely to choose the unscented mock feeder in the bioassay. If the NOC bees expressed a response to an odor during the bioassay due to innate preference for that odor, an imprinting experience during immature development, or exposure to the odor as a teneral adult while still in the nest cell, then the results may have indicated a significant bias towards the scented mock feeder. Indeed, Dobson (1987) found a preference for certain flower, pollen, and pollenkitt odors by inexperienced Colletes fulgidus longiplumosus Stephen (Colletidae), a solitary, ground-nesting bee. However, our study found no evidence for strong innate or imprinted preferences by the NOC bees, because there was a non-significant response to both mock feeders in their bioassays.
To our knowledge, no other conditioning study has compared the performance of female and male solitary bees. Perhaps the importance of testing male bees is often discounted because they only need to fuel themselves and mate, and, therefore, are presumed less prone to learning than females (Church et al. 2001; Muth et al. 2021). But this does not preclude that males also need to learn which flowers are reliable nectar sources or may be attractive to females they seek for mating. Females live longer than males and make repeated trips between the nest and foraging sites. This allows more time for females to learn floral cues associated with rewarding plants and, thereby, may increase reproductive success (Raine and Chittka 2008). A recent study revealed floral preference dissimilarity between the sexes of the same bee species (Roswell et al. 2019), but overall learning of flower odors was not addressed. In this current study, O. lignaria OC males responded similar to OC females in some of these simple trials. Subsequently, the inclusion of males in future similar studies may be meritorious and important to fully understand learning mechanisms and when/if learning is necessary. Both O. lignaria sexes have been shown to have similar patterns of development in their mushroom bodies (Withers et al. 2008), which is the brain region responsible for learning in insects; this suggests equivalent learning capacity. Among other behaviors and corresponding physiologies, however, males and females differ in behavioral motivations and pollination efficiencies (Ne’eman et al. 2006; Roswell et al. 2019), and the evaluation of male and female responses under different circumstances may be insightful (Paldi et al. 2003; Smid and Vet 2016).
With the advances in the study of complex behaviors, neuroanatomy, and neurophysiology related to honey bee olfaction, there is a need to also understand the same aspects for solitary bees for comparative analyses, including a focus on how olfaction and its mechanisms play a role in the evolution of hymenopteran sociality (Giurfa 2007; Paoli and Galizia 2021). Moreover, the fitness effects of learning have yet to be realized for managed solitary bees through investigations that consider ecologically relevant environments and conditions (Smid and Vet 2016; Nieberding et al. 2018), such as when used in commercial crops as mass pollinators.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abramson CI, Aquino IS, Stone SM (1999) Failure to find proboscis conditioning in one-day old Africanized honey bees (Apis mellifera L.) and in adult Uruçu honey bees (Melipona scutellaris). Int J Comp Psychol 12:242–262. https://doi.org/10.46867/C43G6J
Adam A, Hansson BS, Knaden M (2022) Fast learners: One trial olfactory learning in insects. Front Ecol Evol 10:876596. https://doi.org/10.3389/fevo.2022.876596
Amaya-Márquez M, Sergio T, Hernández J, Jimenez JD, Wells H, Abramson CI (2019) Olfactory learning in the stingless bee Melipona eburnean Friese (Apidae: Meliponini). Insects 10:412. https://doi.org/10.3390/insects10110412
Amaya-Márquez M, Hill PSM, Barthell JF, Pham LL, Doty DR, Wells H (2008) Learning and memory during foraging of the blue orchard bee, Osmia lignaria Say (Hymenoptera: Megachilidae). J Kansas Entomol Soc 81:315–327. https://doi.org/10.2317/JKES801.29.1
Bar-Shai N, Keasar T, Shmida A (2011) How do solitary bees forage in patches with a fixed number of food items? Anim Behav 82:1367–1372. https://doi.org/10.1016/j.anbehav.2011.09.020
Bitterman ME, Menzel R, Fietz A, Schäffer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119. https://doi.org/10.1037/0735-7036.97.2.107
Bosch J, Kemp WP (2001) How to manage the blue orchard bee as an orchard pollinator. Sustainable Agriculture Network, National Agricultural Library, Beltsville
Boyle NK, Artz DR, Lundin O, Ward K, Picklum D, Wardell GI, Williams NM, Pitts-Singer TL (2020) Wildflower plantings promote blue orchard bee, Osmia lignaria (Hymenoptera: Megachilidae), reproduction in California almond orchards. Ecol Evol 10:3189–3199. https://doi.org/10.1002/ece3.5952
Brembs B (2003) Operant conditioning in invertebrates. Curr Opin Neurobiol 13:710–717
Campan R, Lehrer M (2002) Discrimination of closed shapes by two species of bee, Apis mellifera and Megachile rotundata. J Exper Biol 205:559–572. https://doi.org/10.1242/jeb.205.4.559
Church D, Plowright C, Loyer D (2001) Discriminations of color and pattern on artificial flowers by male and female bumble bees, Bombus impatiens (Hymenoptera: Apidae). Gt Lakes Entomol 34:85–95. https://scholar.valpo.edu/tgle/vol34/iss2/11
Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7(7):e40848.
