Abstract
This study investigated the thermal, structural, and optoelectronic properties of Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites prepared by the sol-gel method at different calcination temperatures (850 and 950 °C). According to Rietveld’s refinement of the X-ray patterns, the samples crystallized in the cubic spinel structure (\(Fd\overline{3}m\) space group). As the calcination temperature increases, crystallite size and unit cell parameters increase. The FTIR bands of the tetrahedral (A) and octahedral [B] sites shift toward higher wavenumbers with the rise of calcination temperature. From the absorbance and Tauc method, the samples exhibit direct optical transitions. The band-gap energy (Eg) decreases due to the increase in crystallite size. Urbach energies also decrease, implying a decrease in defects and disorders with increasing calcination temperature. In addition, penetration depth, refractive index, extinction coefficient, dielectric constants, conductivity, and loss factor were studied versus wavelength. From these variations in optical parameters, some interesting optoelectronic applications were derived for the as-synthetized Mg0.4Cu0.4Cd0.2Cr2O4 samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Spinel chromites with a general formula of MCr2O4 (M = Cd, Cu, Co, Zn, Fe, Mg…) have attracted great interest for their potential uses. As a result of their wide range of applications and remarkable properties [1,2,3,4], MCr2O4 samples were studied as conductors, humidity detectors and levers, magnetic materials, catalysts, ceramics, semiconductors, and super hard nanomaterials. The MCr2O4 materials were synthesized by different methods, including co-precipitation [5], thermal treatment [6], sol-gel auto-combustion [7], and high-temperature solid state [8] methods. Among these methods, the sol-gel route is used to produce pure spinel chromites with controlled chemical composition and grain size [7, 9]. This method offers several advantages, such as shorter calcination times, lower processing temperatures, enhanced homogeneity, and high purity of the prepared materials [10, 11]. Sol-gel synthesis can also improve the properties of oxides by adjusting several parameters, such as the pH of the initial solution, calcination time, and calcination temperature [12,13,14].
Among spinel chromites, the Mg1-xCuxCr2O4 samples exhibit interesting and rich properties. The structural changes and physical properties of Mg1-xCuxCr2O4 have been studied in previous studies [15, 16]. The structure of this system remains cubic for compositions with x < 0.43. However, for samples with x > 0.43, tetragonal symmetry, driven by cooperative Cu2+ Jahn–Teller distortions, appears. In addition, the properties of Mg1-xCuxCr2O4 spinel chromites are significantly affected by Cu2+ substitution at the tetrahedral A site. In this study, we further explored the Mg1-xCuxCr2O4 chromites. Currently, Mg–Cu chromites with cadmium (Cd) substitution are not systematically studied. Hence, this study aimed to study the properties of Cd-substituted Mg–Cu mixed chromites. According to Shannon [17], Mg2+ and Cu2+ ions have the same ionic radius at the tetrahedral A site. Compared to Mg2+ and Cu2+, the Cd2+ ion has a significantly higher ionic radius. As a result, the substitution of Cd2+ in Mg–Cu chromites can alter their crystal geometry, which has some impact on their physical properties. We have prepared the Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites and studied their structural and optoelectronic properties as a function of calcination temperature at room temperature. Throughout this manuscript, the Mg0.4Cu0.4Cd0.2Cr2O4 samples sintered at 850 and 950 °C are designated as S850 and S950, respectively.
2 Experimental
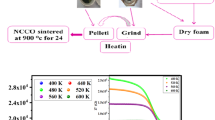
The sol-gel method was used to prepare the Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites as presented in Fig. 1. At a temperature of 90 °C, the nitrates [Cu(NO3)2.3⋅H2O, M = 241.60 g/mol, m = 1.71 g], [Mg(NO3)2⋅6H2O, M = 256.41 g/mol, m = 1.82 g], [Cd(NO3)2⋅4H2O, M = 303.48 g/mol, m = 1.09 g] and [Cr(NO3)3⋅9H2O, M = 400.15 g/mol, m = 14.18 g] were dissolved in deionized water by stirring on a hot plate. After the complete dissolution of these nitrates, controlled amounts of citric acid [C6H8O7.H2O, M = 210.139 g/mol, m = 11.17 g] were added to the solution. Then the pH of the solution was adjusted to about 7 by adding ammonia [NH4OH, M = 35.04 g/mol]. The ethylene glycol [C2H6O2, M = 62.08 g/mol, 21.29 ml] was then added to obtain a viscous solution (gel). After 4 h of continuous heating at 90 °C, a dry foam was formed. The dry foam was then dried at 250 °C for 12 h to eliminate residual solvent. After grinding, we pelletized the obtained powder and calcined it at 600 °C for 24 h to release the enduring gases. Calcined pellets were ground, and the resulting powder was divided into two portions. The powder of each portion was pressed into pellets and calcined separately at 850 and 950 °C for 24 h. The pellets were ground again, and all the characterizations were carried out in powder form for the Mg0.4Cu0.4Cd0.2Cr2O4 samples.
