Abstract
The bioactive packaging polyvinyl alcohol (PVA)/starch films were prepared by incorporating combined antioxidant agents i.e. extracted spent coffee ground (ex-SCG) and citric acid. Effect of citric acid content on chemical compatibility, releasing of antioxidant, antibacterial activities, and physical and mechanical properties of PVA/starch incorporated ex-SCG (PSt-E) films was studied. The results of ATR-FTIR spectra showed that antioxidant agents of ex-SCG can penetrate into the film and the ester bond of blended films by citric acid was also observed. The presence of ex-SCG increased efficiency of antioxidant release and antimicrobial activity. The PSt-E film incorporated 30 wt% citric acid showed minimum inhibitory concentration against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). The incorporation of ex-SCG and citric acid into film showed a synergistic effect on antibacterial activity. The water resistance and kinetic moisture sorption improved with incorporation of citric acid. The tensile strength and biodegradability of samples were in range of 5.63–7.44 MPa and 65.28–86.64%, respectively. Based on this study, PSt-E film incorporated 30 wt% citric acid can be applied as novel food packaging materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, application of biodegradable polymer packaging has attracted much attention for replacement of non-biodegradable materials such a petroleum based synthetic polymers [1, 2]. Starch has been widely used as biodegradable plastics for packaging [3] and coating [4, 5] due to its outstanding properties i.e. abundant availability, inexpensive and biodegradability [6, 7]. However, starch still has some limitations in terms of low water resistance and poor mechanical properties [8, 9]. To overcome these drawbacks, many researchers have focused on the development of properties of starch by blending with other biodegradable polymer such as gelatin [10], chitosan [11, 12], polyvinyl alcohol (PVA) [8], poly(lactic acid) [13] and poly(ε-carprolactone) [14]. Recently, PVA/starch blend has been shown to have high performance as biodegradable plastics for various applications such as disposable packaging materials and biomedical materials [8]. Many techniques have been reported to improve the properties of starch blend, especially the compatibility of between starch and PVA. The non-toxic additives such a citric acid was used as a crosslinking agent [15, 16]. Jose et al. [17] observed that citric acid enhanced the crosslinking of hydroxyl groups in starch and/or PVA. The presence of citric acid in the PVA/starch blend film created the compact morphology and increased thermal stability. Priya et al. [18] reported that the flexibility of PVA/starch film increased with increasing citric acid content. The residue of citric acid in the polymer matrix acted as plasticizer. Furthermore, the PVA/starch film containing 20 wt% citric acid showed the inhibitory effect against of E. coli and S. aureus. De’nobili et al. [19] studied the alginate-based edible film containing citric acid as antioxidant active for food preservation. The incorporation of citric acid into the film decreased the browning development at longer storage time. It is clearly seen that citric acid can improve the overall properties of bioactive film packaging. Nowadays, food spoilage and/or contaminate are one of the most serious health problems worldwide. Thus, bioactive packaging technology has been developed to protect the food stuff from contamination and environmental conditions such as light, moisture and oxygen [20]. The incorporation of antimicrobial or antioxidant agents into polymeric packaging is an alternative way to extend shelf life and control the quality of food products.
The antioxidant agents from natural products such as green tea [21, 22], essential oils [23, 24], grapefruit seed [25] and barley husk [26] have been applied for food packaging. Most of previous studies focused on the antioxidant agents extracted from the main products. Recently, a number of researchers have been investigated the extraction antioxidant from by-product of coffee production i.e. spent coffee ground (SCG) to produce bioactive compounds. The extraction of roasted coffee residue by water and organic solvents was observed by Yen et al. [27]. The used water in extraction process provided the highest yield of antioxidants. The extracted solution consisted of chlorogenic acid and caffeic acid as well as non-phenolic compound namely caffeine, trigonelline, nicotinic acid, and 5-(hydroxymethyl) furfuraldehyde [27]. Parkar et al. [28] also observed that chlorogenic acid and caffeic acid can inhibit the growth of E. coli and S. aureus. Monente et al. [29] showed that SCG provided good inhibition of food borne pathogen such as Gram-positive bacteria and yeast. Based on the literatures, the extracted spent coffee ground (ex-SCG) has a potential of antioxidants agent for the development of bioactive products.
