Abstract
The influence of R2O3 (R = Al, La, Y) on the structure, thermal and some physical properties of 40MgO–30B2O3–30SiO2 glass has been investigated by differential thermal analysis, X-ray diffraction, scanning electron microscopy and Fourier-transform infrared spectroscopy. Fourier-transform infrared spectroscopy results showed that the network of the investigated glasses consists mainly of BO3, BO4, and SiO4 structural units. The change in properties associated with the substituting ions largely correlates with the structural role of these ions in the glass structure. The experimental results suggest that Al3+ ions act as network formers in the investigated composition range, while La3+ and Y3+ ions act as network modifiers and increase the number of non-bridging oxygen in the glass network. The obtained results showed that the glass transition temperature (640–700 ºС), thermal expansion coefficient (5.6–7.5 ppm/ºС), density (2.60–3.34 g/cm3), and molar volume (21.17–24.15 cm3/mol) values of the investigated glasses were increased with increasing R2O3 content. The increase in density is due to the relatively larger molar mass of the substitution oxides than other glass components, while the large ionic radius of the substitution ions causes an increase in the molar volume. The obtained results for the glass transition temperature and thermal expansion coefficient showed an increase in the values for these properties with a decreasing field strength of the substitution ions. These results can be used to develop new materials with the required properties for advanced aerospace and electronic applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Alkaline earth borosilicate glasses and glass-ceramics have recently gained importance both scientifically and technologically due to their unique properties and promising potential applications in instrument engineering and rocket production as heat-resistant electrical insulating coatings [1,2,3], in the energy sector as high-temperature sealants in solid oxide fuel cells [4,5,6], and in the production of heat-resistant and radio-transparent materials for the aerospace and military industries [7,8,9]. However, for most of these materials, the connection between the structure and the properties is not well understood, making it difficult to improve their properties. Therefore, this topic continues to be an active area of research.
Magnesium borosilicate glasses and glass-ceramic materials have recently attracted wide attention due to their excellent properties [6,7,8,9,10,11,12]. This system is a relevant source for the synthesis of new materials that present value and, at the same time, specific properties such as mechanical strength, thermal stability, radio-transparency, high electrical and chemical resistance [11]. The properties of these glasses can be changed over a wide range by introducing trivalent metal oxides that modify the structure of boron and affect the degree of the local order or disorder of the glass network [13]. The introduction of trivalent metal ions in small amounts improves the glass-forming ability, structure, thermal and dielectric properties of the borosilicate glasses [14,15,16,17]. Most of the published work has been devoted to studying the effects of aluminum, yttrium, and lanthanum oxides on the properties of glass and glass-ceramic materials [16,17,18,19,20,21,22,23,24].
Aluminum and yttrium oxides have a dual nature and act either as a glass former or a glass modifier, depending on their concentration in the glass matrix [4, 15,16,17,18,19]. For this dual role, Al2O3 and Y2O3 may inhibit or enhance crystallization in borosilicate glasses [16]. According to Lahl and Singh [4, 16, 17], the introduction of a small amount of Y2O3 (up to 6 mol%) and Al2O3 (up to 10 mol%) suppresses the crystallization process and increases the characteristic temperatures. The addition of Al2O3 to borosilicate glasses improves the glass-forming ability and enhances thermal stability and chemical durability [12]. Yttrium oxide is responsible for the increase in the thermal expansion coefficient, density, elastic moduli and rigidity of the glass network [17]. The role of lanthanum oxide is mainly to reinforce the thermal and mechanical properties of glass and glass-ceramic materials. Lanthanum oxide is widely known to regulate the viscosity (fluidity) of the glass, which aids the sealant in maintaining the needed fluidity after softening and the required mechanical rigidity after ceramization [24]. Understanding the composition-structure-property relationship is crucial for developing heat-resistant glass and glass-ceramic materials for advanced aerospace, military, and electronic applications.