A Panalytical X’Pert Pro System with Cu-Kα radiation (λ = 1.5406 Å) was used to obtain the XRD patterns for the prepared samples from the 15°–85° angular range. The morphology and atomic composition of the samples were examined using scanning electron microscopy “JSM-6380 LA” with an energy-dispersive X-ray (EDX) spectrometer. The Fourier transform infrared (FTIR) spectra were obtained at room temperature using an FTIR-8400 S spectrometer in the wavenumber range from 400 cm1 to 4000 cm-1. At room temperature, UV-VIS-NIR properties of powdered samples have been determined using a UV-3101PC scanning spectrophotometer with a dual beam monochromator in a wavelength range of 200–2400 nm. Two light sources were used to measure the optical properties: UV-visible radiations were emitted by a xenon lamp, while infrared radiations were emitted by a halogen lamp.
3 Results and Discussion
3.1 Thermal Analysis
Figure 2 depicts the percentage weight loss (W%) and the differential thermal analysis (DTA) versus temperature (T) for the Mg0.4Cu0.4Cd0.2Cr2O4 sample calcined at 850 °C. It can be seen that there are primarily two steps in the weight loss process. Firstly, there is a weight loss of ~ 1.5% below 400 °C, with a peak at 355 °C on the dW/dT curve. Weight loss in this range may indicate that enduring gases and moisture have been released [18]. In the second step, weight loss of ~ 9% occurs in the temperature range of 400–830 °C. The DTA curve shows an exothermic peak at 790 °C. The exothermic peak around 790 °C shows the complete crystallization of Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites. Beyond this exothermic peak, no further weight loss or thermal effects occurred, indicating that decomposition does not occur and that stable residues may be attributed to Mg0.4Cu0.4Cd0.2Cr2O4 samples. Both samples show no weight loss and no exothermic peaks beyond 790 °C. This indicates that the initially absorbed entities and water molecules are completely removed, which is indicative of better thermal stability for higher temperature ranges. We, therefore, optimized the calcination temperature of the samples at temperatures above 790 °C (850 and 950 °C).
3.2 Structural Analysis
Figure 3 shows the XRD patterns of Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites calcined at 850 °C (S850) and 950 °C (S950). We can conclude from these patterns that the samples exhibit pure spinel phases without impurities. The peaks were indexed in the space group \(\text{F}\text{d}\overline{3}\text{m}\) (n#227) as (111), (220), (311), (222), (400), (422), (511), (440), (531), (620), (533), (622), and (444). With increasing calcination temperature, the angular positions of the peaks shift to a lower diffraction angle (see the inset in Fig. 3). This leads to the increase in lattice constant (a) as well as cell volume (V = a3). The Rietveld refinement of the experimental patterns can be used to estimate the structural parameters of the synthesized Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites [19]. For the Rietveld refinement, the cations distribution \({{\left({{Mg}_{0.4}^{2+}{Cu}_{0.4}^{2+}Cd}_{0.2}^{2+}\right)}_{A}{\left[{Cr}_{2}^{3+}\right]}_{B}O}_{4}^{2-}\) has been considered for the samples. This cations distribution was proposed based on some studies that have verified the distribution of Mg2+, Cu2+, Cd2+, and Cr3+ ions between both tetrahedral (A) and octahedral [B] sites in some chromite systems such as Mg1−xCuxCr2O4 [16], MgCr2O4 [20], and CdCr2O4 [21]. The atomic positions are taken at 8a (1/8, 1/8, 1/8) for \({\left({{Mg}_{0.4}^{2+}{Cu}_{0.4}^{2+}Cd}_{0.2}^{2+}\right)}_{A}\) cations, 16d (1/2, 1/2, 1/2) for \({\left[{Cr}_{2}^{3+}\right]}_{B}\) cations, and 32e (x, y, z) for O. The reliability R-factors (expected Rexp, Bragg RBragg, profile Rp and weighted profile Rwp) that must be less than 10%, as well as the goodness of fit (χ2 = Rwp/Rexp) that must tend to one, were used to check the Rietveld fitting quality. Table 1 shows the values of all the conventional structural parameters. The Rietveld refinement of the XRD patterns for Mg0.4Cu0.4Cd0.2Cr2O4 samples is illustrated in Fig. 4. This figure shows experimental and calculated patterns as well as the difference between the two. According to Fig. 4, the observed patterns agree well with the calculated ones. As revealed in Table 1, the lattice constant and volume (a and V) increase with calcination temperature. A similar trend of these cell parameters with calcination temperature has been observed for other spinel systems, such as Li0.5Fe1.5Cr1.0O4 [22] and Ni0.3Zn0.7Fe2O4 [23]. Moreover, the obtained cell parameters for Mg0.4Cu0.4Cd0.2Cr2O4 samples have higher values than those found for the Mg1 − xCuxCr2O4 compositions [16, 24]. As shown in the previous study, a transition from a cubic structure to a tetragonal structure has been observed for Mg1−xCuxCr2O4 samples for x alloying from 0 to 1 [16]. In fact, for x < 0.43, the samples present a cubic spinel structure with a gradually decreasing lattice parameter (a). The same behavior was observed in Ref. [24] for Mg1−xCuxCr2O4 samples (with 0.1 ≤ x ≤ 0.4). When x= 0.43, the tetragonal