Currently, there is no information of bioactive film packaging containing the combined active compounds such as citric acid and ex-SCG. Therefore, this research aims to study effect of citric acid and ex-SCG on PVA/starch film properties. The chemical compatibility, mechanical property, water resistance and biodegradability of films are determined. The antimicrobial properties of samples against Gram-positive bacteria (S. aureus) and Gram-negative bacteria (E. coli) are investigated. In addition, the antioxidants release in food simulants of films is also studied.
Experimental
Materials
Cassava starch was purchased from Bangkok Interfood Ltd., Thailand. The starch consisted of 17% amylose and 12.17% moisture. PVA (average molecule weight 1700–1800) was purchased from Laboratory Reagents & Fine Chemical. Citric acid and 2,2,-diphenyl-2-picryl-hydrazyl were purchased from RCI Labscan Limited. SCG was supplied from local coffee shops in Khon Kaen, Thailand. The SCG in wet cake form was dried at temperature of 80 °C for 24 h. The particle size of SCG powder was in the range of 180–250 µm.
Preparation and Characterization of ex-SCG
The extraction method according to previous work [27] was performed on the SCG. 50 g of SCG powder was added to 500 ml of distilled water under mechanical stirring at 100 °C for 5 min. After this period, the solution was filtered through qualitative filter to obtain ex-SCG.
The DPPH scavenging capacity of ex-SCG and citric acid were evaluated using a method modified from that described by Brand-Williams et al. [30]. A volume of 1.5 ml of sample was mixed with 0.0025 ml of 100 mM methanolic of DPPH using a vortex (Scientific Industries G560E 3200 rpm). The mixture was incubated in the dark at room temperature of 28 °C for 30 min. The absorbance of mixture was determined at 517 nm using UV–Vis spectrophotometer (Agilent Cary 60 UV–Vis Spectrophotometer). The percentage of DPPH free radical quenching activity of ex-SCG was calculated following Eq. (1). The DPPH free radical scavenging activity of ex-SCG and citric acid were also expressed as mg/100 ml standard of ascorbic acid [31]. The values of DPPH free radical scavenging activity are summarized in Table 1.
where \({\text{Ab}}{{\text{s}}_{{\text{DPPH}}}}\) and AbsExtract are the absorbances at 517 nm of the DPPH solution and extracted sample, respectively.
Preparation of PVA/Starch Film Enriched with Antioxidants
The PVA/starch films were prepared with compatible blend ratio of 50:50 [18, 32]. The starch (2.5 g) was gelatinized in 50 ml of ex-SCG to form a gel solution. The PVA (2.5 g) was separately dissolved in ex-SCG at 95 °C for 60 min. The solutions of starch and PVA were mixed for another 5 min at 95 °C. The amount of citric acid at 0–30 wt% and glycerol at 20 wt% of the blend film were added to above mixture with continuous stirring. The obtained mixture was poured into 20 mm × 100 mm petri dish and then dried at temperature of 50 °C for 4 h.
Characterization of PVA/Starch Film Enriched with Antioxidants
The structural interaction of samples was analyzed by Attenuated total reflection infrared (ATR-FTIR) spectra (Jasco 4200). The spectra of all samples were tested in the range of 4000–400 cm−1 with 64 scans at a resolution of 2 cm−1.
The antimicrobial activity of sample was evaluated by agar diffusion method. Gram-negative and positive bacteria i.e. E. coli and S. aureus were selected as representative strains of food pathogens. The bacterial were cultured overnight in brain heart fusion broth at 37 °C. The bacterial culture was diluted in a medium to obtain turbidity of approximately 108 CFU/ml. The bacterial inoculums were seeded on Muller Hinton Agar plates by swab plate technique. The inhibition zone (mm) around the sample disc was measured after 24 h of incubation at 37 °C.
DPPH radical scavenging activity of film sample was also determined using the method proposed by Brand-Williams et al. [30]. The sample with dimension of 0.10 × 20 × 150 mm was immersed in 100 ml of distilled water, 10% ethanol and 95% ethanol for 12 h. The extracted film solution (1.5 ml) was mixed with DPPH in ethanol (3 ml). After incubation for 30 min, the mixture was tested for absorbance using UV-spectrometer as mentioned above.
