Abstract
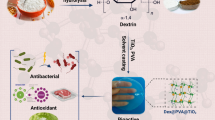
Poly(vinyl alcohol) (PVA) is a synthetic and promising film-forming polymer that is usually used in packaging applications. In this study, PVA nanocomposite films with varying amounts of zinc oxide-doped multiwalled carbon nanotubes (MWCNTs-ZnO) were prepared. The tensile strength of the nanocomposite films was 116% higher than that of the PVA film. The thermal stability, water vapor transmission rate, hydrophobicity, and antibacterial activity of the nanocomposite films were better than those of pure PVA. Tests on water loss in vegetables at room temperature revealed that the vegetable wrapped in packaging films could keep more water for more than 4 days. Tests on the shelf life of chicken meat packed in films suggested that the growth of natural microorganisms in raw chicken kept in the preservation storage of the refrigerator could be inhibited for at least 36 h. The findings of this study indicated that nanocomposite MWCNTs-ZnO/PVA films with good transparency had great potential applications in food packaging.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2016, the European plastics reported that 39.9% of packaging materials are designed to be made of plastics [1]. Generally, a lot of food packaging waste is generated as a result of food consumption [2]. Food packaging waste accounts for almost two-thirds of all packaging waste by volume [3]. Because plastic is easy to form and low in cost, most food packaging materials are plastic products, which cause serious environmental pollution [4]. Therefore, “green” food packaging materials will definitely become a new trend.
Poly(vinyl alcohol) (PVA) is a synthetic and water-soluble polymer [5]. Owing to its features of being non-toxic, biodegradable, non-polluting, and excellent for film formation, PVA is used in food and pharmaceutical packaging, cling film, and others, and it can replace petroleum-based plastics used in food packaging [6]. Food packaging materials are important because they extend food shelf life and ensure food safety. However, pure PVA films no longer meet the demands of food quality assurance.
It was demonstrated that nanoparticles can improve the mechanical properties and barrier capacity of packaging materials used to extend the shelf life of food [7]. Multiwalled carbon nanotubes (MWCNTs) are carbon nanomaterials with unique properties and can enhance the thermal, mechanical, and barrier properties and functionality of food packaging materials [8, 9]. There are researches [10, 11] about nanocomposite MWCNTs/PVA films, and they all found that the mechanical properties of PVA significantly increased owing to the addition of MWCNTs. Huang et al. [12] prepared nanocomposite MWCNTs/CS/PVA films, which they thought could be used in food packaging. Clearly, MWCNTs have promise in food packaging applications. Apart from increased mechanical strength, good antibacterial properties of food packaging films are also especially important for ensuring food safety and extending food shelf life.
As food packaging materials, PVA has been blended with a number of bio-fillers such as corn starch powder [13], chitosan solution [14], bacterial cellulose suspension [15], and solid gelatin [16]. However, bio-fillers could not provide antibacterial properties to nanocomposite films. For long-term protection of foods against exposure to microbial environments, antibacterial properties are particularly important. Food packaging systems contain metallic nanoparticles that exhibit antimicrobial activity; for example, silver nanoparticles [17], zinc oxide nanoparticles (ZnO) [18], and copper nanoparticles [19]. Azizi-Lalabadi et al. [20] detected the microbial qualities of white shrimp packaged with PVA/gelatin nanocomposite containing ZnO nanoparticles doped on 4A zeolite during refrigeration. Amin et al. [21] introduced a nanocomposite pluronic/ZnO/PVA film as an active packaging material with enhanced antimicrobial activity. Because of diffusion, dissolution, and abrasion of packaging surfaces, nanomaterials may contaminate food [22]. However, there are many researches that showed only low concentrations of Zn migrated to food, and the European Food Safety Authority had concluded that ZnO nanoparticles did not migrate from polyolefins and unplasticized polymers [23,24,25,26,27]. So, nanoparticles show great promise in food packaging materials. Due to the different structures, shapes, sizes, and properties of MWCNTs and ZnO, it is easy to cause uneven distribution or agglomeration if the two nanomaterials are added to PVA.