phase appears, and a small region of coexistence persists for 0.43 ≤ x ≤ 0.47, above which the cubic phase disappears [16]. The compounds become increasingly tetragonal for the CuCr2O4 sample with x= 1. In our case, the structure remains cubic for the as-prepared Mg0.4Cu0.4Cd0.2Cr2O4 samples despite the substitution of Cd2+ ions. However, the higher lattice constants for our samples compared to those found for Mg1−xCuxCr2O4 spinel chromites are due to the increase in the average radius of the A site following the substitution of Cd2+ ions. From Table 1, we can also see that the oxygen coordinate is typical of a spinel-type structure. Additionally, as calcination temperature increases, the cation-oxygen bond length at the tetrahedral sites (dA−O) decreases; however, the cation-oxygen bond length at the octahedral sites (dB−O) increases. The values of the bond angles (φA−O−B and φB−O−B) were also presented in Table 1 for Mg0.4Cu0.4Cd0.2Cr2O4 samples. The φA−O−B bond angle is concerned with the A−O−B interactions, while the φB−O−B bond angle is related to the B−O−B interactions. From the data presented in Table 1, the increase in bond angle (φA−O−B) with calcination temperature indicates an increase in the strength of A−B exchange interactions, and the decrease in the bond angle (φB−O−B) indicates a decrease in the strength of B−B exchange interactions [25, 26]. On the other hand, due to the recrystallization of the samples and the increase in their cell volume with calcination temperature [27], the average crystallite size (D) increases (see Table 1).
3.3 Morphological Analysis and Chemical Composition
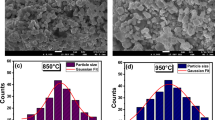
Figure 5a, b shows the scanning electron microscopy (SEM) analysis of Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites calcined at 850 and 950 °C. The SEM micrographs clearly show grains that are found to be roughly cubic and are distributed homogeneously. Furthermore, the effect of calcination temperature on the particle morphology can be seen in Fig. 5a, b. Due to the electrostatics magnetic attraction and calcination effect at high temperatures, individual nanoparticles completely aggregated, and the obtained micrographs showed agglomerated chromite particles. Figure 5c, d shows the size distribution of the samples using image J software. The Gaussian fit of the histograms indicates an average grain size of about 1 μm for the sample calcined at 850 °C and about 1.75 μm for the one calcined at 950 °C. However, these values are higher than those estimated from the XRD data. This is due to the fact that each grain observed by SEM consists of several crystallite domains [28]. The elemental composition of the samples was also analyzed by energy dispersive X-ray analysis (EDX), as shown in Fig. 6a, b. The EDX spectra give the qualitative composition of the samples and also show the peaks only due to the elements Mg, Cu, Cd, Cr, and O as per initial stoichiometry. The chemical composition quantification is presented in Table 2. The values of the measured and nominal atomic compositions of Mg, Cu, Cd, and Cr are close. This indicates that pure Mg0.4Cu0.4Cd0.2Cr2O4 chromites are formed with no traces of any extra elements and without significant loss of any ingredients within the detection limits.
3.4 Optical Properties
3.4.1 Infrared Properties
The Fourier transform infrared (FTIR) spectra for Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites calcined at 850 °C (S850), and 950 °C (S950) are illustrated in Fig. 7. On the FTIR spectra of spinel materials, there are usually two characteristic absorption peaks associated with the intrinsic vibration of oxygen bonds with metal cations at A and B sites [29]. The tetrahedral (A) and octahedral [B] stretching peaks were visible in Fig. 7 for the as-prepared sample. The tetrahedral (νA) and octahedral (νB) frequencies are found as (νA = 616 cm-1 and νB = 506 cm-1 for S850) and (νA = 620 cm-1 and νB = 513 cm-1 for S950). The difference between both νA and νB frequencies is attributed to the difference between dA−O and dB−O bond distances [30], as presented in Table 1. However, as calcination temperature increases, the bands corresponding to the A and B sites shift towards higher wavenumbers. Other spinel materials have shown similar results in previous works [23, 31, 32]. In fact, as calcination temperature increases, both νA and νB move toward a higher wavenumber. The variation of νA and νB is expected since this is closely dependent on the calcination temperature, synthesis process, crystallite size, and cation distribution at octahedral and tetrahedral sites [33]. In addition, the gradual shift of the bands to higher wavenumbers with increasing calcination temperature confirms the improvement of the crystallinity of the samples [34]. From the spectra, we can also observe three other frequency bands at 896, 1020, and 1638 cm-1 that correspond to the elongation and bending vibrations of the C–N=O, CC–C, and C=O groups, respectively [35]. We can also see a broad absorption peak at 3454 cm-1 which is associated with stretching vibration hydrogen-bonded OH groups of remaining water [23, 36, 37].