Moisture content (MC) and total soluble matter (TSM) were determined using the method purposed by Ge et al. [33]. The samples were initially weighted (Mi) and subsequently dried at 105 °C for 24 h. After this, the dried samples were re-weighted (Mo). The weight loss of samples was measured as MC value following Eq. (2). For TSM measurement, the dried samples were immersed in 30 ml distilled water at 25 °C for 24 h. Then, the samples were dried at 105 °C for 24 h and re-weighted (Mt). The ratio of weight loss and initial weight of the dried sample were used to calculate TSM value as expressed in Eq. (3).
The samples were stored at 25 °C with saturated salt solution in separate desiccators. The specific relative humidity values were 32, 43, 52 and 75% using MgCl2, K2CO3, Mg(NO3)2 and NaCl, respectively. After incubation for 14 days, the dried samples were weighted and equilibrium moisture contents were calculated.
The biodegradability of samples were performed by soil burial degradation test [34]. The dimension of specimen was 20 × 20 × 0.15 mm3. The soil was prepared with sand to soil ratio of 1:1 at 30 ± 2 °C and water content of 35 ± 5%. The samples were buried at 10 cm depth in the soil. After 30 days, all samples were collected and washed with distilled water. The samples were dried at 105 °C for 24 h and weighted to calculate their weight loss.
The surface of sample after soil burial test was observed using a scanning electron microscope (SEM) (Hitachi Miniscope model TM-3000). All samples were coated with gold using an ion sputtering device.
The mechanical properties were tested using Universal testing machine (Instron, Model 5567) equipped with a 5 kN load cell following ASTM D 882-10 method (2015). The initial distance between the grips was 50 mm and a crosshead speed of 5 mm s−1 was used.
Results and Discussion
Attenuated Total Reflection Infrared Characterization of PVA/Starch Film Enriched with Antioxidants
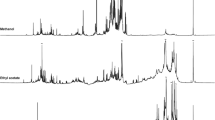
The results of ATR-FTIR spectra of starch, PVA, and the blend films incorporated citric acid at 0–30 wt% are depicted in Fig. 1. The characteristic FTIR spectroscopy of starch is shown in Fig. 1a. The absorption band appeared at 3280 (O–H stretching), 1645 cm−1 (O–H bending of water), 2918 cm−1 (C–H stretching) and 1443 cm−1 (C–H2 stretching). The peaks at 986 and 1158 cm−1 were assigned to C–O stretching [35, 36]. Figure 1b showed the major characteristic peaks of PVA. These peaks were observed at 1740 cm−1 (C=O), 1450 cm−1 (C=O-OR), 1340 cm−1 (C–H2), 1110 cm−1 (C–O–C) and 3400 cm−1 (OH) [37]. The absorption band of PVA/Starch blend film with distilled water (PSt-W) showed both characteristic peaks of PVA and starch without new peak, as depicted in Fig. 1c. This phenomenon indicated that PVA and starch had only physically blended. For PVA/starch blend film with ex-SCG (PSt-E), the peak at 1705 cm−1 was clearly observed, as shown in Fig. 1d. This characteristic peak was assigned to C=O stretching vibration of acid compounds such as chlorogenic acids and caffeic acid [38]. The characteristic peak of carbonyl group of PSt-E incorporated 5–30 wt% citric acid was observed at 1715 cm−1, as shown in Fig. 1e–h. The change of carbonyl peak’s position of PSt-E films was observed when compared with citric acid, as shown in Fig. 2A. The carbonyl peaks of citric acid were observed at 1683, 1720 and 1754 cm−1 which was attributed to carboxyl groups [39]. This behavior indicated that the esterification occurred between starch and citric acid as well as between PVA and citric acid. Furthermore, the intensity of peak at 3200–3400 cm−1 of films significantly decreased with increasing citric content up to 10 wt%. This result indicated that the OH groups of PVA and starch were consumed by citric acid to create ester linkages, as shown in Fig. 2B [40, 41].