The purpose of this study was to combine the properties of MWCNTs and ZnO and prepare a new nanomaterial in the form of zinc oxide-doped multiwalled carbon nanotubes (MWCNTs-ZnO), which not only had the advantages of both MWCNTs and ZnO, but it could also be dispersed easily and uniformly in polymers. There are many researches about PVA, ZnO, and MWCNTs in wound dressing, tissue engineering, food packaging, etc. For example, Abedi et al. [28] fabricated chitosan/MWCNTs/PVA nanofibers, which can be used in cardiac tissue engineering, by blending dissolved chitosan and PVA solution with MWCNTs. Khorasani et al. [29] prepared PVA hydrogels containing dissolved chitosan and ZnO for wound dressing applications. Hence, MWCNTs-ZnO may have great promise in food packaging. MWCNTs-ZnO was mixed with PVA by solution blending to prepare nanocomposite MWCNTs-ZnO/PVA films, which could be used as an antibacterial packaging that had excellent food preservation ability. The water retention and antibacterial properties of these nanocomposite films were tested and evaluated on Chinese cabbage and chicken meat. The physical properties of the films were also characterized: mechanical and thermal properties, water vapor permeability, microstructure, light transmission, and opacity. The magnitude of zinc migration from packaging films to chicken meat was also tested. After additional safety verifications in the future, it is expected that this new nanocomposite process could be used as a reference for preparing food packaging materials.
Experimental section
Materials
PVA (1788, degree of alcoholysis = 87–89%, Mw = 74,800) was purchased from Shanghai Titan Corporation (Shanghai, China). The preparation of MWCNTs-ZnO, a new nanomaterial, was similar to that in our previous research [30]; it was customized by Apex Nanotek Corporation (New Taipei City, Taiwan), a cooperative manufacturer; the weight ratio of MWCNTs to ZnO was 1.2:100. XRD spectra of MWCNTs, ZnO, and MWCNTs-ZnO are shown in Figure S1, and Figure S2 illustrates the morphology of ZnO and MWCNTs-ZnO.
Preparation of nanocomposite MWCNTs-ZnO/PVA films
PVA was stirred and dissolved in distilled water at 80 ºC on a DF-101S digital hotplate–magnetic stirrer (Gongyi Yuhua Instrument Co. Ltd., China). MWCNTs-ZnO was ultrasonically dispersed in distilled water. Afterward, the suspension of MWCNTs-ZnO was added to the aqueous PVA solution. The concentration of PVA was 19%. The content of MWCNTs-ZnO in PVA was 0, 0.3, 0.6, and 0.9%. To achieve a completely homogenized MWCNTs-ZnO/PVA solution, it was ultrasonically dispersed for 15 min. Finally, the film was made using a specially tailored tool with a thickness of 20 µm on a glass plate (25 × 25 cm2). The film was dried, first at 85 °C for 2 h, then at 105 °C for 1 h to remove all moisture. Ultimately, nanocomposite MWCNTs-ZnO/PVA films were produced. Scheme 1 shows the flow for preparing a nanocomposite MWCNTs-ZnO/PVA film.
Characterization
Mechanical tests were conducted using a microcomputer-controlled electronic universal testing machine (Furbs Xiamen Furbusi Testing Equipment Co. Ltd., Xiamen, China), according to the standard method of ASTM D882-02. Mechanical properties, including tensile strength and elongation at break, were evaluated. The test speed was 10 min/mm. Data obtained from five samples were averaged, and the average value was used for subsequent analyses.
Differential scanning calorimetry (DSC) on samples was carried out on a DSC-200 F3 calorimeter (Netzsch, Germany) under nitrogen atmosphere. Samples were sealed hermetically in DSC pans and heated from room temperature to 250 °C at a rate of 10 °C/min to examine their melting temperature (Tm) and melting enthalpy (ΔHm). Fan [31] showed that a rapid heating could increase the sensitivity of DSC. So, the glass transition temperature (Tg) was determined as a result of rapid heating from room temperature to 250 °C. The faster the heating rate, the lower the resolution and the higher the sensitivity. To have both good sensitivity and high resolution, the heating rate of 20 °C/min has been performed.
Thermogravimetric analysis (TGA) was performed using TGA instruments (HTG-1, HENVEN, China). The heating of samples was done in air to simulate the natural degradation of food packaging materials. The test condition was from room temperature to 650 °C at a scan rate of 10 °C/min. The thermal decomposition temperature (Td) and DTG (derivative of TGA) were obtained.
Water contact angles were measured using a contact angle meter (JC2000D, Shanghai, China). Samples were mounted on a platform, onto which 2 μL of distilled water was dropped from a syringe. The computer software recorded the contact angle data, which were used to evaluate the hydrophobicity of PVA and nanocomposite MWCNTs-ZnO/PVA films.
Water vapor transmission rate (WVTR) was determined by gravimetry, according to ASTM E96. The water vapor through the film was measured at 23 °C and 70% RH by using a test instrument W3/060 (Labthink, Jinan, China). WVTR (g/m2h) was calculated using the following Eq. (1):
where Wg was the weight measured at different periods of time, t was the test time, and A was the permeation area of samples.