Therefore, the aim of this work was to study the influence of R2O3 (R = Al, La, Y) on the structure, thermal and some physical properties of 40MgO–30B2O3–30SiO2 glass using Fourier-transform infrared spectroscopy (FTIR), dilatometry and differential thermal analysis, and the crystallization process is further demonstrated by heat treatment and X-ray diffraction studies.
2 Materials and methods
The details of the batch composition of the investigated glasses with their label are given in Table 1. Reagent grade chemicals of Mg(OH)2 (99.8%, NikoMag), H3BO3 (99.9%, Eti Mine Works), SiO2 (99.9%, Polar Quartz), Al2O3 (99%, Mykolaiv Alumina Plant), La2O3 (99%, Sigma-Aldrich) and Y2O3 (99.9 Alfa-Aesar) were used as starting raw materials. The glass batches were prepared by mixing an appropriate mole fraction of the desired oxide ingredients in an agate mortar with a pestle to ensure complete homogeneity. The homogeneous glass batches were melted in the alumina crucibles with a volume of 50 mL in an electric furnace with silicon carbide heaters at the temperature of 1400 ºС for 1 h in an air atmosphere. The homogeneous melts were quickly cast onto a preheated stainless-steel mold to obtain glasses which were then transferred into a muffle furnace preset to 600 °C.
Powders with an average particle size of 50 μm from the annealed glasses were measured by differential thermal analysis (DTA) in platinum crucibles at a constant heating rate of 10 °C/min (Derivatograf Q-1500D) from room temperature to 1000 °C in an air atmosphere. The glass powders were sieved through a set of standard sieves, and the fraction that passed the 270 mesh sieve (53 μm) and retained by the 325 mesh sieve (45 μm) was used for differential thermal analysis. The reference substance was high purity alumina powder, and the temperature error was ± 5 °C. The temperature difference between the glass transition (Tg) and the first exothermic peak onset (Tc), indicating the value of glass stability (ΔT), was calculated. Higher values of ΔT indicate better stability against crystallization.
Crystalline phases precipitated during heat treatment were identified by X-ray diffractometer DRON-3М using Co-Kα radiation in the 10 < 2Θ < 90 range. X-ray diffraction measurements were carried out at room temperature on powder samples. The microstructure of the crystalline phases developed in the glass samples after heat treatment was examined by scanning electron microscopy (SEO-SEM Inspect S50-B) operating at 20 kV. FTIR transmitting spectra were recorded in the 1600–400 cm− 1 region with a resolution of 2 cm− 1 using the KBr pellet technique (Thermo Nicolet Avatar 370 FTIR Spectrometer). Each sample (2 mg of glass) was mixed with 200 mg of KBr in an agate mortar and then pressed into pellets with 13 mm diameter. The spectrum for each sample represents an average of 20 scans, which were normalized to the spectrum of the blank KBr pellet.
The thermal expansion coefficient (TEC) and dilatometric softening point (Td) of the glass samples were determined using a dilatometer (Dilatometer1300 L, Italy) at a heating rate of 3 °C/min. The TEC value was calculated between 20 and 400 °C (Fig. 1), and repeated measurements indicated the thermal expansion coefficient with an error of ± 0.2 ppm/ºС. The density of the glasses was determined at room temperature by the Archimedes principle using distilled water as the immersion liquid and a digital balance of sensitivity 10− 4 g. The weight of each glass sample was measured three times, and an average was taken to minimize the sources of error. The volume resistivity of the investigated glasses was measured on flat-parallel plates in a cell with graphite electrodes in the temperature range of 100–400 °C at a heating rate of 5°/min using the teraohmmeter Е6–13А.
3 Results and Discussion
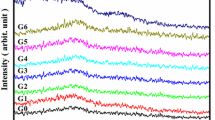
All glasses obtained after melting at 1400 °C were transparent and bubble-free. The amorphous nature of the glass samples was checked by X-ray diffraction study, which shows (Fig. 2) a broad halo pattern typical for a fully amorphous structure.