Releasing of Antioxidants
DPPH scavenging assay was applied to evaluate DPPH scavenging capacity of the films as depicted in Fig. 3. The PSt-E film without citric acid had DPPH scavenging activity at 60.09%. This result confirmed the presence of antioxidant agents of ex-SCG in the film. Panusa et al. [42] reported that the major antioxidant components of ex-SCG were chlorogenic acids and caffeic acid. The DPPH scavenging activity of PSt-E film increased with increasing citric acid content. The citric acid is a food antioxidant for butter, vegetable oil, fat and phospholipid proportion in milk [43]. Figure 4 showed the antioxidant releasing of PSt-E, PSt-E containing 30 wt% citric acid and PSt-W containing 30 wt% citric acid in three food simulants such as distilled water, 10% ethanol and 95% ethanol which represented aqueous food, alcoholic food and fatty food, respectively [44]. Similar trend of DPPH scavenging activity was observed for all simulant foods. The presence of ex-SCG in film showed that the DPPH scavenging activity was higher than that of PSt-W incorporated 30 wt% citric acid. The DPPH scavenging capacity value of PSt-E film incorporated 30 wt% citric acid was fourfold greater than that of PSt-W film incorporated citric acid at the same ratio. The bioactive film containing antioxidant agents can thus be applied for prevention of lipid oxidation and maintaining quality of foods [45].
Antimicrobial Activity of PVA/Starch Film Enriched with Antioxidants
The antimicrobial activities of PSt-E films containing 0–30 wt% citric acid are summarized in Table 2. The minimum inhibitory concentration against E. coli and S. aureus were observed in the PSt-E film containing 30 wt% citric acid. It is well known that citric acid is not lipophilic and undissociated acids. Therefore, it hardly penetrated into the cells of bacteria. The main effect of citric acid on antibacterial properties was acidulation. The cell maintained the acidic ions which caused the damage of enzyme inside the cell, resulting in damaging the extracellular membrane. Furthermore, the citric acid also acted as antioxidant indirectly by chelating effect. The chelating agents of citric acid could bind and remove essential metal ions for microbial growth [46, 47]. It was clearly seen that the presence of 30 wt% citric acid in blend film resulted in the largest inhibitory zone for S. aureus. This observation implied that citric acid was more active in inhibiting the growth of Gram-positive bacteria than Gram-negative bacteria. The obtained result is in good agreement with previous study [18]. In Gram-negative bacteria, the outer membrane consisted of lipopolysaccharides with high chelating charge on the surface as compared with the Gram-positive bacteria which composed of peptidoglycan and teichoic acid [48]. Consequently, Gram-negative bacteria were more resistant to inhibit growth of microbial than Gram-positive bacteria. Interestingly, the synergistic behavior of combination between citric acid and ex-SCG was observed in antibacterial activity of film as shown in Table 2. The inhibition zones for S. aureus and E. coli of PSt-E incorporated 30 wt% citric acid were found to be larger than those of PSt-E and PSt-W incorporated 30 wt% citric acid. The ex-SCG consisted of antibacterial agents such as chlorogenic acid, caffeic acid as well as other bioactive components. Lima et al. [49] observed that caffeic acid combined with antibiotics such as gentamicin and imipenem showed the synergistic effect on inhibition of Pseudomonas aeruginosa. The caffeic acid can inhibit against the bacterial RNA polymerase enzyme. Furthermore, the chlorogenic acids improved the permeability of outer and plasma membranes as well as the leakage of cytoplasm molecules [50]. Furthermore, the caffeine and melanoidins were also responsible for the growth inhibition of both Gram-negative bacteria and Gram-positive bacteria [29]. It was clearly seen that the essential compounds in SCG integrated with citric acid could provide the synergism behavior for antibacterial. The synergistic antibacterial activity of PVA/starch film incorporated ex-SCG and citric acid is very attractive for bioactive packaging to control and preserve food quality.
Water Resistance of PVA/Starch Film Enriched with Antioxidants
The water resistance is the important factor for packaging films to maintain moisture within the product and dimension stability of packaging [51]. Therefore, the water solubility and moisture content of the film are very important parameters for food packaging applications. Table 3 shows the values of MC and TSM for PSt-E incorporated 0–30 wt% citric acid. The MC and TSM values were in range of 18.24–22.44% and 41.13–55.48%, respectively. Both MC and TSM significantly decreased with increasing citric acid up to 10 wt%. It was due to the crosslinking effect of citric acid on PVA and starch. The crosslinking via esterification decreased the number of hydroxyl groups per unit of sample resulting in the reduction of hydrogen bond interaction [32, 40]. The improvement of water resistance is beneficial for various applications of bioactive film.
