Abstract
The traditional plastic packaging harms the environment, necessitating the need for environmentally friendly alternatives. The plant mediated amalgamation process for copper nanoparticles using Argemone maxicana offers an economically feasible and non-toxic approach. κ-Carrageenan, derived from red seaweeds, serves as an ideal matrix for creating nanocomposite materials. The resultant nanocomposite films have improved thermal stability, elastic properties, water vapour resistance, and UV resistance qualities. With inhibitory zones against S. aureus and E. coli, they also exhibit strong activities against bacteria. Additionally, grapes (12 days) and cottage cheese (7 days) were preserved using these films, and the food’s quality was effectively maintained without any additional care. Overall, this method lessens the environmental effect of traditional plastic materials while providing an environmentally acceptable packing for food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Form last few decades, plastic is being used as packing materials. The main reasons for the widespread use of plastics are their non-reactive nature, thermal resistance, high stability, durability, economical and easily affordable [1]. In the present age, every place is full of plastic products even our own houses. However, the degradation of plastic is very difficult or even takes million years for complete degradation. These days plastic waste is stored in landfills but this is not a permanent solution. This may lead to underground water pollution and impact subterranean organisms. As awareness increases, people become more concerned about environmental pollution and its hazardous consequences. We are endeavouring to find environmentally friendly and biodegradable alternative materials. The main focus is now shifted toward biopolymers, the materials that are derived from natural resources. Biopolymers can be synthesized from algae, fungus, plants, and mammals and microorganisms.
Commonly used natural polymers are chitosan, agar, alginates, polyamides, starch, protein, carbohydrates, pectin, lignin and melanin etc. Carrageenan a biopolymer is utilized for fabricating the nanocomposite film. Carrageenan is a specific type of polymer obtained from different members of red algae. Carrageenan mainly applied as thickener in the food sector as well as gelling and stabilizing agent [2]. Carrageenan is found to be more advantageous than other encapsulating materials. Carrageenan is used in drug delivery systems, pharmaceuticals, textiles, cosmetics, printing and other industries because it degrade easily and biocompatible [3]. Carrageenan possesses antifungal, antibacterial, carrier and film forming properties, but do not possess sufficient mechanical, thermal or barrier properties [4]. Different metal nanoparticles are incorporated to various biopolymers that enhances the thermal and mechanical traits of biopolymers [5].
In this work, we employed copper nanoparticles in conjunction with carrageenan for synthesizing nanocomposite films. Copper (Cu) is used since ancient times to make utensils and also in medicines due to their antimicrobial properties. Copper is chemically less reactive, 100% recyclable, resistant to corrosion and used in agriculture, wood preservation, anti-fouling paints, textile, wound healing etc. It is an essential element in human nutrition. The common approach that was used for formation of nanoparticles is chemical based. These processes are time consuming and chemicals are not safe for both human health and ecosystem. These days green synthesis of nanoparticles is preferred due to environment friendliness. Different plant parts are utilised in the fabrication process. Numerous metal oxide nanoparticles were created with green method ZnO [6, 7], Ag and CuO nanoparticles [8]. However, limited research has been conducted on one pot (in-situ) fabrication of copper nanoparticles via green method.
The nanoparticles are fabricated via using plant extract as a reducing agent. Argemone maxicana commonly known as Mexican prickly poppy, leaf extract was used in amalgamation of copper nanoparticles. This plant has been traditionally used since ancient times for treating jaundice, chest pain, asthma, liver ailments, and as a remedy for blood disorders. We can’t consume this plant but is a common weed located in the scorched region all over India. Biosynthesized biopolymeric food packaging films are gaining popularity as an eco-friendly substitute of conventionally used petroleum-based polymers. These films are reasonably priced, renewable and free from hazardous chemicals [9]. Synthesized films possess improved UV barrier properties when contrasting to neat carrageenan and films containing nanoparticles that are not synthesized with the films. UV-resistance ability of these films keeps the packed food items having log lasting freshness and prevents microorganism growth. Metal oxide nanoparticles have antimicrobial traits over a wide range of pathogenic microbes [10]. The antimicrobial properties of the nanocomposite films containing copper nanoparticles were also studied. Our hypothesis lies in the fact that the mechanical, thermal, and antibacterial qualities of nanocomposite films synthesized using carrageenan will be enhanced with addition of copper nanoparticles, making them an efficient and sustainable alternative for food packaging with increased UV barrier characteristics. The primary goal of this research was to analyse Carr/Cu nanocomposite films by utilising different characterization techniques and different parameters required for food packaging applications. Films were used for the storage of grapes at room temperature and grapes can be stored for 12 days without any specific treatment.