3.4.2 Absorbance and Band Gap Energy
The UV–VIS–NIR spectra of Mg0.4Cu0.4Cd0.2Cr2O4 samples calcined at 850 °C (S850) and 950 °C (S950) are shown in Fig. 8. Both samples exhibit UV absorption bands, which suggests that they may absorb UV light [38, 39]. The samples also exhibit broad absorption bands in the visible range (between 400 and 800 nm). Thus, they can be used in solar cell devices and as excellent photocatalytic materials [40]. In addition, the samples exhibit absorption bands in the NIR region, which makes them suitable candidates for near-infrared optoelectronic devices [41]. The bands’ gap energies can be calculated for Mg0.4Cu0.4Cd0.2Cr2O4 samples from Tauc’s law as follows [42, 43]:
Here, B, hν, Eg, α, and are the constant relative to the disorder degree in the samples, the photon energy, the band-gap energy, and the absorption coefficient, respectively. The value of the exponent “n” has a physical meaning: when n = 2, the optical transition is direct; however, if n = 1/2, the optical transition is indirect. According to the Beer-Lambert law [44], the absorption coefficient (α) is calculated using thickness (d) and absorbance (A) as follows :
Where b is a constant (b = 2.303). The band gap energy can then be estimated as follows:
We presented in Fig. 9a, b the variations of (αhν)1/2 and (αhν)2 versus hν for Mg0.4Cu0.4Cd0.2Cr2O4 samples calcined at 850 and 950 °C. Using the linear extrapolation of the plots to zero absorption spectra, we obtained the values of the indirect (Egi) and direct (Egd) band gap energies as (Egi= 0.45 eV and Egd= 2.44 eV for S850) and (Egi= 0.26 eV and Egd= 2.23 eV for S950). It is clear from these values that both energies decrease with increasing calcination temperature. Using the following equations, we can determine the type of optical transitions in the samples:
Based on the estimated values of Egi and Egd, the curves of ln(αhν) vs. ln(hν-Eg) were presented in Fig. 10a, b. The linear fit of the curves gives values of n much lower than two for the indirect transition. However, the obtained values for the direct optical transition are close to 1/2. Consequently, the as-synthesized samples exhibit direct optical transitions. The band gap energies of Mg0.4Cu0.4Cd0.2Cr2O4 samples are presented in Table 3. We found that the Egd value decreased from 2.44 eV for S850 to 2.23 eV for S950. Generally, band gap energy can be affected by several factors, including grain size, impurities, and structural parameters [35, 45]. The decrease in band gap energy in the case of our samples is due to the increase of grain size with rising calcination temperature. In fact, grain boundaries are reduced at higher calcination temperatures with increasing grain size. This reduces the scattering of carriers at grain boundaries, resulting in lower Eg energy. The obtained band gap energies for the Mg0.4Cu0.4Cd0.2Cr2O4 spinels are compared in Table 3 to other published values [8, 46,47,48,49,50,51,52]. In fact, materials with large Eg values absorb ultraviolet photons (see Table 3, for example, the values provided for some semiconductors [46,47,48] and some other spinel chromites [8, 49,50,51,52]). While sunlight contains only a small amount of ultraviolet photons (about 5%), and most room-light lamps emit only visible photons [53]. As a result, materials are now being studied for their spectral response to visible light [46, 47]. Obviously, the estimated Eg values for the as-synthesized Mg0.4Cu0.4Cd0.2Cr2O4 samples are lower than the reported ones in Refs. [8, 49,50,51,52]. In fact, a lower band gap results in more visible light absorption in photocatalytic reactions [54]. In this sense, our samples may be suitable candidates for visible light absorption. In addition, it should be noted that the Eg values for the Mg0.4Cu0.4Cd0.2Cr2O4 samples exceed the expected energy requirement for water separation (Eg > 1.23 eV). Consequently, our samples can also be used as photocatalytic materials [55].
3.4.3 Penetration Depth and Extinction Coefficient
The optical penetration depth (δ) is a parameter that illustrates the depth to which incident radiation can penetrate a compound. This coefficient is estimated from the α(λ) values as follows [56]:
The δ(λ) curves presented in Fig. 11a for the Mg0.4Cu0.4Cd0.2Cr2O4 samples calcined at 850 °C (S850) and 950 °C (S950) show three regions. In the UV domain (region I), the δ values decrease and remain constant in the VIS domain (region II). As we move into region III (in the NIR domain), the δ values gradually increase up to 2400 nm. This increasing trend is accompanied by large bands for the two compounds. The extinction coefficient (k) can be estimated as follows [57]:
The plots of the k(hυ) curves for the Mg0.4Cu0.4Cd0.2Cr2O4 ferrites are shown in Fig. 11b. The coefficient k decreases when hυ increases due to the decrease in incident photon energy near the surface [58].