3.1 Differential Thermal Analysis
Differential thermal analysis (DTA) was employed to determine the thermal behavior of the investigated glasses. DTA curves of the glass powder samples in the temperature range of 500–1000 ºC are shown in Fig. 3, and the thermal analysis values of the curves are given in Table 2. As shown in Fig. 3, each curve is characterized by endothermic and exothermic peaks, corresponding to the glass transition temperature and crystallization temperature. In this work, the onset of the endothermic peak on the DTA curves was taken as Tg. The glass transition temperatures of the investigated glasses are reported in Table 2. It was observed that the Tg values increase with increasing the R2O3 (R = Al, La, Y) content (Fig. 4). Tg linearly increases with the addition of La2O3 and Y2O3 into the composition of 40MgO–30B2O3–30SiO2 glass. However, non-linear trends are observed in the case of glasses containing Al2O3. The observed increase in the Tg values in the investigated glasses can be explained based on Ray’s analysis of the composition-property relationships in inorganic oxide glasses [25]. According to Ray, the glass transition temperature is a sensitive structural parameter to even small substitutions of the glass network forming ions with network modifiers, owing to their high coordination number. The role played by the substitution ion in the glass network may be classified as network former, intermediate, and modifier by general criteria based on bond strength and ionic field strength. Based on Dietzel’s ionic field strength theory [26], the metal ions act as network formers or intermediate when ionic field strength (I = Z/r2, where Z is the cation charge and r is the ion radius) is greater than 5 Å−2. In contrast, when the ionic field strength is less than 5 Å−2, the metal ions act as network-modifying ions in the glass structure. When the ionic field strength is equal to 5 Å−2, the effect of metal ions depends on the concentration of monovalent or divalent metal ions in the glass [27]. When a cation enters the glass structure as a network-modifying ion, a coordination number similar to that of the pure oxide structure should be expected [28]. The ionic field strengths of Al3+, La3+ and Y3+ are 11.53 Å−2, 2.90 Å−2 and 3.78 Å−2 [21], respectively. Therefore, according to Ray, greater values of Tg would be expected with the addition of La3+ and Y3+ ions as network modifiers in seven- and eight-fold coordination, respectively, than with Al3+ entering the glass structure as intermediate ions, i.e., four-fold and six-fold coordination [21]. These conclusions are consistent with the results of this study. According to the DTA data, the addition of La2O3 or Y2O3 into the composition of 40MgO–30B2O3–30SiO2 glass greatly increases the glass transition temperature and reduces the stability of the glass against crystallization. In the case of MgAl glass samples, increasing Al2O3 content increases the stability of the glasses against crystallization, while the glass transition temperature values change by only 15 degrees. When Al2O3 is added to alkaline earth borosilicate glasses, it exhibits a strong preference to enter the three-dimensional network in tetrahedral coordination [29]. In this case, Al3+ ions require charge compensation to form AlO4 tetrahedra, which is achieved by high concentrations of Mg2+ ions, which would otherwise serve to form non-bridging oxygens, are instead used to compensate for the charge of [AlO4] tetrahedra [29, 30]. In this regard, a slight increase in the Tg and ΔT values with an increase in the Al2O3 content can also be associated with the transformation of part of Mg2+ ions from network modifiers to charge compensators to compensate for the charge of AlO4 tetrahedra [31].
The addition of Al2O3, La2O3, or Y2O3 into the composition of 40MgO–30B2O3–30SiO2 glass leads to an increase in the crystallization temperature and a change in the shape of the exothermic peak. The above results show that the additives affect the glass structure and crystallization process of the investigated glasses.