Moisture Sorption Isotherm of PVA/Starch Film Enriched with Antioxidants
The moisture sorption of PSt-E films with 0, 10 and 30 wt% citric acid at various percentages of relative humidity is depicted in Fig. 5. The similar trend of moisture sorption was observed in all relative humidity. The films rapidly absorbed moisture in the first stage i.e. 0–4 h. The moisture sorption significantly decreased with the incorporation of 10 wt% citric acid into PSt-E films. Furthermore, the moisture sorption isotherm parameters of PSt-E films were also evaluated using GAB (Guggenheim–Anderson–de Boer) model as expressed in Eq. (4).
where M is the equilibrium moisture content at a given water activity (\({a_w}\)), \({a_w}\) is RH/100, \({m_o}\) is the monolayer value (g water/ g solid) and C and K are the GAB constants.
Figure 6 shows the moisture sorption isotherms of the samples. The isotherm sorption of all samples tended to increase with increasing relative humidity. Similar trends were observed in thermoplastic wheat flour/poly(lactic acid) blends containing citric acid [52]. The GAB model parameters are summarized in Table 4. The monolayer value decreased with incorporated citric acid content at 10 wt%. The monolayer value indicated the maximum amount of absorbed water in a single layer per gram of dry material. This parameter indicated the sorbing sites of water in sample. This result implied that the addition of citric acid in PSt-E film reduced the hygroscopic characteristic.
Soil Burial Degradation of PVA/Starch Film Enriched with Antioxidants
Biodegradability of polymers is one the important function of their application. The soil burial method was applied to evaluate weight loss of specimens by moisture and microorganism [34]. Figure 7 shows the degree of degradation of film samples in range of 65.28–86.64% at 30 days. The weight loss of samples decreased with increasing citric content. The presence of ester bond in film resisted the penetration of moisture and increased hydrophobicity which resulted in decreasing of the biodegradation rate as well as microbial attack. This result was consistent with the surface morphology of film samples before and after soil burial test as shown in Fig. 8. Before testing, the roughness of the surface of film decreased with increasing citric content and the surface became quite smooth with no crack. After the soil burial test, cracks were observed on the surface of film in all samples. The amount of cracks on surface reduced and smoother surfaces were observed with increasing citric acid content. The obtained result was in good agreement with the previous work [53].
The change of chemical structures of films after soil burial test was also investigated using FTIR, as shown in Fig. 9. All samples showed the similar trend of result. The absorbance at 1320–1750 cm−1 significantly changed due to the hydrolysis of ester linkages by the shortened aliphatic chains [54, 55]. The changes in chemical structures of films confirmed the characteristic of biodegradable films.
Tensile Properties of PVA/Starch Film Enriched with Antioxidants
Tensile strength and modulus of film samples are presented in Fig. 10A and B. The tensile strength and modulus values were in range of 5.63–7.44 MPa and 11.34–17.59 MPa, respectively. The mechanical properties of film were significantly improved by addition of 10 wt% citric acid. This observation indicated that the 10 wt% citric acid was the optimal content for crosslinking of polymer matrix. It was observed that increasing of citric acid content from 20 to 30 wt%, the mechanical properties of films decreased. The obtained result was in accordance with MC and TSM results, which indicated that the residual citric acid in the blend films acted as plasticizer and decreased the interaction of molecules [56]. The tensile strength and modulus values of PSt-E incorporated citric acid were comparable with those of bioactive film based PVA and/or starch containing antioxidants i.e. PVA incorporated apple pomace (2.0–10.9 MPa) [57], PVA/starch containing anthocyanin and limonene (4.51 MPa) [58] and starch containing extracted propolis (5.5–6.3 MPa) [59].
Conclusion
PVA/starch films containing extracted spent coffee ground (ex-SCG) and different amounts of citric acid (0–30 wt%) were prepared using a solvent casting process. Interestingly, the synergistic effect was found at 30 wt% of citric acid loading in PSt-E film with the highest DPPH scavenging capacity and the largest inhibitory zone for E. coli and S. aureus. Obviously, increasing citric acid content resulted in the decrease of MC, TSM and hygroscopic characteristic of films. The presence of ester bond in film resisted the penetration of moisture and increased the hydrophobicity, leading to the decrease of biodegradation rate and microbial attack. The esterification between citric acid and polymer matrix was also confirmed by ATR-FTIR spectroscopy. Tensile strength slightly decreased for blend film containing citric acid content higher than 10 wt% due to the plasticizer effect of residual citric acid. The results provided a new insight into the development of green antimicrobial materials for food packaging.