Dobson HEM (1987) Role of flower and pollen aromas in host-plant recognition by solitary bees. Oecologia 72:618–623. https://doi.org/10.1007/BF00378991
Dukas R, Real LA (1991) Learning foraging tasks by bees: a comparison between social and solitary species. Anim Behav 42:269–276. https://doi.org/10.1016/S0003-3472(05)80558-5
Dyer AG, Chittka L (2004) Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. J Comp Physiol A 190:759–763. https://doi.org/10.1007/s00359-004-0547-y
Dyer AG, Spaethe J, Prack S (2008) Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. J Comp Physiol A 194:617–627. https://doi.org/10.1007/s00359-008-0335-1
Farina WM, Balbuena MS, Herbert LT, Goñalons CM, Vázquez DE (2019) Effects of the herbicide glyphosate on honey bee sensory and cognitive abilities: individual impairments with implications for the hive. Insects 10:354. https://doi.org/10.3390/insects10100354
Farooqui T, Robinson K, Vaessin H, Smith BH (2003) Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci 23:5370–5380. https://doi.org/10.1523/JNEUROSCI.23-12-05370.2003
Free JB (1993) Insect pollination of crops. Academic Press, San Diego
Giurfa M (2007) Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A 193:801–824. https://doi.org/10.1007/s00359-007-0235-9
Giurfa M, Sandoz J-C (2012) Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem 19:54–66. https://doi.org/10.1101/lm.024711.111
Giurfa M, Giurfa de Brito A, Giurfa de Brito T, de Britohez Sanchez MG (2021) Charles Henry Turner and the cognitive behavior of bees. Apidologie 52:684–695. https://doi.org/10.1007/s13592-021-00855-9
Guerrieri FJ, d’Ettorre P (2008) The mandibular opening response: quantifying aggression elicited by chemical cues in ants. J Exp Biol 211:1109–1113. https://doi.org/10.1242/jeb.008508
Heinrich B (1984) Learning in invertebrates. In: Marler P, Terrace HS (eds) The biology of learning. Springer-Verlag, Berlin, pp 135–147
Heinrich B, Mudge PR, Deringis PG (1977) Laboratory analysis of flower constancy in foraging bumblebees: Bombus ternarius and B. terricola. Behav Ecol Sociobiol 2:247–265. https://doi.org/10.1007/BF00299738
Horne M (1995) Pollen preference and its relationship to nesting success of Megachile rotundata (Hymenoptera: Megachilidae). Ann Entomol Soc Am 88:862–867. https://doi.org/10.1093/aesa/88.6.862
Howard SR (2021) Wild non-eusocial bees learn a colour discrimination task in response to simulated predation events. Sci Nat 108:28. https://doi.org/10.1007/s00114-021-01739-9
Ings TC, Raine NE, Chitta L (2009) A population comparison of the strength and persistence of innate colour preference and learning speed in the bumblebee Bombus terrestris. Behav Ecol Sociobiol 63:1207–1218. https://doi.org/10.1007/s00265-009-0731-8
Institute SAS (2013) Version 9.4. SAS Institute Inc., Cary
Kimsey LS (1980) The behaviour of male orchid bees (Apidae, Hymenoptera, Insecta) and the question of leks. Anim Behav 28:996–1004. https://doi.org/10.1016/S0003-3472(80)80088-1
Knudsen JT, Tollsten L, Bergström LG (1993) Floral scents – a checklist of volatile compounds isolated by head-space techniques. Phytochem 33:253–280. https://doi.org/10.1016/0031-9422(93)85502-I
Ko C-Y, Nai Y-S, Lo W, Chen C-T, Chen Y-W (2022) Low-level fluvalinate treatment in the larval stage induces impaired olfactory associative behavior of honey bee workers in the field. Insects 13:273. https://doi.org/10.3390/insects13030273
Laloi D, Pham-Delègue MH (2004) Bumble bees show asymmetrical discrimination between two odors in a classical conditioning procedure. J Insect Behav 17:385–396. https://doi.org/10.1023/B:JOIR.0000031538.15346.e1