3.2 X-ray Diffraction Analysis
In order to identify the crystalline phase corresponding to each exothermic peak on DTA curves, the glass powder was heat-treated at around the crystallization temperature (Tc) in the air for 5 h. The crystallization temperatures, determined by DTA, were 960 °C for Mg0, 980 °C for MgAl10, 765 and 990 °C for MgLa10, and 990 °C for MgY10. Figure 5 shows the corresponding X-ray diffraction patterns. In the case of the glass samples Mg0 and MgAl10, magnesium borate (Mg2B2O5) precipitated as the only crystalline phase. Meanwhile, the intensity of the diffraction peaks of the Mg2B2O5 crystal precipitated in the glass is significantly reduced with the addition of Al2O3 into the composition of the Mg0 glass. The addition of La2O3 enhanced the crystallization tendency and reduced the thermal stability of the base glass. In the case of the MgLa10 glass sample with the addition of 10 mol % La2O3, the new crystalline phase formed in the sample obtained by a heat treatment at 990 °C for 5 h is lanthanum borate (LaBO3). The X-ray diffraction pattern of the MgLa10 glass sample heat-treated at 765 °C for 5 h does not show any crystalline phases. In the case of the MgY10 glass sample with 10 mol % Y2O3, the crystalline phase formed in the sample obtained by a heat treatment at 990 °C for 5 h is lanthanum borate (YBO3). The abovementioned results imply that La2O3 and Y2O3 are nucleating agents that promote the formation of the RBO3 (R = La; Y) crystalline phase, while Al2O3 mainly inhibits the formation of the Mg2B2O5 crystalline phase in the base glass.
3.3 SEM Analysis
SEM micrographs of investigated glass samples are illustrated in Fig. 6, showing the samples heat-treated for 5 h at 960 °C for Mg0, 980 °C for MgAl10, 990 °C for MgLa10, and 990 °C for MgY10. The addition of different trivalent metal oxides to the MgO–B2O3–SiO2 system has an obvious effect not only on the main crystalline phase but also on the crystal morphology. It can be clearly observed that the bulk crystallization and the morphology of crystals in samples Mg0 and MgAl10 are fine long needle-like crystals about 0.5–1.5 μm in length and 0.1–0.3 μm in diameter, while the sample MgLa10 is spherical crystals about 0.2–2 μm in diameter. Since the X-ray diffraction analysis confirmed the presence of crystalline phases in the heat-treated samples, it is obvious that fine needle crystals in samples Mg0 and MgAl10 correspond to the Mg2B2O5 crystalline phase, while spherical crystals in the sample MgLa10 correspond to the LaBO3 phase. In the case of the sample MgY10, the clusters of YBO3 polyhedral crystals form irregularly shaped particles of size 0.5–2 μm.
3.4 FTIR Analysis
Fourier-transform infrared spectroscopy (FTIR) is sensitive to the local structure of glass and is very useful for elucidating structural changes in glass according to its composition [32]. The influence of R2O3 on the structural properties of 40MgO–30B2O3–30SiO2 glass was investigated by detecting their FTIR spectra (Fig. 7) in the range 1600–400 cm− 1. The silicate and borate groups have been observed to play a dominant role in the spectra of the glasses. The absorption bands centered at 1260 cm− 1 and 1420 cm− 1 are due to the vibration of the boroxol rings and the stretching vibrations of B–O–B in [BO3] triangles, respectively [24, 33,34,35,36]. The band in the range 850–1100 cm− 1 is attributed to the asymmetric stretching vibration of B–O–B in [BO4] tetrahedra, and its width becomes wider with increasing modifier content [34,35,36]. Indeed, the equimolar substitution of B2O3 by La2O3 or Y2O3 into the composition of magnesium borosilicate glass leads to the appearance of [BO4] tetrahedra of the progressively greater number of non-bridging oxygens, causing the absorption band to shift to lower frequencies. The absorption band centered at 1080 cm− 1 is due to the antisymmetric stretching vibration of Si–O–Si in [SiO4] tetrahedra [34,35,36] and the stretching vibration of Si–O–B bond, which connects [SiO4] and [BO4] tetrahedral structural units [35,36,37]. The absorption peaks located at 800 cm− 1 and 780 cm− 1 may be related to the bending vibration of Al–O–Al in [AlO4] and the symmetric stretching vibration of Si–O–Si and Si–O–Al linkages, respectively [32, 35,36,37]. Corrosion of the alumina crucible by molten glass leads to the leaching of a part of Al2O3 from the crucible material into the glass melt [11], which contributes to the formation [AlO4] tetrahedra units in all glass compositions, even in those glass batches that did not contain Al2O3. As all glass compositions have the same melting conditions, the amount of dissolved Al2O3 is in the same range. Only additional studies of glasses melted in platinum crucibles can give a quantitative assessment of the effect of dissolved Al2O3 [38] on the properties of magnesium borosilicate glasses. The strong absorption band centered around 720 cm− 1 is attributed to the bending vibration of B–O–B in [BO3] triangles [24, 34,35,36]. The peaks at 470 cm− 1 and 515 cm− 1 are mostly associated with bending vibrations of Si–O–Si and Si–O–Al linkages, respectively [17, 32].