Scanning electron microscopy (SEM, TESCAN, Czech) was used to observe the surface morphology of PVA and nanocomposite MWCNTs-ZnO/PVA films; the morphology included the flaw, clusters of nano-fillers, phase separations, and other phenomena. The film was sputtered with a thin layer of gold, which was necessary to obtain distinct surface SEM images. The scanning or accelerating voltage was 20 kV. The dispersion of nano-fillers in nanocomposites was obtained using an energy-dispersive spectrometer (EDS, BRUKER, German) operated at 500 × magnification.
The light transmission and opacity of films were determined using a UV–Vis spectrophotometer (P4, MAPADA, Shanghai), according to the method reported by Syahida et al. [32]. The light transmission was measured at a visible light range, with air used as blank. The test was repeated five times. The opacity of the sample was calculated using the following relationship (2):
The antibacterial activity of PVA and nanocomposite MWCNTs-ZnO/PVA films was determined against Escherichia coli (quantitative test), according to the method described by Tsou et al. [33, 34]. Each sample was cultured with an E. coli suspension in a serum bottle, and it was shaken at a speed of 120 rpm/min at 37 °C for 24 h. The antibacterial activity of the film was gauged from the counted number of bacteria. The bacterial suspension with no film was set as the control. The antibacterial rate was calculated using the following formula:
Tests on the rate of water loss (RWL) in vegetables were conducted on the basis of gravimetry. The water on the surface of fresh vegetable was wiped with a filter paper, and the vegetables were weighed (W0). They were then packaged using a film and left at room temperature (25 °C and 70% RH). The vegetable not packaged with a film served as the control group. After four days, they were weighed again (Wdry). RWL was calculated from the following Eq. (3):
Tests on the shelf life of chicken meat were performed by observing the antibiotic activity of sample films against the growth of natural germs in the raw chicken, according to the method reported by Wang et al. [35]. The raw chicken was cut into small cubes; some were packaged with pure PVA and nanocomposite films, and some were left without packaging. Then, they were all kept in the preservation storage of the refrigerator, and the bacterial concentration was measured at different periods of time: 0, 12, 36, and 48 h. Those without film packaging served as the blank group.
Evaluation of the possible migration of nanomaterials from packaging films to food wrapped in them was based on a method called contact tests described by Avella [7]. The shape of raw chicken was the same as that in tests for the shelf life of chicken meat, and it was packaged similarly. The samples were all refrigerated at 4 °C for 4 days. Then, the packaging film was removed, and the sample was placed in a porcelain cup and heated in a furnace at 500 °C for 1 h. After the sample was cooled, the obtained ash was analyzed using EDS at 100 × magnification to determine the amount of Zn released.
Results and discussion
Mechanical properties
Figure 1a presents data on tensile strength and elongation at break of PVA and nanocomposite MWCNTs-ZnO/PVA films. MWCNTs are inorganic nanomaterials, which can improve the polymer matrix mechanical properties [36]. Hence, the addition of 0.3% MWCNTs-ZnO significantly increased the nanocomposite film tensile strength by 116%, in comparison with the PVA film tensile strength. Li et al. [37] and Wang et al. [38] found that Zn+ may combine with functional groups (such as hydroxyl) and form coordination bonds. So, in addition to the reinforcing effect of nanomaterials on PVA, a strong bonding might also exist between the hydroxyl on the ZnO surface and that in PVA. However, agglomeration formed when the filler content continued to increase, which caused the tensile strength to decrease [39], but it was still higher than that of the PVA film.
The elongation at break of nanocomposite MWCNTs-ZnO/PVA films continued to improve significantly, and it was about 81% higher than that of pure PVA film when the MWCNTs-ZnO content was 0.9%. MWCNTs-ZnO could help stretch the chains or improve the degree of polymer orientation [40,41,42]. At the same time, the interaction between the many hydrogen bonds in MWCNTs-ZnO and PVA might form inter-chain bonds that reinforced cohesion of the PVA network, and this strengthened the nanocomposite, so a greater stretching force would be needed to break it. Therefore, the strength and flexibility could be boosted by MWCNTs-ZnO. This result is similar to another report, which dealt with a high content of MWCNTs [43], but it is different from most reports. However, PVA blended with MWCNTs-ZnO has not yet been reported. The combination of MWCNTs and ZnO led to the aforementioned new mechanism.