2 Materials and Experimental Procedure
2.1 Materials
All of the reagents of high purity, which were acquired from Merck Chemicals, were utilised exactly as purchased. (kappa) carrageenan that is used as food additive (white powder in a 1.5% aqueous solution at 75 °C with 12% water, 15% ash, having an average viscosity of 75 cps), copper sulphate (159.6 g/mol), and Argemone albiflora leaf procured from surrounding desert areas were used to make the copper/carrageenan nanocomposite films. As a plasticizer, glycerol was utilised, and pH is maintained by sodium hydroxide.
2.2 Experimental Procedure
2.2.1 Synthesis of Green Extract
The fresh leaves of Argemone maxicana were collected and washed thoroughly. Leaves were dried at room temperature and cut into smaller pieces. 4 g freshly chopped leaves of Argemone maxicana in 100 mL of double distilled water were boiled for 30 min. at 100 °C [11]. After cooling the extract at room temperature, whatman filter paper (grade 5) was used for filtering Cu nanoparticles.
2.3 Carrageenan/Cu Nanocomposite Film’s Synthesis
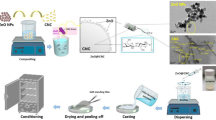
Bio-nanocomposite film was synthesized by following solution casting technique with some modifications [12]. 0.1 mM CuSO4 solution was prepared and permitted constant mixing at 70 °C on a magnetic stirrer. Plant extracts of different concentrations 30, 60 and 90 mL were added to the CuSO4 solution dropwise. The initial colour of copper sulphate solution initially was light bluish that changed from light blue to yellow with increased plant concentration which indicated the reduction of copper. Once the fixed amount of plant extract was added, to keep the pH 10 NaOH solution was added. Carrageenan (4 g) was added in distilled water (150 mL) and 1 mL of glycerol was mixed. The resultant mixture was continuously stirred for 30 min. Subsequently, this mixture was added into petri plates and dried for 2 days. Reaction scheme is given in Fig. 1. The film samples synthesized with different concentrations of plant extract were named as Carr/Cu—30, Carr/Cu—60 and Carr/Cu—90 containing 30, 60 and 90 mL of plant extract correspondingly.
2.4 Characterization of Films
2.4.1 Optical Properties (UV–Visible Spectroscopy)
Film’s optical properties and their per cent transmittance were measured by observing the light absorption spectra. For blank reading empty cuvette was used. A fragment of film was cut into (3 cm × 3 cm) square pieces, then situated immediately within spectrophotometer holder. Per cent transmittance of the film was calculated at different wavelengths [13].
2.4.2 FTIR and XRD of Film Samples
FTIR analysis was done to identify the different functional groups present in the sample. The film sample was cut into (4 cm × 4 cm) size and positioned on spectrometer. The spectra of three different pieces of these copper containing bio nanocomposite films are obtained with an FTIR spectrometer ranging in wavelength from 4000 to 400 cm−1.
The crystalline nature of the film was analysed by calculating crystalline index (CI).
XRD spectrum of film’s (1 cm × 1 cm) rectangular pieces was recorded. The crystalline size of these nanoparticles was computed utilising the Debye–Scherer formula.
2.4.3 Morphological Properties of the Film (FESEM)
FESEM was used to examine the topography of the film sample and powdered κ-carrageenan. For this purpose, the film sample (1 cm × 1 cm) size square pieces. The elemental composition of films was determined using EDS along with FE-SEM.
2.4.4 Thickness and Mechanical Features
Thickness of films (10 cm × 10 cm) was calculated. Five different locations were selected from the film and film’s thickness was determined with a portable micrometre having an accuracy of 0.01 mm. These values were used for the calculation of tensile strength. Universal testing machine (UTM) was applied in obtaining the mechanical traits of the films. 2 cm × 10 cm pieces of the films were used. The machine was operated at a load of 30 KN and 50 mm/min load rate. The tensile strength (TS) and elongation at break (%) of films was analysed for dissimilar film samples [14] using given equations.
where ΔL and L0 were the extended and original lengths (mm) of the sample, correspondingly; F was the tension of the film at break (N), x is thickness (mm) and W is the width (mm) of film.
2.4.5 Thermal Stability (TGA)
Thermal stability was investigated with thermo gravimetry of the different Carr/Cu nanocomposite films. Approximately 6.00 mg of films was heated from (30 °C) to 500 °C at 10 °C/min. This analysis was carried out at a 20 mL/min flow of nitrogen gas. The decrease in sample weight was calculated as a function of temperature. A central finite method was opted for the calculation of TGA (DTG) values.
W(t + Δt) and W(t − Δt) are the wt. of sample left at time t + Δt and t − Δt [15].
2.5 Different Parameters Studied
2.5.1 Water Vapour Permeability (WVP)
The analysis of WVP of film samples was done gravimetrically as per ASTM E 96-95 standard with some changes [16]. The film of 8 cm × 8 cm square pieces and directly positioned on vapour permeation cups containing 15 mL distilled water and screwed firmly to stop water from seeping through. The setup was placed and temperature is maintained at (25 °C) and 50% RH conditions [17]. Every hour, cup’s weight was recorded for 8 h. and decrease in weight every cup was calculated. Water vapour permeability was analysed from given equation.