3.5 Physical Properties of the Investigated Glasses
The physical properties of oxide glasses are closely related to their structure, which is determined by their composition. The physical properties of the glasses were investigated by measuring the volume resistivity, thermal expansion coefficient, dilatometric softening point, density, and calculating the molar volume values to shed light on the structural behavior of the investigated glasses. The obtained values are given in Table 2. It can be seen from the dilatometry results that the glasses containing Y2O3 have higher Td values, as do the glasses containing La2O3. However, the highest TEC values were found in glasses containing La2O3 than those containing Y2O3. The lowest values of Td and TEC were observed for glasses containing Al2O3. The higher TEC values in glasses containing La2O3 compared to other glasses can be related to the larger ionic radii of La3+ ions. The obtained results showed that the density (2.60–3.34 g/cm3) and molar volume (21.17–24.15 cm3/mol) values of the investigated glasses were increased with increasing R2O3 content. The increase in the density is due to the relatively larger molar mass of trivalent metal oxides than other glass components, while the large ionic radius of the substitution ions causes an increase in the molar volume. At the temperature of 150 ºС, the volume resistivity of the investigated glasses is in the range of 1011–1012 Ohm·cm, which indicates their high electrical insulation properties.
4 Conclusion
Magnesium borosilicate glass was prepared with substitutions of Al2O3, Y2O3, and La2O3 for B2O3. The effect of these substitutions on the structural, thermal, and physical properties of the investigated glass was determined. The main building units forming the glass network are BO3 (peak at 720, 1280, 1420 cm− 1), BO4 (peak at 1080 cm− 1), and SiO4 (peak at 470, 990 cm− 1). The effect of R2O3 substitutions on the properties of magnesium borosilicate glass depends on the structural role of R3+ ions. The experimental results suggest that Al3+ ions act as network formers in the investigated composition range, while La3+ and Y3+ ions act as network modifiers and increase the number of non-bridging oxygen in the glass network. The increase in density is due to the relatively larger molar mass of the substitution oxides than other glass components, while the large ionic radius of the substitution ions causes an increase in the molar volume. The results for Tg and TEC showed increasing values for these properties with a decreasing field strength of the substitution ions, which is consistent with expectations based on the literature criteria. Among the glasses investigated in the present work, glasses containing La2O3 showed higher TEC values. The higher TEC in these glasses may be due to the large ionic radius and low field strength of La3+. The highest thermal stability is observed for glasses containing Al2O3 compared to other glasses. The tendency to crystallize is higher in glasses containing La2O3 compared to glasses containing Y2O3 or Al2O3. The obtained results in this study indicate that these glasses can be potential candidates for advanced aerospace and electronic applications as heat-resistant electrical insulating glass- and glass-ceramic-to-metal seals and coatings.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