3.4.4 Refractive Index
In the visible range, the refractive index (n) is expressed as follows [59]:
Where R is the reflectance, and k is the extinction coefficient. In spectral dispersion applications, the refractive index (n) is one of the most fundamental parameters. Figure 12 illustrates the variations of n(λ) curves for Mg0.4Cu0.4Cd0.2Cr2O4 samples calcined at 850 °C (S850) and 950 °C (S950). As can be seen in Fig. 12, significant absorption bands are evident in the UV–VIS–NIR regions, which reflect the high optical quality of our samples. According to the Cauchy relation, the refractive index (n) can be expressed as a function of the wavelength (λ) as follows [58]:
Where n0, n1, and n2 are the Cauchy parameters defined as precise refractive index parameters in the low absorption spectral range. The curves of n versus 1/λ2 were fitted using Eq. (9) as displayed in the insets of Fig. 12. The Cauchy parameters were determined as follows: (n0 = 1.48, n1 = 0.39 (nm2) and n2 = 0.50 (nm4) for S850) and (n0 = 1.72, n1 = 0.38 (nm2) and n2 = 0.48 (nm4) for S950). Additionally, the dispersion energy (Ed), as well as the effective single oscillator (E0), are two crucial parameters for describing inter-band optical transition intensities. Based on the Wemple-Didomenico relation [60], the E0 and Ed energies were estimated as follows:
From the curves of \(\left(\frac1{(n^2-1)}\right)\) versus \(\left(h\upsilon\right)^2\), the values of E0 and Ed were estimated in Fig. 13 for the Mg0.4Cu0.4Cd0.2Cr2O4 samples. The linear part of the curve corresponds to a slope equal to , and the intercept with the vertical axis corresponds to \(\left(\frac1{E_0E_d}\right)\) and the intercept with the vertical axis corresponds to \(\left(\frac{E_0}{E_d}\right)\). The values of E0 and Ed were then found as (E0 = 1.490 eV and Ed=1.377 eV for S850) and (E0 = 1.569 eV and Ed= 2.099 eV for S950). Similarly, the static refractive index (n’0) and the zero-frequency dielectric constant (εop) were calculated using the following relation:
This equation gives values of εop and n′0 as (εop = 1.92 and n′0= 1.39 for S850) and (εop = 2.34 and n′0= 1.53 for S950). Particularly, the estimated n′0 values are close to those estimated from the Cauchy fit for the n0 parameter. Additionally, we used the following relation to calculate the oscillator wavelength (λ0) and the average oscillator strength (S0) [61]:
Figure 14 shows the curves of \(\left(\frac1{n^2-1}\right)\) versus \(\left(\frac1{\lambda^2}\right)\). The values of λ0 and S0 were estimated from the linear fit to the curves as (λ0 = 803 nm and S0 = 1.655 × 10−6 m−2 for S850) and (λ0 = 785 nm and S0 = 2.408 × 10−6 m−2 for S950).
3.4.5 Conductivity, Dielectric Constants, and Loss Factor
The optical conductivity (σop) for Mg0.4Cu0.4Cd0.2Cr2O4 samples calcined at 850 °C (S850) and 950 °C (S950) was plotted in Fig. 15a versus wavelength (λ). The conductivity (σop) was evaluated as follows [62]:
Where c, n, α, and k denote the speed of light, refractive index, absorption coefficient, and extinction coefficient, respectively. From Fig. 15a, we can notice that the optical conductivity increases at low wavelengths, and then it decreases as λ increases. The high values of σop in the low wavelength region confirm the high photo-response of the prepared samples. On the other hand, the complex dielectric permittivity, \(\epsilon \left(\lambda \right)\), is given as a function of the refractive index (n) and the extinction coefficient (k) as follows [63]:
The real (ε1) and imaginary (ε2) of the dielectric permittivity can be expressed according to the following two equations:
The curves of these two dielectric constants are presented in Fig. 15b, c as a function of wavelength (λ). Due to the low extinction coefficient k(λ), the variation of ε1(λ) shows a behavior similar to that of the refractive index n(λ). However, the constant ε2(λ) increases with increasing wavelength. The behavior of the two dielectric constants (ε1 and ε2) is in reasonable agreement with the results presented in the literature for other samples [64]. The optical loss factor tan(δ) is given as a function of ε1 and ε2 according to the following equation [65]:
The values of tan(δ) versus wavelength increase with calcination temperature as presented in Fig. 15d.