Laloi D, Sandoz JC, Picard-Nizou AL, Marchesi A, Pouvreau A, Taséi JN, Poppy G, Pham-Delègue MH (1999) Olfactory conditioning of the proboscis extension in bumble bees. Entomol Exp Appl 90:123–129. https://doi.org/10.1046/j.1570-7458.1999.00430.x
Matsumoto Y, Menzel R, Sandoz J-C, Giurfa M (2012) Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods 211:159–167. https://doi.org/10.1016/j.jneumeth.2012.08.018
McCabe SI, Hartfelder K, Santana WC, Farina WM (2007) Odor discrimination in classical conditioning of proboscis extension in two stingless bee species in comparison to Africanized honeybees. J Comp Physio A 193:1089–1099. https://doi.org/10.1007/s00359-007-0260-8
Mullin CA, Chen J, Fine JD, Frazier MT, Frazier JL (2015) The formulation makes the honey bee poison. Pestic Biochem Physiol 120:27–35. https://doi.org/10.1016/j.pestbp.2014.12.026
Muth F, Papaj DR, Leonard AS (2016) Bees remember flowers for more than one reason: pollen mediates associative learning. Anim Behav 111:93–100. https://doi.org/10.1016/j.anbehav.2015.09.029
Muth F, Tripodi AD, Bonilla R, Strange JP, Leonard AS (2021) No sex difference in learing in wild bumblebees. Behav Ecol 32:638–645. https://doi.org/10.1093/beheco/arab013
Ne’eman G, Shavit O, Shaltiel L, Shmida A (2006) Foraging by male and female solitary bees with implications for pollination. J Insect Behav 19:383–401. https://doi.org/10.1007/s10905-006-9030-7
Nieberding CM, Van Dyck H, Chittka L (2018) Adaptive learning in non-social insects: from theory to field work, and back. Curr Opin Insect Sci 27:75–81. https://doi.org/10.1016/j.cois.2018.03.008
Page RE, Erber J, Fondrk MK (1998) The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 182:489–500
Paldi N, Zilber S, Shafir S (2003) Associative learning of honeybees to differential rewards in multiple contexts – effect of odor component and mixture similarity. J Chem Ecol 29:2515–2538. https://doi.org/10.1023/A:1026362018796
Paoli M, Galizia GC (2021) Olfactory coding in honeybees. Cell Tissue Res 383:35–58. https://doi.org/10.1007/s00441-020-03385-5
Peterson SS, Artz DR (2014) Production of solitary bees for pollination in the United States. In: Morales-Ramos J, Rojas G, Shapiro Ilan DI (eds) Mass production of beneficial insects. Academic Press, Amsterdam, pp 653–682
Pitts-Singer TL (2007) Olfactory response of megachilid bees, Osmia lignaria, Megachile rotundata, and M. pugnata, to individual cues from old nest cavities. Environ Entomol 36:402–408. https://doi.org/10.1093/ee/36.2.402
Pitts-Singer TL, Cane JH (2011) The alfalfa leafcutting bee, Megachile rotundata: the world’s most intensely managed solitary bee. Annu Rev Entomol 56:221–237. https://doi.org/10.1146/annurev-ento-120709-144836
Pitts-Singer TL, James RR (2005) Emergence success and sex ratio of commercial alfalfa leafcutting bees from the United States and Canada. J Econ Entomol 98:1785–1790. https://doi.org/10.1093/jee/98.6.1785
Pokorny T, Vogler I, Losch R, Schlutting P, Juarez P, Bissantz N, Ramírez SR, Eltz T (2017) Blown by the wind: the ecology of male courtship display behavior in orchid bees. Ecol 98:1140–1152. https://doi.org/10.1002/ecy.1755
Raine NE, Chittka L (2008) The correlation of learning speed and natural foraging success in bumble-bees. Proc R Soc B Biol Sci 275:803–808. https://doi.org/10.1098/rspb.2007.1652
Raine NE, Chittka L (2012) No trade-off between learning speed and associative flexibility in bumblebees: a reversal learning test with multiple colonies. PLoS ONE 7(9):e45096.