References
Guo G, Zhang C, Du Z, Zou W, Li H (2015) J Polym Environ 23(2):183–189
Puglia D, Dominici F, Kenny JM, Santulli C, Governatori C, Tosti G, Benincasa P (2016) J Polym Environ 24(1):37–47
Trongchuen K, Ounkaew A, Kasemsiri P, Hiziroglu S, Mongkolthanaruk W, Wannasutta R, Pongsa U, Chindaprasirt P (2018) Starch Stärke. https://doi.org/10.1002/star.201700238
Zhao M, Wang Y, Liu L, Liu L, Chen M, Zhang C, Lu Q (2017) Polym Compos. https://doi.org/10.1002/pc.24519
Zhang C, Milhorn A, Haile M, Mai G, Grunlan JC (2017) Green Mater 5(4):182–186
Kasemsiri P, Dulsang N, Pongsa U, Hiziroglu S, Chindaprasirt P (2017) J Polym Environ 25(2):378–390
Pirooz M, Navarchian AH, Emtiazi G (2017) J Polym Environ 26(4):1702–1714
Tian H, Yan J, Rajulu AV, Xiang A, Luo X (2017) Int J Biol Macromol 96(Supplement C):518–523
Mittal A, Garg S, Kohli D, Maiti M, Jana AK, Bajpai S (2016) Carbohydr Polym 151(Supplement C):926–938
Acosta S, Chiralt A, Santamarina P, Rosello J, González-Martínez C, Cháfer M (2016) Food Hydrocoll 61(Supplement C):233–240
Talón E, Trifkovic KT, Vargas M, Chiralt A, González-Martínez C (2017) Carbohydr Polym 175(Supplement C):122–130
Valencia-Sullca C, Vargas M, Atarés L, Chiralt A (2018) Food Hydrocoll 75(Supplement C):107–115
Ferri J, Garcia-Garcia D, Sánchez-Nacher L, Fenollar O, Balart R (2016) Carbohydr Polym 147:60–68
Correa AC, Carmona VB, Simão JA, Mattoso LHC, Marconcini JM (2017) Carbohydr Polym 167:177–184
Seligra PG, Jaramillo CM, Famá L, Goyanes S (2016) Carbohydr Polym 138:66–74
Wu Z, Wu J, Peng T, Li Y, Lin D, Xing B, Li C, Yang Y, Yang L, Zhang L (2017) Polymers 9(3):102
Jose J, Al-Harthi MA (2017) Iran Polym J 26(8):579–587
Priya B, Gupta VK, Pathania D, Singha AS (2014) Carbohydr Polym 109(Supplement C):171–179
De’Nobili MD, Soria M, Martinefski MR, Tripodi VP, Fissore EN, Rojas AM (2016) J Food Eng 175:1–7
Dominguez-Martinez BM, Martínez-Flores HE, Berrios JDJ, Otoni CG, Wood DF, Velazquez G (2017) J Polym Environ 25(3):683–691
Chen C-W, Xie J, Yang F-X, Zhang H-L, Xu Z-W, Liu J-L, Chen Y-J (2017) J Food Process Preserv. https://doi.org/10.1111/jfpp.13374
López de Dicastillo C, Nerín C, Alfaro P, Catalá R, Gavara R, Hernández-Muñoz P (2011) J Agric Food Chem 59(14):7832–7840
Abdollahi M, Rezaei M, Farzi GA (2012) J Food Eng 111(2):343–350
Tongnuanchan P, Benjakul S, Prodpran T (2012) Food Chem 134(3):1571–1579
Kanmani P, Rhim J-W (2014) Carbohydr Polym 102:708–716
Pereira de Abreu DA, Maroto J, Villalba Rodríguez K, Cruz JM (2012) J Sci Food Agric 92(2):427–432
Yen W-J, Wang B-S, Chang L-W, Duh P-D (2005) J Agric Food Chem 53(7):2658–2663
Parkar SG, Stevenson DE, Skinner MA (2008) Int J Food Microbiol 124(3):295–298
Monente C, Bravo J, Vitas AI, Arbillaga L, De Peña MP, Cid C (2015) J Funct Foods 12:365–374
Brand-Williams W, Cuvelier ME, Berset C (1995) LWT 28(1):25–30
Kim D-O, Lee KW, Lee HJ, Lee CY (2002) J Agric Food Chem 50(13):3713–3717
Yoon S-D, Park M-H, Byun H-S (2012) Carbohydr Polym 87(1):676–686
Ge L, Zhu M, Xu Y, Li X, Li D, Mu C (2017) Food Bioprocess Technol 10(9):1727–1736
Ibrahim H, Farag M, Megahed H, Mehanny S (2014) Carbohydr Polym 101:11–19