3.4.6 Urbach Energy
The values of the Urbach energy (Eu) were estimated for the as-prepared samples by using the following equation [66]:
Where α0 represents a constant. The Urbach energy (Eu) is a parameter used to measure the degree of disorder and defects in materials [67, 68]. This parameter refers to the localized state of the conduction band and the extended valence band transition [69]. From Eq. (18), the Urbach energy (Eu) can be expressed as a function of the absorption coefficient (α) according to the following equation:
By plotting ln(α) versus (hv) in Fig. 16, the Eu value was estimated as follows:
In the case of the Mg0.4Cu0.4Cd0.2Cr2O4 samples, the Eu values were obtained as 0.822 and 0.555 eV for S850 and S950, respectively. These values are even more than the Urbach energies obtained at room temperature for some standard semiconductors materials such as ZnO (Eu= 67 meV) [70], GaAs (Eu= 44.33 meV) [71], and CuO (Eu is less than 8 meV) [72]. This reveals the importance of disorder and defects in the as-prepared samples when comparing them to these semiconductor materials. However, the estimated Eu values for the Mg0.4Cu0.4Cd0.2Cr2O4 spinels agree well with those found for other spinels samples such as NiFe2O4 [73], Cd0.5Zn0.5F2O4 [74], and ZnFe2-xLaxO4 [75]. On the other hand, the Urbach energy for the sample calcined at 950 °C is lower than that of the sample calcined at 850 °C. This indicates that increasing the calcination temperature leads to fewer disorder and defects. The steepness parameter, σ(T), was also estimated using the following relation [76]:
With kB, σ, and T represent the Boltzmann constant, the steepness parameter, and the room temperature, respectively. The steepness parameter is a physical property of the band gap. It describes the broadening of the absorption edge caused by interactions between electrons-phonons or excitons–phonons [41]. At room temperature, the σ values were estimated for our samples as 0.031 and 0.047 for S850 and S950, respectively. The σ parameter is also inversely correlated with the electron-phonon interaction energy (Ee−ph) as follows [41].
According to this equation, the Ee−ph values were estimated as 21.51 eV and 14.18 eV for the samples calcined at 850 and 950 °C, respectively.
4 Conclusion
In conclusion, the Mg0.4Cu0.4Cd0.2Cr2O4 spinel chromites were synthesized successfully using the sol-gel method at different calcination temperatures (850 and 950 °C). Structural analysis indicates the crystallization of these samples in the cubic spinel structure (\(Fd\overline{3}m\) space group). With increasing calcination temperature, the crystallite size, the cell parameters, and the frequency of absorption bands increase. Due to the increase in crystallite size with calcination temperature, the band-gap energy (Eg) decreases from 2.44 eV for the sample calcined at 850 °C (S850) to 2.23 eV for the sample calcined at 950 °C (S950). Moreover, the Urbach energy (Eu) decreased from 0.822 eV for S850 to 0.555 eV for S850, which implies that the degree of disorder and defects decreases with increasing calcination temperature. The dispersion energy parameters were determined from the Wemple–Didomenico relation, and the Cauchy parameters were estimated based on the refractive index variation. The prepared samples also showed significant variations in extinction coefficient, penetration depth, conductivity, dielectric constants, and loss factor. In terms of applications, Mg0.4Cu0.4Cd0.2Cr2O4 chromites can be used in solar cell devices. They may also be classified as suitable materials for UV–VIS–NIR light absorption and photocatalytic materials.
Data Availability
Upon a reasonable request, the data that support this study’s findings are available from the corresponding author.
References
M. Bayhan, N. Kavasoglu, Sens. Actuator B 117, 261 (2006)