G/m2 was used to measure WVTR. The letters S, L stand for mean thickness, while ∆P stands for the films’ partial water vapour pressure differential [16].
2.5.2 Moisture Content (MC)
For moisture content analysis (3 cm × 3 cm) pieces of different samples were weighted (Mi). Samples were dried for 24 h. at 105 °C and weighed again (Md). Moisture content was analysed by the derivation given below.
2.5.3 Total Soluble Matter (TSM) Studies
For TSM studies, the (dry) were placed in 50 mL for 24 h in DW (distilled water). Drying of these samples was done for 24 h. at 105 °C and weighed again (Mt). The TSM was calculated from given equation [18].
2.5.4 Biodegradability Study
The biodegradability of the sample was analysed from soil burial test [19]. Square pieces film samples were sliced (4 cm × 4 cm) and weighted. Three samples with varying amounts of plants were covered with soil having pH 7.5 at a temperature between 20 and 25 °C and watered regularly to maintain humidity 60–70%. Film sample was brought out from the soil, washed properly and allow to dry at 50 °C for 1 day and weight every week for 1 month [20].
2.6 Antimicrobial Study
The film’s antimicrobial effect against E. coli and S. aureus were studied by disk diffusion approach with slight modifications [21, 22]. For the nutrient Broth (NB) preparation we have taken 100 mL of distilled water to which NB was dissolved. Weighed the NB and then autoclaved them. Now S. aureus and E. coli was incubated in NB at 37 °C to 40 °C for 24 to 48 h. Nutrient Agar preparation was done by dissolving them autoclaved in 250 mL of distilled water. Poured the agar into the 6 petri plates (which were autoclaved) and let them solidify. After solidification, performed the inoculation of bacterial strains onto the 6 petri plates by clean cotton and making lawns and marking them accordingly. Film were cut precisely into 5 mm pieces and placed carefully in the centre of the plates. These plates were incubated at 37 °C to 40 °C for 24–48 h.
2.7 Application of Nanocomposite Film
A carrageenan and metal oxide nanocomposite film were used earlier by many people for the packaging of different fruits like bananas, cherries, grapes and strawberries. We used Carr/Cu nanocomposite films for storing grapes and cottage cheese. The synthesized nanocomposite films were used for the storage of Cottage cheese and grapes were bought from the local market. Cottage cheese was sliced into 5 × 5 cm pieces and placed in a container composed of glass. Carr/Cu films were employed to keep the cottage cheese. At room temperature, the glass container was properly cleaned and dried. A piece of cottage cheese was put inside the container and then Carr/Cu film was placed over it and secured with a rubber band. The container was then kept at 4 °C in a lab refrigerator by Haier Biomedical for 8 days.
Grapes was brought from nearby market then used distilled water for washing. After that grapes were washed and air-dried at room temperature for 2 h. We used common food packing boxes with lid having holes for the passage of air, moisture and oxygen exchange. These holes were blocked by the synthesized film to prevent moisture loss. The packaged grapes were stored at 20 °C for 12 days.
3 Statistical Analysis
Three replicated experimental units, individually made films were used to measure the characteristics of the films i.e. oxygen permeability, water vapour permeability, mechanical and thermal properties. The results of the present analysis were presented as mean ± SD (standard deviation) values. ANOVA, or one-way analysis of variance, was used for statistical analysis.
4 Results and Discussion
4.1 Characterizations
4.1.1 Optical Properties
Film’s optical properties was analysed by UV–Visible spectroscopy ranging from 200 to 700 nm. Transmittances percentage was analysed at 660 nm and 280 nm using UV–Visible spectrophotometer. The results were shown in Table 1. In-situ synthesized nanocomposite films exhibit noticeable decline in contrast to films made entirely of carrageenan and ones that include ex-situ synthesised nanoparticles from the previous literature [16]. The UV barrier traits are very important as they prevent decolouration, oxidation of fats, nutritional deficit, and fungal growth in the packed food items [17]. The graph of UV–Visible analysis was given in Fig S1 and the same pattern was observed for earlier synthesized Carr/Cu nanocomposite films [16]. Similar peak for copper nanoparticles was observed at 222 nm in earlier studies [23].