V.A. Rozenenkova, S.S. Solntsev, N.A. Mironova, Glass Ceram. 70, 269 (2013). https://doi.org/10.1007/s10717-013-9558-x
K.Y. Frolenkov, Prot. Met. Phys. Chem. Surf 45, 444 (2009). https://doi.org/10.1134/S2070205109040121
E. Karasik, Y. Hordieiev, East. Euro. J. Enterp. Technol 6, 53 (2021). https://doi.org/10.15587/1729-4061.2021.244004
K. Singh, N. Gupta, O.P. Pandey, J. Mater. Sci 42, 6426 (2007). https://doi.org/10.1007/s10853-006-1188-z
M.S. Salinigopal, N. Gopakumar, P.S. Anjana, Silicon 12, 101–107 (2020). https://doi.org/10.1007/s12633-019-00103-x
S. Rodríguez-López, M.J. Pascual, Crystals 11(7), 737 (2021). https://doi.org/10.3390/cryst11070737
L. Han, J. Song, Q. Zhang et al., Silicon 10, 2685–2693 (2018). https://doi.org/10.1007/s12633-018-9806-3
S.S. Solntsev, Russ J. Gen. Chem. 81, 992–1000 (2011). https://doi.org/10.1134/S1070363211050306
A. Zaichuk, A. Amelina, Y. Kalishenko et al., J. Korean Ceram. Soc. 58, 483–494 (2021). https://doi.org/10.1007/s43207-021-00125-5
G. Dou, M. Guo, Y. Li et al., J. Mater. Sci. : Mater. Electron. 26(6), 4207–4211 (2015). https://doi.org/10.1007/s10854-015-2968-5
Yu.S. Hordieiev, E.V. Karasik, A.V. Zaichuk, Silicon (2022). https://doi.org/10.1007/s12633-022-01745-0
S. Banijamali, T. Ebadzadeh, J. Non Cryst. Solids 441, 34 (2016). https://doi.org/10.1016/j.jnoncrysol.2016.03.014
A.M. Alsharari, A. Alatawi, S.A. Issa et al., Mater. Res. Express 6(12), 125201 (2019). https://doi.org/10.1088/2053-1591/ab5632
Yu.S. Hordieiev, АA. Amelina, Voprosy Khimii i Khimicheskoi Tekhnol. 5, 43–49 (2021). https://doi.org/10.32434/0321-4095-2021-138-5-43-49
Y.S. Hordieiev, E.V. Karasik, АA. Amelina, Voprosy Khimii i Khimicheskoi Tekhnol 3, 83–89 (2021). https://doi.org/10.32434/0321-4095-2021-136-3-83-89
N. Lahl, K. Singh, L. Singheiser, K. Hilpert, D. Bahadur, J. Mater. Sci. 35, 3089 (2000). https://doi.org/10.1023/A:1004851418274
S. Singh, G. Kalia, K. Singh, J. Mol. Struct. 1086, 239 (2015). https://doi.org/10.1016/j.molstruc.2015.01.031
K.H. Mahmoud, A.S.A. Alsubaie, E.A.A. Wahab, F.M. Abdel-Rahim, K.S. Shaaban, Silicon 14, 3419 (2022). https://doi.org/10.1007/s12633-021-01125-0
P.K. Ojha, S.K. Rath, K. Sudarshan, S.K. Sharma, P.K. Pujari, T.K. Chongdar, J. Inorg. Organomet. Polym. Mater. 27, 231 (2017). https://doi.org/10.1007/s10904-017-0674-x
S.A. Algarni, A.A. El-Maaref, B.M. Alotaibi, N. Alharbiy, A.F.A. El-Rehim, E.A.A. Wahab, K.S. Shaaban, J. Inorg. Organomet. Polym. Mater. (2022) https://doi.org/10.1007/s10904-022-02321-0
F. Branda, F. Arcobello-Varlese, A. Costantini, G. Luciani, J. Non-Cryst Solids 246(1–2), 27–33 (1999). https://doi.org/10.1016/S0022-3093(99)00039-3
A.M. Al-Baradi, E.A.A. Wahab, K.S. Shaaban, Silicon 14, 5277 (2022). https://doi.org/10.1007/s12633-021-01286-y
K.S. Shaaban, M.S.I. Koubisy, H.Y. Zahran, I.S. Yahia, J. Inorg. Organomet. Polym. Mater. 30, 4999 (2020). https://doi.org/10.1007/s10904-020-01640-4