R.M. Gabr, M.M. Girgis, A.M. El-Awad, Mater. Chem. Phys. 30, 169 (1992)
H. Martinho, N.O. Moreno, J.A. Sanjurjo, C. Rettori, A.J. García-Adeva, D.L. Huber, S.B. Oseroff, W. Ratcliff, S.W. Cheong, P.G. Pagliuso, J.L. Sarrao, G.B. Martins, Phys. Rev. B 64, 024408 (2001)
C.L. Honeybourne, R.K. Rasheed, J. Mater. Chem. 6, 277 (1996)
K. George, S. Sugunan, Catal. Commun. 9, 2149 (2008)
S.A. Bakar, E. Saion, A. Bahrami, N. Soltani, M.R. Zare, J. Phys. Chem. Solids 128, 378 (2019)
M. Javed, A.A. Khan, J. Kazmi, M.A. Mohamed, M.N. Khan, M. Hussain, R. Bilkees, Mater. Res. Bull. 138, 111242 (2021)
P. Choudhary, D. Varshney, J. Magn. Magn. Mater. 454, 274 (2018)
M.H. Habibi, F. Fakhri, J. Therm. Anal. Calorim. 115, 1329 (2014)
N. Mechi, A. Mallah, S. Hcini, M.L. Bouazizi, M. Boudard, A. Dhahri, J. Supercond. Nov. Magn. 33, 1547 (2020)
F. Alresheedi, S. Hcini, M.L. Bouazizi, M. Boudard, A. Dhahri, J. Mater. Sci. 31, 8248 (2020)
S.A.S. Ebrahimi, S.M. Masoudpanah, J. Magn. Magn. Mater. 357, 77 (2014)
N. Kouki, S. Hcini, M. Boudard, R. Aldawas, A. Dhahri, RSC Adv. 9, 1990 (2019)
S. Hcini, N. Kouki, A. Omri, A. Dhahri, M.L. Bouazizi, J. Magn. Magn. Mater. 464, 91 (2018)
M.C. Kemei, S.L. Moffitt, D.P. Shoemaker, R. Seshadri, J. Phys. 24, 046003 (2012)
D.P. Shoemaker, R. Seshadri, Phys. Rev. B 82, 214107 (2010)
R.D. Shannon, Acta Crystallogr. A 32, 751 (1976)
S.B. Somvanshi, M.V. Khedkar, P.B. Kharat, K.M. Jadhav, Ceram. Int. 46, 8640 (2020)
H.M. Rietveld, J. Appl. Crystallogr. 2, 65 (1969)
M. Javed, A.A. Khan, M.S. Ahmed, S.N. Khisro, J. Kazmi, R. Bilkees, M.N. Khan, M.A. Mohamed, Phys. B 599, 412377 (2020)
J.H. Chung, Y.S. Song, S. Park, H. Ueda, Y. Ueda, S.H. Lee, J. Korean Phys. Soc. 62, 1900 (2013)
R.P. Patil, P.P. Hankare, K.M. Garadkar, R. Sasikala, J. Alloy. Compd. 523, 66 (2012)
M. Rahimi, P. Kameli, M. Ranjbar, H. Salamati, J. Nanopart. Res. 15, 1865 (2013)
S. Qi Wu, G. Sang, Q. Wang, L. Wang, T. Huang, Zhu, Y. Li, Ceram. Int. (2023). https://doi.org/10.1016/j.ceramint.2023.02.063
G. Kumar, R.K. Kotnala, J. Shah, V. Kumar, A. Kumar, P. Dhiman, M. Singh, Phys. Chem. Chem. Phys. 19, 16669 (2017)
R. Sharma, P. Thakur, M. Kumar, N. Thakur, N.S. Negi, P. Sharma, V. Sharma, J. Alloy. Compd. 684, 569 (2016)
A. Gholizadeh, E. Jafari, J. Magn. Magn. Mater. 422, 328 (2017)
E. Oumezzine, S. Hcini, E.K. Hlil, E. Dhahri, M. Oumezzine, J. Alloy Compd. 615, 553 (2014)
F. Hcini, S. Hcini, B. Alzahrani, S. Zemni, M.L. Bouazizi, Appl. Phys. A 126, 362 (2020)
M.D. Rahaman, M.D. Mia, M.N.I. Khan, A.K.M.A. Hossain, J. Magn. Magn. Mater. 404, 238 (2016)
Y. Han, A. Sun, X. Pan, W. Zhang, X. Zhao, J. Supercond. Nov. Magn. 32, 3823 (2019)
N. Kouki, S. Hcini, R. Aldowas, M. Boudard, J. Supercond. Nov. Magn. 32, 2209 (2019)
S. Nasrin, S.M. Khan, M.A. Matin, M.N.I. Khan, A.K.M.A. Hossain, Md.D. Rahaman, J. Mater. Sci. 30, 10722 (2019)
M.P. Reddy, R.A. Shakoora, A.M.A. Mohamed, M. Gupta, Q. Huang, Ceram. Int. 42, 4221 (2016)
S. AbuBakar, N. Soltani, W.M.M. Yunus, E. Saion, A. Bahrami, Solid State Commun. 192, 15 (2014)
E. AlArfaj, S. Hcini, A. Mallah, M.H. Dhaou, M.L. Bouazizi, J. Supercond. Nov. Magn. 31, 4107 (2018)
S. Torkian, A. Ghasemi, R.S. Razavi, Ceram. Int. 43, 6987 (2017)
K. Souifi, O. Rejaiba, O. Amorri, M. Nasri, B. Alzahrani, M.L. Bouazizi, K. Khirouni, J. Khelifi, J. Inorg. Organomet. Polym. 32, 4515 (2022)
G. Raddaoui, O. Rejaiba, M. Nasri, K. Khirouni, B. Alzahrani, M.L. Bouazizi, J. Khelifi, J. Mater. Sci. 33, 21890 (2022)
J. Wang, C. Zhang, H. Liu, R. McLaughlin, Y. Zhai, S.R. Vardeny, X. Liu, S. McGill, D. Semenov, H. Guo, R. Tsuchikawa, V.V. Deshpande, D. Sun, Z.V. Vardeny, Nat. Commun. 10, 129 (2019)
O. Rejaiba, K. Khirouni, M.H. Dhaou, B. Alzahrani, M.L. Bouazizi, J. Khelifi, Opt. Quantum Electron. 54, 315 (2022)
S. Debnath, R. Das, J. Mol. Struct. 1199, 127044 (2020)
K. Gagandeep, B.S. Singh, Lark, H.S. Sahota, Nucl. Sci. Eng. 134, 208 (2000)