4.1.2 FTIR and XRD of Nanocomposite Films
The Carr/Cu film’s FTIR was examined between 400 and 4000 cm−1. These studies was done for examine the way in which Cu NPs and biopolymer interact. Figure 2 gives the result of FTIR analysis of Carr/Cu nanocomposite films. From the previous literature Cu NPs show a peak at 3200–3600 cm−1 representing stretching of O–H group [8] and a peak at 1481 cm−1 was due to absorbed water [24]. The intensity increased in every peak with inclusion of biopolymer because of van der Waal interactions between biopolymer and NPs [17]. All films exhibit peaks from 2800 to 3700 cm−1 for kappa-carrageenan. Peak at 3599.88 cm−1 corresponds to stretching of carrageenan’s N–H group and starching vibrations due to C–H group present in κ-carrageenan is represented by the peak at 2851.68 [25]. Band at 1508.66 corresponds to –NH bending and stretching. The peak at 968.42 corresponds to the saccharide units present in carrageenan [17].
XRD was done to analyse the crystalline nature of Carr/Cu nanocomposite films as presented in Fig. 3. The film exhibit characteristics peaks at 2θ around 30.88°, 43.04°, 53.37°, 60.64°, 68.49° and 75.86° indicating planes of 110, 111, 020, 113, 113 and 004 respectively [26]. The crystalline index of film was calculated from XRD spectrum is 0.22. The crystalline size of the nanoparticles estimated from Debye–Scherrer equation was found 27.7 nm. It was evident from previous studies that no specific peak was observed in pure carrageenan film due to its amorphous traits [24].
4.1.3 Morphological Properties (FESEM)
The topographic image of κ-carrageenan and Carr/Cu films was presented in Fig. 4 that was analysed by FESEM analysis. The crystalline nature of powdered carrageenan was clearly shown in Fig. 4a. The FESEM images for different synthesized films was given in The Fig. 4b for Carr/Cu—30, Fig. 4c for Carr/Cu—60 and Fig. 4d for Carr/Cu—90 film samples shows that. Some agglomerations are found which indicate the electrostatic interaction of negatively charged disaccharide units of κ-carrageenan with positively charged copper ions [27].
The elemental arrangement of Carr/Cu film was analysed with Energy-dispersive X-Ray spectroscopy (EDS). The nanocomposite film’s spectra show the presence of sulphur (S), carbon (C), oxygen (O) and copper (Cu) in the carrageenan matrix which confirms the distribution of Cu NPs in nanocomposite film Fig. S2.
4.1.4 Thickness and Mechanical Properties
The average thicknesses of all samples synthesized with different amount of plant extract were estimated using a hand micrometre. Film’s mechanical properties were analysed by calculating elastic modulus (EM), tensile strength (TS) and elongation at break (E) are given in Table 2. The mechanical attributes of films increase with the addition of copper nanoparticles. In previous studies, tensile strength of films with 0.2 M CuCl2 was 30.4 MPa [24], 0.2 M CuCl2 was 55.5 MPa [16] and 2 M CuCl2·2H2O was 60.2 MPa [28]. Our films show better results with 1 mM CuSO4 solution than all mentioned film samples. The bar graph related to TS, E and ES has been given in Fig S4.
4.1.5 Thermal Stability (TGA)
The thermal stability of Carr/Cu films was analysed by thermo gravimetry. Figure 5 illustrates the curves representing TGA and DTG of different nanocomposite films. TGA thermogram (Fig. 5a) and DTG (Fig. 5b) show the weight loss percentage with concerning to temperature. TGA curve shows the thermal degradation in two steps. The first degradation occurred at 80 °C for all three samples because of the vaporised moisture absorbed by the films [29]. The second degradation occurred at 300 °C and corresponds to the degradation of glycerol and biopolymer carrageenan [30]. DTG curve peak was used to calculate the maximum decomposition temperature for different films. Tmax for the three samples were 238.95 °C, 240.60 °C and 241.66 °C respectively. For pure carrageenan film, Tmax was 229 °C calculated in previous studies [16]. There was an increase in thermal degradation temperature with addition of copper nanoparticles from 229 to 241.66 °C. After final destruction at 500 °C, the residual left and temperature corresponding to 50% degradation were given in Table S2.
4.2 Different Parameter Studied
4.2.1 Water Vapour Permeability (WVP)
The film’s WVP describes its ability to allow water vapours to pass via it. Water vapour barrier properties help in maintaining the freshness of packed food for a longer period and extend its shelf-life. Neat carrageenan film’s WVP and Carr/Cu nanoparticle films were given in Table S3. WVP of neat carrageenan film was 1.29 × 10–9 g m/m2 Pa s [17] but it decreases to 1.08 × 10–9 g m/m2 Pa s after incorporation of copper nanoparticles. The Carr/Cu film’s WVP decreases which confirms the even arrangement of copper NPs in the carrageenan membrane [31].
4.2.2 Moisture Content (MC)
Moisture content of various films is tabulated in Table S3. Moisture content (MC) of the Carr/Cu nanocomposite film decreases as the number of nanoparticles increases. This decreased moisture content extends the storage time of packed products; prevents microbiological development and biochemical response that are responsible for food degradation [32]. As we increased the amount of plant extract a larger number of nanoparticles get dispersed in the biopolymer membrane which in turn significantly decreases the moisture content.