Reisenman CE, Giurfa M (2008) Chromatic and achromatic stimulus discrimination of long wavelength (red) visual stimuli by the honeybee Apis mellifera. Arthropod-Plant Interact 2:137–146. https://doi.org/10.1007/s11829-008-9041-8
Richards KW (1984) Alfalfa leafcutter bee management in western Canada, 1495/E:1–53. Agriculture Canada, Ottawa
Riveros AJ, Gronenberg W (2009) Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften 96:851–856. https://doi.org/10.1007/s00114-009-0532-y
Roswell M, Dushoff J, Winfree R (2019) Male and female bees show large differences in floral preference. PLoS ONE 4(4):e0214909.
Roussel E, Padie S, Giurfa M (2011) Aversive learning overcomes appetitive innate responding in honeybees. Anim Cogn 15:135–141. https://doi.org/10.1007/s10071-011-0426-1
Sandoz JC, Pham-Delègue MH, Renou M, Wadhams LJ (2001) Asymmetrical generalization between pheromonal and floral odours in appetitive olfactory conditioning of the honey bee (Apis mellifera L.). J Comp Physiol A 187:559–568. https://doi.org/10.1007/s003590100228
Sezen E, Dereszkiewicz E, Hozan A, Bennett MM, Ozturk C, Smith BH, Cook CN (2021) Heritable cognitive phenotypes influence appetitive learning but not extinction in honey bees. Ann Entomol Soc Am 114:606–613. https://doi.org/10.1093/aesa/saab023
Smid HM, Vet EM (2016) The complexity of learning, memory and neural processes in an evolutionary context. Curr Opin Insect Sci 15:61–69. https://doi.org/10.1016/j.cois.2016.03.008
Smith BH (1991) The olfactory memory of the honeybee Apis mellifera I. Odorant modulation of short- and intermediate-term memory after single-trial conditioning. J Exp Biol 161:367–382. https://doi.org/10.1242/jeb.161.1.367
Smith BH, Wright GA, Daly KC (2006) Learning-based recognition and discrimination of floral odors. In: Dudareva N, Pichersky E (eds) Biology of floral scent. Taylor and Francis, Boca Raton, pp 263–295
Strange JP, Delaney DA, Tarpy DR, James RR (2017) Novel satellite loci reveal high genetic diversity yet low population structure for alfalfa leafcutting bees in North America. Conserv Genet 18:679–687. https://doi.org/10.1007/s10592-017-0943-9
Takeda K (1961) Classical conditioned response in the honey bee. J Insect Physiol 6:168–179. https://doi.org/10.1016/0022-1910(61)90060-9
Villar ME, Marchal P, Viola H, Giurfa M (2020) Redefining single-trial memories in the honeybee. Cell Rep 30:2603–2613. https://doi.org/10.1016/j.celrep.2020.01.086
von Frisch K (1950) Bees: Their vision, chemical senses, and language. Cornell University Press, Binghamton
Vorel CA, Pitts-Singer TL (2010) The proboscis extension reflex not elicited in megachilid bees. J Kansas Entomol Soc 83:80–83. https://doi.org/10.2317/0022-8567-83.1.80
Wertlen AM, Niggebrügge C, Vorobyev M, Hempel de Ibarra N (2008) Detection of patches of coloured discs by bees. J Exp Biol 211:2101–2104. https://doi.org/10.1242/jeb.014571
Withers GS, Day NF, Talbot EF, Dobson HEM, Wallace CS (2008) Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria (Megachilidae). Development Neurobiol 68:73–82. https://doi.org/10.1002/dneu.20574
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgements
The authors thank USDA ARS technician Ellen Klomps and ARS summer employees Michael Barker, Nicole Boehme, Sarah Clark, Elizabeth Sharp, Hannah Turner, and Shannon Wooley for their assistance in preparing and completing these bioassays. Appreciation is extended to Ricardo Ramirez, Kimberly Sullivan, Natalie Boyle, Morgan Dunn, Lindsie McCabe and anonymous reviewers for their helpful comments on this manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Funding
Research was funded by the USDA ARS Pollinating Insects Research Unit.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed primarily by Cory Stanley-Stahr for a dissertation chapter. The manuscript was written by both authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stanley-Stahr, C.A., Pitts-Singer, T.L. Establishment of an Olfactory Conditioning Assay for Two Solitary, Cavity-Nesting Bees. J Insect Behav 36, 210–221 (2023). https://doi.org/10.1007/s10905-023-09822-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-023-09822-x