Thermogravimetric analysis
DTG curves and data for PVA and its nanocomposite films are represented in Fig. 2 and Table 1, respectively. The thermal degradation of PVA and its nanocomposite films showed four weight-loss regions. The first region (I) at a temperature of about 90 °C was due to the evaporation of water [44]. The second region (II) was the degradation of the PVA side chain, which occurred at around 200–400 °C [45]. However, the first weight-loss region for MWCNTs-ZnO and ZnO was at a temperature range of 50–260 °C (Figure S3), indicating that the second weight-loss peak for all MWCNTs-ZnO/PVA nanocomposites was at a temperature lower than that for pure PVA. The third region (III) occurred at 400–500 °C, pertaining to the C–C backbone cleavage in PVA [46]. The fourth peak (IV) was attributed to the transformation of C into CO2, as the test was done under atmospheric conditions. As shown in Table 1 that 0.3 and 0.6% MWCNTs-ZnO had little effect on PVA for the fourth peak (IV), but when MWCNTs ZnO content was 0.9, the pyrolysis temperature of PVA was significantly reduced. This may be due to the agglomeration of MWCNTs-ZnO and the thermal decomposition of ZnO. The thermal decomposition temperature for PVA nanocomposite films in the second region (II) decreased with increase in the MWCNTs-ZnO content, which was because the first decomposition temperature of ZnO in the new nanomaterial was about 180 °C. It can be observed in Figure S3 that the fastest degradation rate for ZnO occurred at 170 °C and 500 °C. Therefore, it confirmed that the thermal stability changed with the introduction of MWCNTs-ZnO.
Differential scanning calorimetry
Figure 3 shows the DSC curves for PVA and its nanocomposite films. Thermal parameters (Tg, Tm, and ΔHm), for both nanocomposite films and PVA, are listed in Table 2. The addition of MWCNTs-ZnO significantly increased Tg from 74.3 to 76.9 °C, owing to the nano-filler impeding the motion of polymer chains [30]. This indicated that MWCNTs-ZnO could improve the Tg of PVA, which allowed the film to become more temperature-resistant. The crystallinity (Xc) was evaluated from the following relationship (4):
where α was the content of MWCNTs-ZnO; ΔHm was the measured melting enthalpy; ΔHm0 was the enthalpy of 100% PVA crystals (161 J/g) [47]. Xc of the nanocomposites was lower than that of PVA because the nano-fillers increased the steric hindrance of polymer chains [30]. Tm and Xc of 0.3(MWCNTs-ZnO)/PVA were lower than those of pure PVA. This may also because the nano-fillers could destroy the crystals [48], so the size of PVA spherulites became smaller. However, when the MWCNTs-ZnO content increased to 0.6–0.9%, PVA may be promoted to produce more nucleus, and then improve the crystallinity and melting point of PVA.
Water contact angle
Water contact angle is an important parameter for evaluating the wettability of food packaging films, particularly in the case of PVA, as it is water-soluble [32]. Figure 4 and Table 3 show the results of water contact angle. PVA is water-soluble; thus, it was expected that its water contact angle was lower than 90°. All nanocomposite films had higher water contact angles than pure PVA, and they showed nearly hydrophobic values, which indicated that MWCNTs-ZnO could increase the water contact angle of PVA. MWCNTs-ZnO was exposed at the surface, so the water contact angle was greater than 90°.
Water vapor transmission rate
A low WVTR signifies that films maintain the quality of food [35]. Table 3 shows WVTR results for PVA and its nanocomposites. WVTR was significantly reduced with the addition of MWCNTs-ZnO, and the value was minimum when the nano-filler content was 0.6%, which indicated that MWCNTs-ZnO could improve the barrier property of the film against water vapor. That is because nano-fillers caused increased tortuous pathways for the water vapor through the film [49]. However, defects appeared when there was filler agglomeration with increased content, which led WVTR to increase slightly. It was clear that at different periods of time, WVTR for MWCNTs-ZnO/PVA was lower than that for PVA (Fig. 5). So, the water barrier properties of PVA were improved by the addition of MWCNTs-ZnO.
Scanning electron microscopy
SEM images of films, at the same magnification (2.00 kx), are shown in Fig. 6. The PVA film was smooth, but the others had some particles on the surface. MWCNTs-ZnO was dispersed well in PVA when the nanomaterial content was 0.3 and 0.6%. However, when the content was increased to 0.9%, MWCNTs-ZnO was clearly agglomerated into large particles that were exposed on the surface.
EDS results on the distribution of Zn in PVA and its nanocomposites are shown in Fig. 7a’–d’. In pure PVA film, there was no Zn. However, it was obvious that Zn was present in MWCNTs-ZnO/PVA nanocomposite films, and it was dispersed uniformly when the MWCNTs-ZnO content was 0.1%. There was little agglomeration in 0.6(MWCNTs-ZnO)/PVA and 0.9(MWCNTs-ZnO)/PVA. It also confirmed the decrease in tensile strength and the increase in water contact angle and WVTR.