4.2.3 Total Soluble Matter (TSM) Studies
TSM values were given in Table S3. The same trends were observed in values of TSM as in moisture content. TSM values decrease with an increase in the amount of NPs present in the Carr/Cu film. The addition of nanoparticles tends to establish a more hydrophobic mixture and decreased TSM values [33].
4.2.4 Biodegradability Studies
Three different film samples had been dumped in ground in three different containers having different concentrations of plant extract. The biodegradability setup was shown in Fig S4 and weight loss was given in Table S4. Film samples were removed from the soil, washed with distilled water, dried and weighed every week continuously for 1 month [19]. The degradation of film samples was evident from the Fig. 6. The film sample with minimum plant extract (Cu—30) degraded completely in 3 weeks while the other two samples degraded completely in 4 weeks. This show that when the films come in contact with soil it will take only 4 weeks for complete degradation.
4.3 Antimicrobial Properties
Antimicrobial properties of Carr/Cu nanocomposite films was tested on E. coli and S. aureus food grade pathogens bacteria and the results was given in Table S5 and the bar graph in Fig. 7.
Strong antimicrobial properties were shown by all the film samples. The reason of the high antimicrobial activity was that the oxygen forming complex and water present in bacterial membrane generate Copper ions from copper nanoparticles. The copper ions with positive charge react with bacterial cell wall (negative charge) and proteins [34]. These nanoparticles present in Carr/Cu film damage the cell wall, generate reactive oxygen species and lipid peroxidation that resulted in destruction of cell [22]. The zone of inhibition for different film samples was clearly shown in Fig. 8.
5 Application for Food Packaging
5.1 Cottage Cheese (Paneer) Storage
Cottage cheese was stored for 8 days and in the first 7 days, there was no change in the flavour, texture, or fragrance of the cottage cheese, but on the 8th day, there was a small alteration as shown in Fig. 9. Dairy products can be safely stored in Carr/Cu films for 1 week. From the previous literature, we did not find such type of film for cottage cheese packaging. A bio-nanocomposite composed of carboxymethyl cellulose/polyvinyl alcohol/CuO was used for preservation of processed cheese [20]. Bio-nanocomposite was applied directly on the cheese surface. But there was nothing mentioned, about how to use this cheese and whether the applied bio-nanocomposite was edible or not. The Carr/Cu nanocomposite film (Carr/Cu—90) was easy to handle, not in direct contact with packed products, cost-effective and biodegradable. These films were synthesized with minimum copper sulphate concentration and carrageenan is already used as a thickener and gelling agent in the food industry. There was no specific treatment required before packaging of food.
5.1.1 Analysis of Different Properties of Stored Cottage Cheese
The MC, acidity, chewiness, hardness, colour, odour, pH and taste of the cottage cheese after storage, all these factors are studies thoroughly. These parameters were analysed with slight modifications in earlier used methods [20].
During the storage of cottage cheese there was slight change in MC, pH, hardness, acidity, colour, odour and taste. The moisture content remained approximately same due to barrier properties of the Carr/Cu film evident from the WVP studies of the film. The change in all the properties was given in Table 3. A value was assigned for the colour, aroma, and flavour using a starting value of 10 and this value is set as control for this analysis. For the cottage cheese stored in plastic packing, all above mentioned properties start changing after 3rd day but the Carr/Cu films increased the storage period of cottage cheese for 7 days.
5.2 Packing of Grapes
The grapes were stored for 12 days and the grapes start changing their colour from green to yellow after 6th day and start decaying on 12th day as shown by arrows in Fig. 10. When any particular packaging is not present, grapes start decaying within 2 days. When Carr/Cu nanocomposite film was used for packaging it gives better results without any specific treatment as given by others. A carrageenan/TiO2 film was used for the packing of bananas at 20 °C for 12 days. The banana starts changing its colour from yellow to black after 6 days. For keeping bananas fresh for 12 days they used film consisting titanium nanotube doped with copper oxide along with carrageenan [31]. A Chitosan/Ag nanoparticle film was utilized for strawberry packaging for 12 days but they irradiate the packed strawberry with γ-rays using an underwater calibrator to keep it fresh for the long time [35]. This method required well-trained persons and not cost-effective. The nanocomposite films synthesized, Carr/Cu films are cost effective, safe and biodegradable.
5.2.1 Weight Loss (%), Total Soluble Solid (TSS), Titratable Acidity (TA) and Vitamin C Content Analysis
The weight loss percentage was measured by taking the weight of the grapes at different time of storage. The given equation was applied in calculating the weight loss percentage [36].
In the given formula, Wi is the initial weight and W0 is the weight on 0th day. The weight loss pattern for the grapes covered with Carr/Cu nanocomposite film was given in Table 4.