N. Sasmal, M. Garai, A.R. Molla, A. Tarafder, S.P. Singh, B. Karmakar, J. Non-Cryst Solids 387, 62–70 (2014). https://doi.org/10.1016/j.jnoncrysol.2013.12.030
N.H. Ray, J Non-Cryst Solids 15(3), 423–434 (1974). https://doi.org/10.1016/0022-3093(74)90148-3
P.W. McMillan, Glass-ceramics, 2nd edn. (Academic Press, London, 1979)
L. Deng, X. Zhang, M. Zhang, X. Jia, Z. Zhang, B. Li, J. Alloys Compd. 785, 932–943 (2019). https://doi.org/10.1016/j.jallcom.2019.01.260
S.M. Salman, S.N. Salama, H.A. Abo-Mosallam, Silicon 3, 199–205 (2011). https://doi.org/10.1007/s12633-011-9099-2
N.J. Smith, C.G. Pantano, Appl. Phys. A Mater. Sci. Process. 116, 529 (2014). https://doi.org/10.1007/s00339-014-8467-3
C. Chen, C. Zhong, Y. Zhang, A. Li, S. Huang, H. Zeng, Q. Zu, Ceram. Int. 48, 22444 (2022). https://doi.org/10.1016/j.ceramint.2022.04.259
Z. Wang, J. Zhang, M. Zhong, C. Wang, Metall. Mater. Trans. B 53, 1364 (2022). https://doi.org/10.1007/s11663-022-02507-4
H. Gui, C. Li, C. Lin, Q. Zhang, Z. Luo, L. Han, J. Liu, T. Liu, A. Lu, J. Eur. Ceram. Soc. 39, 1397 (2019). https://doi.org/10.1016/j.jeurceramsoc.2018.10.002
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, S.A. Makhlouf, K.S. Shaaban, Silicon 13, 2289 (2021). https://doi.org/10.1007/s12633-020-00798-3
J. Wan, J. Cheng, P. Lu, J. Wuhan Univ. Technol. Mat. Sci. Edit 23, 419 (2008). https://doi.org/10.1007/s11595-007-3419-9
W. Luo, Z. Bao, W. Jiang, J. Liu, G. Feng, Y. Xu, H. Tang, T. Wang, Ceram. Int. 45(18), 24750–24756 (2019). https://doi.org/10.1016/j.ceramint.2019.08.215
S. Huang, S. Li, F. Wu, Y. Yue, J. Inorg. Organomet. Polym. Mater. 25, 816 (2015). https://doi.org/10.1007/s10904-015-0164-y
X. Gao, Q. Zhang, J. Yu, W. Tang, Y. Li, A. Lu, J. Non Cryst. Solids 481, 98 (2018). https://doi.org/10.1016/j.jnoncrysol.2017.10.032
N.A. Wójcik, S. Ali, D. Möncke, N.S. Tagiara, E.I. Kamitsos, H. Segawa, M. Eriksson, B. Jonson, J. Non Cryst. Solids 521, 119532 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.119532
Acknowledgements
The authors gratefully acknowledge the financial support from the Ministry of Education and Science of Ukraine (project No. 0120U101969).
Funding
This work was supported by the Ministry of Education and Science of Ukraine (project No. 0120U101969).
Author information
Authors and Affiliations
Contributions
YSH wrote the manuscript, conceived and designed the experiments, performed experimental work; AVZ provided the reagents and assisted in the experimental work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The manuscript has not been published elsewhere and is not under consideration by any other journals.
Competing Interest
The authors declare that they have no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hordieiev, Y.S., Zaichuk, A.V. Study of the Influence of R2O3 (R = Al, La, Y) on the Structure, Thermal and some Physical Properties of Magnesium Borosilicate Glasses. J Inorg Organomet Polym 33, 591–598 (2023). https://doi.org/10.1007/s10904-022-02526-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02526-3