Light transmission and opacity
The opacity of food packaging directly influences the preference of consumers and plays an important role in packaging materials. Figure 8 displays the light transmittance in PVA and nanocomposite MWCNTs-ZnO/PVA films at selected wavelengths of ultraviolet light (200–320 nm) and visible light (400–800 nm). Table 4 provides the result of opacity tests. It indicates that the transmission in 0.3(MWCNTs-ZnO)/PVA film was lower than that in neat PVA. The lesser the opacity, the greater the transparency. MWCNTs could cause a significant increase in light absorbance and opacity of films due to light scattering effects [50]. The decrease in the number of defects, increase in crystal size, decrease in film thickness, and improvement in surface morphology of films would improve optical transmission [51]. Data on Xc (Table 2) and EDS (Fig. 7) indicated that 0.6(MWCNTs-ZnO)/PVA and 0.9(MWCNTs-ZnO)/PVA films exhibited better properties. Hmar et al. [52] also prepared nanocomposites with good transparency, which contained ZnO nanosheet-multiwalled carbon nanotubes. But the agglomeration of nano-fillers in the polymer matrix also reduced the transparency of PVA [53]. Hence, the transmission and transparency for 0.9(MWCNTs-ZnO)/PVA film decreased. In conclusion, according to opacity tests, the transparency of all samples was in the following descending order: 0.6(MWCNTs-ZnO)/PVA > PVA > 0.9(MWCNTs-ZnO)/PVA > 0.3(MWCNTs-ZnO)/PVA.
Antibacterial property
ZnO plays a key role as an antibacterial agent. Sirelkhatim et al. [54] reported that ZnO could weaken mitochondria, and it could lead to intracellular outflow and release of gene expression associated with oxidative stress, inhibit the growth of microbial cells, and eventually lead to death. Table 5 lists data on the antibacterial ability of PVA and nanocomposite MWCNTs-ZnO/PVA films against E. coli for a period of 24 h. The antibacterial activity became apparent with the addition of MWCNTs-ZnO. The bacteriostatic rate was as high as 98.63% even when only 0.3% MWCNTs-ZnO was added. It showed that nanocomposite MWCNTs-ZnO/PVA films had a very good antibacterial ability, and they could ensure well that food was not attacked by E. coli within 24 h.
Vegetable water loss
The film with better performance (i.e., nanocomposite 0.6(MWCNTs-ZnO)/PVA film) was selected for tests on the vegetable water loss. Figure 9 presents data on RWL in vegetables with and without film wrapping at room temperature for 4 days. After 4 days, the total RWL for the control group and the vegetable wrapped with pure PVA was 53.7 and 37.9%, respectively. Because the vegetable without a packaging film was in direct contact with air, RWL was fast; signs of water loss were clearly visible (Fig. 9). However, the vegetable packed in 0.6(MWCNTs-ZnO)/PVA film did not show much water loss or dehydration, and it was fresher than the other vegetables. Therefore, it was demonstrated that 0.6(MWCNTs-ZnO)/PVA film could prevent the vegetable from losing a great amount of water at room temperature for 4 days. This material may have great promise for preserving fresh vegetables.
Shelf life of chicken meat
According to the research by Bolton et al. [55], the shelf life of chicken meat chilled at 4 °C under aerobic conditions was less than 5 days. The shelf life of fresh chicken stored in the refrigerator at 4 °C was about 3 days. Thus, the meat is generally spoilt when bacterial counts reach 107–8 CFU/g [55]. Bacteria from the surrounding must pass through a packaging film to reach the chicken. However, nanomaterials in nanocomposite PVA films would disrupt the bacterial cell wall, causing the bacteria to die (Fig. 10a). Thus, the film could protect the chicken from the bacteria in the environment. To verify the resistance of nanocomposite films against bacteria, the amount of bacteria in raw chicken was measured for 48 h. A comparison of control and PVA films revealed that all nanocomposite films were capable of inhibiting bacterial growth, demonstrating their excellent ability to preserve the chicken. Overall, the growth of bacteria in raw chicken wrapped with PVA films containing MWCNTs-ZnO was not apparent for a period of time between 12 and 36 h. But after 36 h, microbial growth was obvious, and it increased from 36 to 48 h (Fig. 10b). So, it was demonstrated that nanocomposite MWCNTs-ZnO/PVA films could preserve the quality of fresh chicken.