The TSS of the grapes was calculated by using refractometer. For this purpose we take 10 g pulp and centrifuged for 25 min [37]. This suspension was used to calculate TSS. The content of TSS was maintained by the Carr/Cu film so prevent fruit spoilage. The TSS content of the fruit is an indicator of fruit ripening. The titratable acidity (TA) was measured by treating the above suspension with 0.1 M NaOH [38]. The film maintained the acidity of the fruit for longer time and helps to keep the fruit fresh for long time.
Vitamin C content of grapes was determined by using 10 g pulp. The Carr/Cu film help to maintain the amount of ascorbic acid for long time in comparison to other packing materials. Ascorbic acid content was important for maintaining the nutritional value of the fruits. The Carr/Cu films are capable of maintaining vitamin C content, TSS and TA (Table 4) which help to keep the grapes fresh during storage.
6 Conclusion
Copper–carrageenan nanocomposite films synthesized from Argemone Mexicana extract or green method. Argemone mexicana is a widespread annual herb available in dry regions near water bodies and not edible. This plant was used from ancient times for medicinal purposes. Carrageenan was used in the Carr/Cu film and is a biopolymer extracted from red algae and extensively utilised in the food sector. Copper sulphate solution with a malar concentration of 1 mM was used for the synthesis of Carr/Cu films which was much less than previously synthesized films. Carr/Cu films show better UV barrier properties with minimum metal salt concentration. The film possesses higher tensile strength than the film synthesized with 1 M and 2 M metal salt concentrations. These films show good antimicrobial properties which help in maintaining the freshness of food. Carr/Cu films biodegrade within 4 weeks after burial in the soil. Carr/Cu films were applicable in the packing of cottage cheese and grapes i.e. dairy products and fruits. The storage time of cottage cheese was increased to 7 days at 4 °C without any other treatment. The grapes remained fresh for 12 days at room temperature (20 °C). The earlier synthesized films used for the packaging of fruits were given special treatment for extending the shelf life of packed products. Our Copper- carrageenan film was synthesized by green methods which are free from harmful chemicals and are economical, biodegradable and environment friendly. These films are a better alternative for the plastic packaging.
Data Availability
The data was provided upon request.
References
W. Zhang, S. Roy, P. Ezati, D.P. Yang, J.W. Rhim, Tannic acid: a green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. (2023). https://doi.org/10.1016/j.tifs.2023.04.004
A. Khan, R. Priyadarshi, T. Bhattacharya, J.W. Rhim, Carrageenan/alginate-based functional films incorporated with Allium sativum carbon dots for UV-barrier food packaging. Food Bioprocess Technol. 16, 1–15 (2023). https://doi.org/10.1007/s11947-023-03048-7
W. Chi, W. Liu, J. Li, L. Wang, Simultaneously realizing intelligent color change and high haze of κ-carrageenan film by incorporating black corn seed powder for visually monitoring pork freshness. Food Chem. 402, 134257 (2023). https://doi.org/10.1016/j.foodchem.2022.134257
Y. Han, M. Zhou, D.J. McClements, F. Liu, C. Cheng, J. Xiong, M. Zhu, S. Chen, Investigation of a novel smart and active packaging materials: nanoparticle-filled carrageenan-based composite films. Carbohydr. Polym. 301, 120331 (2023). https://doi.org/10.1016/j.carbpol.2022.120331
N. Bhatia, A. Kumari, A. Sharma, R. Sharma, Bio-inspired green formulation of various metal nanoparticles: a review. J. Mater. Sci. Res. Rev. 8(4), 1–38 (2021)
N. Chauhan, N. Thakur, A. Kumari, C. Khatana, R. Sharma, Mushroom and silk sericin extract mediated ZnO nanoparticles for removal of organic pollutants and microorganisms. S. Afr. J. Bot. 153, 370–381 (2023). https://doi.org/10.1016/j.sajb.2023.01.001
V. Dhiman, M. Jangra, S. Kumar, P. Choudhary, S. Chand, A. Kumari, R. Sharma, N. Kondal, Structural and defect-related optical characteristics of Viola odorata extract mediated ZnO. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2022.11.106
N. Bhatia, A. Kumari, N. Thakur, G. Sharma, R.R. Singh, R. Sharma, Phytochemically stabilized chitosan encapsulated Cu and Ag nanocomposites to remove cefuroxime axetil and pathogens from the environment. Int. J. Biol. Macromol. 212, 451–464 (2022). https://doi.org/10.1016/j.ijbiomac.2022.05.143