Polat S, Uslu M-K, Aygün A, Certel M (2013) J Food Eng 116(2):267–276
Prachayawarakorn J, Pomdage W (2014) Mater Des 61:264–269
Jayasekara R, Harding I, Bowater I, Christie G, Lonergan GT (2004) Polym Test 23(1):17–27
Lyman DJ, Benck R, Dell S, Merle S, Murray-Wijelath J (2003) J Agric Food Chem 51(11):3268–3272
Xie JX, Chang JB, Wang XM (2001) Infra-red spectrum applications in organic chemistry and medicinal chemistry. Scientific Publication, Beijing, pp 296–297
Shi R, Bi J, Zhang Z, Zhu A, Chen D, Zhou X, Zhang L, Tian W (2008) Carbohydr Polym 74(4):763–770
Shi R, Zhang Z, Liu Q, Han Y, Zhang L, Chen D, Tian W (2007) Carbohydr Polym 69(4):748–755
Panusa A, Zuorro A, Lavecchia R, Marrosu G, Petrucci R (2013) J Agric Food Chem 61(17):4162–4168
Hraš AR, Hadolin M, Knez Ž, Bauman D (2000) Food Chem 71(2):229–233
Requena R, Vargas M, Chiralt A (2017) Eur Polym J 92(Supplement C):185–193
López-de-Dicastillo C, Gómez-Estaca J, Catalá R, Gavara R, Hernández-Muñoz P (2012) Food Chem 131(4):1376–1384
Russell NJ, Gould GW (2012) Food preservatives. Springer, New York
Demirci A, Ngadi MO (2012) Microbial decontamination in the food industry: novel methods and applications. Woodhead Publishing Ltd., Cambridge
Mani-López E, García HS, López-Malo A (2012) Food Res Int 45(2):713–721
Lima VN, Oliveira-Tintino CD, Santos ES, Morais LP, Tintino SR, Freitas TS, Geraldo YS, Pereira RL, Cruz RP, Menezes IR (2016) Microb Pathog 99:56–61
Lou Z, Wang H, Zhu S, Ma C, Wang Z (2011) J Food Sci 76(6):M398–M403
Guerrero P, Stefani PM, Ruseckaite RA, de la Caba K (2011) J Food Eng 105(1):65–72
Abdillahi H, Chabrat E, Rouilly A, Rigal L (2013) Ind Crops Prod 50(Supplement C):104–111
Seligra PG, Medina Jaramillo C, Famá L, Goyanes S (2016) Carbohydr Polym 138(Supplement C):66–74
Hulwani A, Zain M, Kahar M, Wahab AB, Ismail H (2018) J Polym Environ 26(2):691–700
Rudnik E, Briassoulis D (2011) J Polym Environ 19(1):18–39
Uranga J, Leceta I, Etxabide A, Guerrero P, de la Caba K (2016) Eur Polym J 78(Supplement C):82–90
Gaikwad KK, Lee JY, Lee YS (2016) J Food Sci Technol 53(3):1608–1619
Liu B, Xu H, Zhao H, Liu W, Zhao L, Li Y (2017) Carbohydr Polym 157:842–849
Araújo GKP, Souza SJ, Silva MV, Yamashita F, Gonçalves OH, Leimann FV, Shirai MA (2015) Int J Food Sci Technol 50(9):2080–2087
Acknowledgements
This work was financial supported by New Research Group, Research fund of the faculty of Engineering, Khon Kaen University and the Applied Engineering for Important Crops of the North East Research Group, Khon Kaen University. The authors also would like to acknowledge the support of Hitachi Scholarship Research and Tokyo Institute of Technology. The authors thank Prof. Shinji Ando at Tokyo Institute of Technology for helpful advice and utilization of ATR-FTIR and SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ounkaew, A., Kasemsiri, P., Kamwilaisak, K. et al. Polyvinyl Alcohol (PVA)/Starch Bioactive Packaging Film Enriched with Antioxidants from Spent Coffee Ground and Citric Acid. J Polym Environ 26, 3762–3772 (2018). https://doi.org/10.1007/s10924-018-1254-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1254-z