Test on the migration of zinc from packaging films to food
Chicken meat protein also contains abundant nutrients and elements such as sodium (Na), magnesium (Mg), potassium (K), and phosphorus (P) [56]. Gold (Au) was also indicated in Table 6 because all samples had been sputtered with a thin layer of gold, which was necessary prior to the EDS test. For the chicken meat packaged with MWCNTs-ZnO/PVA nanocomposite films for 4 days, only a trace amount of zinc (Zn) was detected in the chicken wrapped with a film containing more than 0.6 phr MWCNTs-ZnO (Table 6). However, there was much water, as well as many other organic ingredients in the chicken. Samples were tested after a high-temperature calcination, so the actual amount of Zn may be much smaller. In addition, if 0.3(MWCNTS-ZnO)/PVA nanocomposite film was used for food packaging, no Zn transfer occurred within 4 days, and the chicken preservation experiment proved that when the film was used for more than 36 h, the chicken would be corroded by bacteria and become unsafe. Zn is an essential element in the human diet; however, too much Zn is harmful to human health [57]. Therefore, MWCNTS-ZnO/PVA nanocomposite film may be used for food packaging, but further research is also needed.
Conclusion
In this study, we prepared nanocomposite MWCNTs-ZnO/PVA films and tested the possibility of applying them for food packaging. The results showed that when the MWCNTs-ZnO content was 0.6%, the water vapor barrier and transmittance were the best. This was attributed to the fact that more nanomaterials were distributed in the substrate but less agglomerated. The optimal MWCNTs-ZnO content in PVA increased the tortuous path for water molecules through the nanocomposite film. Hence, 0.6(MWCNTs-ZnO)/PVA was selected for tests on rates of water loss in vegetables; it was found that the film could effectively retain more water in the vegetables than without the use of a packaging film or than the neat PVA film within 4 days. The results from tests on the shelf life of chicken meat showed that nanocomposite MWCNTs-ZnO/PVA films could be used effectively to slow down the growth of natural bacteria in raw chicken within 36 h. In addition, the test on the migration of zinc from packaging films to chicken meat indicated that there was no Zn released into the meat, when the MWCNTs-ZnO content was 0.3%. Therefore, the films may also be used for the preservation of fruits, vegetables, meat, and packaging frozen foods. In summary, MWCNTs-ZnO could enhance certain properties of PVA and impart antibacterial properties to nanocomposite MWCNTs-ZnO films. After further standard tests on safety were conducted, these films may have potential application in the field of food packaging.
References
Lara BRB, Araújo ACMA, Dias MV et al (2019) Morphological, mechanical and physical properties of new whey protein isolate/ polyvinyl alcohol blends for food flexible packaging. Food Packag Shelf Life 19:16–23
Xiao W, Sun Z, Liu J, Dong J (2018) Study of the influence of preparation conditions of γ-PGA ester as a food packaging material on the biodegradation performance. In: Zhao P, Ouyang Y, Xu M, Yang L, Ren Y (eds) Applied sciences in graphic communication and packaging. Lecture notes in electrical engineering, vol 477. Springer, Singapore. https://doi.org/10.1007/978-981-10-7629-9_98
Marsh K, Bugusu B (2007) Food packaging-roles, materials, and environmental issues. J Food Sci 72:R39–R55
Setiawan AH, Aulia F (2017) Development of more friendly food packaging materials base on polypropylene through blending with polylacticacid. AIP Conf Proc 1803(1):020039
Chetouani A, Elkolli M, Bounekhel M, Benachour D (2017) Chitosan/oxidized pectin/PVA blend film: mechanical and biological properties. Polym Bull 74:4297–4310
Yun Y-H, Yoon S-D (2010) Effect of amylose contents of starches on physical properties and biodegradability of starch/PVA-blended films. Polym Bull 64:553–568
Avella M, De Vlieger JJ, Errico ME et al (2005) Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem 93:467–474
Septiani NLW, Yuliarto B, Iqbal M et al (2015) The methanol response sensing properties using MWCNT-ZnO composite. Adv Mater Res 1112:116–119
Kotsilkov S, Ivanov E, Vitanov N (2018) Release of graphene and carbon nanotubes from biodegradable poly(lactic acid) films during degradation and combustion: risk associated with the end-of-life of nanocomposite food packaging materials. Materials 11(12):2346
Manohara SR, Samal SS, Rudreshappa GE (2016) Humidity sensing properties of multiwalled carbon nanotubePolyvinyl alcohol nanocomposite films. Nanosci Nanotechnol-Asia 6:128–134