M. Hadidi, S. Jafarzadeh, M. Forough, F. Garavand, S. Alizadeh, A. Salehabadi, A.M. Khaneghah, S.M. Jafari, Plant protein-based food packaging films; recent advances in fabrication, characterization, and applications. Trends Food Sci. Technol. (2022). https://doi.org/10.1016/j.tifs.2022.01.013
R. Sharma, R. Sharma, R.R. Singh, A. Kumari, Evaluation of biogenic zinc oxide nanoparticles from Tinospora cordifolia stem extract for photocatalytic, anti-microbial, and antifungal activities. Mater. Chem. Phys. 297, 127382 (2023). https://doi.org/10.1016/j.matchemphys.2023.127382
D.G. Téllez-de-Jesús, N.S. Flores-Lopez, J.A. Cervantes-Chávez, A.R. Hernández-Martínez, Antibacterial and antifungal activities of encapsulated Au and Ag nanoparticles synthesized using Argemone mexicana L extract, against antibiotic-resistant bacteria and Candida albicans. Surf. Interfaces 27, 101456 (2021). https://doi.org/10.1016/j.surfin.2021.101456
W. Zhang, W. Jiang, Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 155, 1252–1261 (2020). https://doi.org/10.1016/j.ijbiomac.2019.11.093
S. Shankar, J.P. Reddy, J.W. Rhim, H.Y. Kim, Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 117, 468–475 (2015). https://doi.org/10.1016/j.carbpol.2014.10.010
S. Shankar, J.W. Rhim, Preparation of nanocellulose from micro-crystalline cellulose: the effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 135, 18–26 (2016). https://doi.org/10.1016/j.carbpol.2015.08.082
J.W. Rhim, Physical-mechanical properties of agar/κ-carrageenan blend film and derived clay nanocomposite film. J. Food Sci. 77(12), N66–N73 (2012). https://doi.org/10.1111/j.1750-3841.2012.02988
S. Shankar, L.F. Wang, J.W. Rhim, Preparation and properties of carbohydrate-based composite films incorporated with CuO nanoparticles. Carbohydr. Polym. 169, 264–271 (2017). https://doi.org/10.1016/j.carbpol.2017.04.025
J.W. Rhim, L.F. Wang, Preparation and characterization of carrageenan-based nanocomposite films reinforced with clay mineral and silver nanoparticles. Appl. Clay Sci. 97, 174–181 (2014). https://doi.org/10.1016/j.clay.2014.05.025
A. Ounkaew, P. Kasemsiri, K. Kamwilaisak, K. Saengprachatanarug, W. Mongkolthanaruk, M. Souvanh, U. Pongsa, P. Chindaprasirt, Polyvinyl alcohol (PVA)/starch bioactive packaging film enriched with antioxidants from spent coffee ground and citric acid. J. Polym. Environ. 26, 3762–3772 (2018). https://doi.org/10.1007/s10924-018-1254-z
H. Ibrahim, M. Farag, H. Megahed, S. Mehanny, Characteristics of starch-based biodegradable composites reinforced with date palm and flax fibers. Carbohydr. Polym. 101, 11–19 (2014). https://doi.org/10.1016/j.carbpol.2013.08.051
A.M. Youssef, F.M. Assem, H.S. El-Sayed, S.M. El-Sayed, M. Elaaser, M.H. Abd El-Salam, Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese. RSC Adv. 10(62), 37857–37870 (2020). https://doi.org/10.1039/D0RA07898K
R. Sharma, P. Singh, R. Dharela, G.S. Chauhan, K. Chauhan, Thiourea functionalized β-cyclodextrin as green reducing and stabilizing agent for silver nanocomposites with enhanced antimicrobial and antioxidant properties. New J. Chem. 41(21), 12645–12654 (2017). https://doi.org/10.1039/C7NJ00759K
N. Bhatia, A. Kumari, N. Chauhan, N. Thakur, R. Sharma, Duchsnea indica plant extract mediated synthesis of copper oxide nanomaterials for antimicrobial activity and free-radical scavenging assay. Biocatal. Agric. Biotechnol. 47, 102574 (2023). https://doi.org/10.1016/j.bcab.2022.102574
A. Henglein, Formation and absorption spectrum of copper nanoparticles from the radiolytic reduction of Cu (CN) 2. J. Phys. Chem. B 104(6), 1206–1211 (2000). https://doi.org/10.1021/jp992950g
A.A. Oun, J.W. Rhim, Carrageenan-based hydrogels and films: effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 67, 45–53 (2017). https://doi.org/10.1016/j.foodhyd.2016.12.040
H. Hezaveh, I.I. Muhamad, Modification and swelling kinetic study of kappa-carrageenan-based hydrogel for controlled release study. J. Taiwan Inst. Chem. Eng. 44(2), 182–191 (2013). https://doi.org/10.1016/j.jtice.2012.10.011
S.K. Karuppannan, R. Ramalingam, S.M. Khalith, M.J.H. Dowlath, G.D. Raiyaan, K.D. Arunachalam, Characterization, antibacterial and photocatalytic evaluation of green synthesized copper oxide nanoparticles. Biocatal. Agric. Biotechnol. 31, 101904 (2021). https://doi.org/10.1016/j.bcab.2020.101904