S.M.H. Qaid, B.A. Al-Asbahi, H.M. Ghaithan, M.S. AlSalhi, A.S. Al dwayyan, J. Coll. Interface Sci. 563, 426 (2020)
P. Thakur, R. Sharma, V. Sharma, P. Sharma, Mater. Chem. Phys. 193, 285 (2017)
A. Fujishima, X. Zhang, D.A. Tryk, Surf. Sci. Rep. 63, 515 (2008)
J. Xie, H. Wang, M. Duan, L. Zhang, Appl. Surf. Sci. 257, 6358 (2011)
N.R. Dhineshbabu, V. Rajendran, N. Nithyavathy, R. Vetumperumal, Appl. Nanosci. 6, 933 (2016)
G. Thennarasu, A. Sivasamy, J. Chem. Technol. Biotechnol. 90(3), 514 (2015)
A. Venugopal, R. Sarkari, C. Anjaneyulu, V. Krishna, M.K. Kumar, N. Narender, A.H. Padmasri, Appl. Catal. A Gen. 469, 398 (2014)
M.A. Kassem, A.A. El-Fadl, A.M. Nashaat, H. Nakamura, J. Alloys Compd. 790, 853 (2019)
A.A. El-Fadl, A.M. Nashaat, M.A. Kassem, Mater. Res. Expr. 6, 1150a7 (2019)
H. Tajizadegan, A. Heidary, O. Torabi, M.H. Golabgir, A. Jamshidi, Int. J. Appl. Ceram. Technol. 13(2), 289 (2016)
J. Li, G. Lu, Y. Wang, Y. Guo, Y. Guo, J. Coll. Interface Sci. 377(1), 191 (2012)
F. Hcini, S. Hcin, M.M. Almoneef, M.H. Dhaou, M.S. Alshammari, A. Mallah, S. Zemni, N. Lefi, M.L. Bouazizi, J. Mol. Struct. 1243, 130769 (2021)
A.M. El Nahrawy, B.A. Hemdan, A.M. Mansour, A. Elzwawy, A.B. AbouHammad, Silicon 14, 6645 (2022)
B.A. Hemdan, A.M. El Nahrawy, A.F.M. Mansour, A..B.. Abou Hammad, Environ. Sci. Pollut. Res. 26, 9508 (2019)
F. Hcini, S. Hcini, M.A. Wederni, B. Alzahrani, H. Al Robei, K. Khirouni, S. Zemni, M.L. Bouazizi, Phys. B 624, 413439 (2022)
A.M. Mansour, B.A. Hemdan, A. Elzwawy, A.B. Abou, Hammad, A.M. El Nahrawy, Sci. Rep. 12, 9855 (2022)
S.H. Wemple, M. Didomenico, Phys. Rev. B 3, 1338 (1971)
N. Tounsi, A. Barhoumi, F.C. Akkari, M. Kanzarin, H. Guermazi, S. Guermazi, Vacuum 121, 9 (2015)
H. Yokokawa, N. Sakai, T. Kawada, M. Dokiya, Solid State Ion 52, 43 (1992)
R. Mguedla, A. Ben Jazia Kharrat, N. Moutia, K. Khirouni, N. Chniba-Boudjadaa, W. Boujelben, J. Alloys Compd. 836, 155186 (2020)
E.A. Davis, N.F. Mott, Philos. Mag. A 22, 903 (1970)
Z. Raddaoui, B. Smiri, A. Maaoui, J. Dhahri, R. M’ghaieth, N. Abdelmoulad, K. Khirouni, RSC Adv. 8, 27870 (2018)
A.M. Mansour, M. Nasr, H.A. Saleh, G.M. Mahmoud, Appl. Phys. A 125, 625 (2019)
Y. Feng, S. Lin, S. Huang, S. Shrestha, G. Conibeer, J. Appl. Phys. 117, 125701 (2015)
S.A. Moyez, S. Roy, J. Nanoparticle Res. 20, 5 (2017)
A. Bougrine, A. El Hichou, M. Addou, J. Ebothé, A. Kachouane, M. Troyon, Mater. Chem. Phys. 80, 438 (2003)
M. Caglar, S. Ilican, Y. Caglar, F. Yakuphanoglu, Appl. Surf. Sci. 255, 4491 (2009)
S.R. Johnson, T. Tiedje, J. Appl. Phys. 78, 5609 (1995)
X. Zeng, M. Zhukova, S. Faniel, J. Proost, D. Flandre, J. Mater. Sci. 31, 4563 (2020)
S.K. Paswan, S. Kumari, M. Kar, A. Singh, H. Pathak, J.P. Borah, L. Kumar, J. Phys. Chem. Solids 151, 109928 (2021)
T.S. Soliman, A.S. Abouhaswa, J. Mater. Sci. 31, 9666 (2020)
H. Lemziouka, A. Boutahar, R. Moubah, L.H. Omari, S. Bahhar, M. Abid, H. Lassri, Vacuum 182, 109780 (2020)
S. Husain, A.O.A. Keelani, W. Khan, Nano-Struct. Nano-Objects 15, 17 (2018)
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number (IF2/PSAU/2022/01/22494).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MLB: Methodology, Conceptualization, Investigation, Data curation, and Writing-original draft. SH: Data analysis, Investigation, Writing-review, and revision. KK: Data analysis, Software, and revision. FN: Review, Supervision and editing. AHA: Supervision and Data analysis. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests that are relevant to the content of this article to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouazizi, M.L., Hcini, S., Khirouni, K. et al. Structural and Optoelectronic Properties of Mixed Mg–Cu–Cd Spinel Chromites Prepared by the sol–gel Method Under Different Calcination Temperatures. J Inorg Organomet Polym 33, 2127–2141 (2023). https://doi.org/10.1007/s10904-023-02658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02658-0