Yee MJ, Mubarak NM, Khalid M et al (2018) Synthesis of polyvinyl alcohol (PVA) infiltrated MWCNTs buckypaper for strain sensing application. Sci Rep 8:1–16
Huang D, Wang A (2013) Non-covalently functionalized multiwalled carbon nanotubes by chitosan and their synergistic reinforcing effects in PVA films. RSC Adv 3:1210–1216
Tudorachi N, Cascaval C, Rusu M, Pruteanu M (2000) Testing of polyvinyl alcohol and starch mixtures as biodegradable polymeric materials. Polym Test 19:785–799
Kanatt SR, Rao MS, Chawla SP, Sharma A (2012) Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll 29:290–297
Millon LE, Wan WK (2006) The polyvinyl alcohol–bacterial cellulose system as a new nanocomposite for biomedical applications. J Biomed Mater Res Part B Appl Biomater 79B:245–253
Pal K, Banthia AK, Majumdar DK (2007) Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. Aaps PharmSciTech 8:E142–E146
Tsou C-H, Lee H-T, Hung W-S et al (2016) Synthesis and properties of antibacterial polyurethane with novel Bis (3-pyridinemethanol) silver chain extender. Polymer 85:96–105
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Tsou C-H, Lee H-T, Hung W-S et al (2017) Effects of different metals on the synthesis and properties of waterborne polyurethane composites containing pyridyl units. Polym Bull 74:1121–1143
Azizi-Lalabadi M, Ehsani A, Ghanbarzadeh B, Divband B (2020) Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: microbial and sensory qualities of packaged white shrimp during refrigeration. Int J Food Microbiol 312:1–10
Amin KM, Partila AM, Abd El-Rehim HA, Deghiedy NM (2020) Antimicrobial ZnO nanoparticle-doped polyvinyl alcohol/pluronic blends as active food packaging films. Part Part Syst Charact 37:2000006
Wyser Y, Adams M, Avella M et al (2016) Outlook and challenges of nanotechnologies for food packaging. Packag Technol Sci 29:615–648
Emamifar A, Kadivar M, Shahedi M, Soleimanian-Zad S (2011) Effect of nanocomposite packaging containing Ag and ZnO on inactivation of lactobacillus plantarum in orange juice. Food Control 22:408–413
Panea B, Ripoll G, González J et al (2014) Effect of nanocomposite packaging containing different proportions of ZnO and Ag on chicken breast meat quality. J Food Eng 123:104–112
Espitia PJP, Soares NDFF, dos Reis-Coimbra JS et al (2012) Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol 5:1447–1464
EC (2016) Commission Regulation (EU) 2016/1416 of 24 August 2016 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food (Text with EEA relevance). Official Journal of the Eur Union L230:22–42
Garcia CV, Shin GH, Kim JT (2018) Metal oxide-based nanocomposites in food packaging: applications, migration, and regulations. Trends Food Sci Technol 82:21–31
Abedi A, Bakhshandeh B, Babaie A et al (2021) Concurrent application of conductive biopolymeric chitosan/polyvinyl alcohol/MWCNTs nanofibers, intracellular signaling manipulating molecules and electrical stimulation for more effective cardiac tissue engineering. Mater Chem Phys 258:123842
Khorasani MT, Joorabloo A, Moghaddam A et al (2018) Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int J Biol Macromol 114:1203–1215
Tsou C-H, Yao W-H, Lu Y-C et al (2017) Antibacterial property and cytotoxicity of a poly(lactic acid)/nanosilver-doped multiwall carbon nanotube nanocomposite. Polymers 9:100
Fan JH (2020) Application of DSC method in measuring the glass transition temperature of polyvinyl alcohol. Weilun Commun 40:56–59
Nurul Syahida S, Ismail-Fitry MR, Ainun ZMA, Nur Hanani ZA (2020) Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag Shelf Life 23:100437
Tsou C-H, Wu C-S, Hung W-S et al (2019) Rendering polypropylene biocomposites antibacterial through modification with oyster shell powder. Polymer 160:265–271
Tsou CH, Yao WH, Hung WS et al (2018) Innovative plasma process of grafting methyl diallyl ammonium salt onto polypropylene to impart antibacterial and hydrophilic surface properties. Ind Eng Chem Res 57:2537–2545
Wang W, Yu Z, Alsammarraie FK et al (2020) Properties and antimicrobial activity of polyvinyl alcohol-modified bacterial nanocellulose packaging films incorporated with silver nanoparticles. Food Hydrocoll 100:105411
Zheng Q, Javadi A, Sabo R et al (2013) Polyvinyl alcohol (PVA)–cellulose nanofibril (CNF)–multiwalled carbon nanotube (MWCNT) hybrid organic aerogels with superior mechanical properties. RSC Adv 3:20816–20823