C. Karthikeyan, K. Varaprasad, S.K. Venugopal, S. Shakila, B.R. Venkatraman, R. Sadiku, Biocidal (bacterial and cancer cells) activities of chitosan/CuO nanomaterial, synthesized via a green process. Carbohydr. Polym. 259, 117762 (2021). https://doi.org/10.1016/j.carbpol.2021.117762
F. Li, Y. Liu, Y. Cao, Y. Zhang, T. Zhe, Z. Guo, X. Sun, Q. Wang, L. Wang, Copper sulfide nanoparticle-carrageenan films for packaging application. Food Hydrocoll. 109, 106094 (2020). https://doi.org/10.1016/j.foodhyd.2020.106094
P. Kanmani, J.W. Rhim, Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 106, 190–199 (2014). https://doi.org/10.1016/j.carbpol.2014.02.007
A.A. Oun, J.W. Rhim, Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 127, 101–109 (2015). https://doi.org/10.1016/j.carbpol.2015.03.073
P. Ezati, Z. Riahi, J.W. Rhim, Carrageenan-based functional films integrated with CuO-doped titanium nanotubes for active food-packaging applications. ACS Sustain. Chem. Eng. 9(28), 9300–9307 (2021). https://doi.org/10.1021/acssuschemeng.1c01957
R. Balasubramanian, S.S. Kim, J. Lee, J. Lee, Effect of TiO2 on highly elastic, stretchable UV protective nanocomposite films formed by using a combination of k-Carrageenan, xanthan gum and gellan gum. Int. J. Biol. Macromol. 123, 1020–1027 (2019). https://doi.org/10.1016/j.ijbiomac.2018.11.151
L. Ge, M. Zhu, Y. Xu, X. Li, D. Li, C. Mu, Development of antimicrobial and controlled biodegradable gelatin-based edible films containing nisin and amino-functionalized montmorillonite. Food Bioprocess Technol. 10, 1727–1736 (2017). https://doi.org/10.1007/s11947-017-1941-0
K. Chauhan, R. Sharma, R. Dharela, G.S. Chauhan, R.K. Singhal, Chitosan-thiomer stabilized silver nano-composites for antimicrobial and antioxidant applications. RSC Adv. 6(79), 75453–75464 (2016). https://doi.org/10.1039/C6RA13466A
S. Shankar, D. Khodaei, M. Lacroix, Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries. Food Hydrocoll. 117, 106750 (2021). https://doi.org/10.1016/j.foodhyd.2021.106750
Z. Su, M. Hu, Z. Gao, M. Li, Z. Yun, Y. Pan, Z. Zhang, Y. Jiang, Apple polyphenols delay senescence and maintain edible quality in litchi fruit during storage. Postharvest Biol. Technol. 157, 110976 (2019). https://doi.org/10.1016/j.postharvbio.2019.110976
S. Mei, B. Fu, X. Su, H. Chen, H. Lin, Z. Zheng, C. Dai, D.P. Yang, Developing silk sericin-based and carbon dots reinforced bio-nanocomposite films and potential application to litchi fruit. LWT 164, 113630 (2022). https://doi.org/10.1016/j.lwt.2022.113630
X. Jiang, H. Lin, J. Shi, S. Neethirajan, Y. Lin, Y. Chen, H. Wang, Y. Lin, Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 252, 134–141 (2018)
Acknowledgements
The authors show their genuine gratitude to the Researchers Supporting Project number (RSP2024R391) at King Saud University, Riyadh, Saudi Arabia. We are truly appreciative to and grateful to Sophisticated Analytical Instrumentation Facility (SAIF) lab QQ69 + H8Q, Punjab University, Sector 14, Chandigarh, India, 160014 for their valuable support for conducting FESEM characterization of the Carr/Cu film. Their skills as well as resources have greatly contributed to the success of this research. We heartily appreciate Materials Research Center (MRC) at Malaviya National Institute of Technology (MNIT), JLN Marg, Jaipur—302017 for analysis of the mechanical traits of the synthesized films. The assistance provided by them has been invaluable in our quest to understand the functional integrity of synthesized films. We are sincerely thankful for their support and cooperation.
Funding
This study was supported by King Saud University (Grant No. RSP2024R391).
Author information
Authors and Affiliations
Contributions
S.K. performed the experiments and written the original draft, A.K. investigate the results, J.K. performed the review and writing, R.J. supervised the whole work, M.S. performed the review and writing, N.L. performed the experiment, N.K. investigate the results, A.K. provided the technical support, R.S. performed the experiments, supervised and written the original draft.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, S., Kumari, A., Ahmed, J. et al. Enhancing UV Protection and Antimicrobial Properties in Food Packaging Through the Use of Copper Nanoparticles and κ-Carrageenan Based Nanocomposite Film. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03231-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03231-z