Li B, Liu T, Wang Y, Wang Z (2012) ZnO/graphene-oxide nanocomposite with remarkably enhanced visible-light-driven photocatalytic performance. J Colloid Interface Sci 377:114–121
Wang Y-W, Cao A, Jiang Y et al (2014) Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl Mater Interfaces 6:2791–2798
Yao Y, De GMR, Duan H et al (2020) Infusing high-density polyethylene with graphene-zinc oxide to produce antibacterial nanocomposites with improved properties. Chin J Polym Sci 38:898–907
Kim Y, Kim M, Choi JK, Shim SE (2015) Mechanical and electrical properties of PVA nanocomposite containing sonochemically modified MWCNT in water. Polym Korea 39:136–143
Yeh J, Wu T, Lai Y et al (2011) Ultradrawing properties of ultrahigh-molecular weight polyethylene/functionalized carbon nanotube fibers and transmittance properties of their gel solutions. Polym Eng Sci 51:2552–2563
Abdolrahimi M, Seifi M, Ramezanzadeh MH (2018) Study the effect of acetic acid on structural, optical and mechanical properties of PVA/chitosan/MWCNT films. Chin J Phys 56:221–230
Das SK, Hasan M, Islam JMM et al (2017) Characterization of solution casting derived carbon nanotube reinforced poly (vinyl alcohol) thin films. Int J Plast Technol 21:338–350
Wang J, Gao C, Zhang Y, Wan Y (2010) Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater Sci Eng C 30:214–218
GeorgeRamanaBawa-Siddaramaiah JKVAS (2011) Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int J Biol Macromol 48:50–57
Gong X, Tang CY, Pan L et al (2014) Characterization of poly (vinyl alcohol)(PVA)/ZnO nanocomposites prepared by a one-pot method. Compos Part B Eng 60:144–149
Bonelli N, Poggi G, Chelazzi D et al (2019) Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J Colloid Interface Sci 536:339–348
Wen Y-H, De Guzman MR, Lin X et al (2020) Antibacterial nanocomposites of polypropylene modified with silver-decorated multiwalled carbon nanotubes. NANO 15:2050112–1-2050112–15
Rhim J, Lee S, Hong S (2011) Preparation and characterization of agar/clay nanocomposite films: the effect of clay type. J Food Sci 76:N40–N48
Kavoosi G, Dadfar SMM, Dadfar SMA et al (2014) Investigation of gelatin/multi-walled carbon nanotube nanocomposite films as packaging materials. Food Sci Nutr 2:65–73
Sugumaran S, Bellan CS, Muthu D et al (2015) Novel hybrid PVA–InZnO transparent thin films and sandwich capacitor structure by dip coating method: preparation and characterizations. RSC Adv 5:10599–10610
Hmar JJL, Majumder T, Roy JN, Mondal SP (2015) Flexible, transparent, high dielectric and photoconductive thin films using ZnO nanosheets-multi-walled carbon nanotube-polymer nanocomposites. J Alloys Compd 651:82–90
Gharoy Ahangar E, Abbaspour-Fard MH, Shahtahmassebi N et al (2015) Preparation and characterization of PVA/ZnO nanocomposite. J Food Process Preserv 39:1442–1451
Sirelkhatim A, Mahmud S, Seeni A et al (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242
Bolton DJ, Meredith H, Walsh D, McDowell DA (2014) The effect of chemical treatments in laboratory and broiler plant studies on the microbial status and shelf-life of poultry. Food Control 36:230–237
Yasmine P (2009) Nutrient values for Australian and overseas chicken meat. Nutr Food Sci 39:685–693
Demirezen D, Uruc K (2006) Comparative study of trace elements in certain fish, meat and meat products. Meat Sci 74:255–260
Acknowledgements
The authors would like to acknowledge the financial support from the following organizations: Wuliangye Group Co. Ltd. (CXY2019ZR001); Sichuan Province Science and Technology Support Program (2019JDRC0029); Zigong City Science and Technology (2017XC16; 2019CXRC01; 2020YGJC13); Opening Project of Material Corrosion and Protection Key Laboratory of Sichuan Province (2017CL03; 2019CL05; 2018CL08; 2018CL07; 2016CL10); Opening Project of Sichuan Province, the Foundation of Introduced Talent of Sichuan University of Science and Engineering (2017RCL31; 2017RCL36; 2017RCL16; 2019RC05; 2019RC07; 2014RC31; 2020RC16); the Opening Project of Key Laboratories of Fine Chemicals and Surfactants in Sichuan Provincial Universities (2020JXY04). Appreciation is also extended to Sichuan Jinxiang Sairui Chemical Co. Ltd; Apex Nanotek Co. Ltd.; Ratchadapisek Sompote Fund for Postdoctoral Fellowship (Chulalongkorn University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, YH., Tsou, CH., de Guzman, M.R. et al. Antibacterial nanocomposite films of poly(vinyl alcohol) modified with zinc oxide-doped multiwalled carbon nanotubes as food packaging. Polym. Bull. 79, 3847–3866 (2022). https://doi.org/10.1007/s00289-021-03666-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03666-1